Abstract
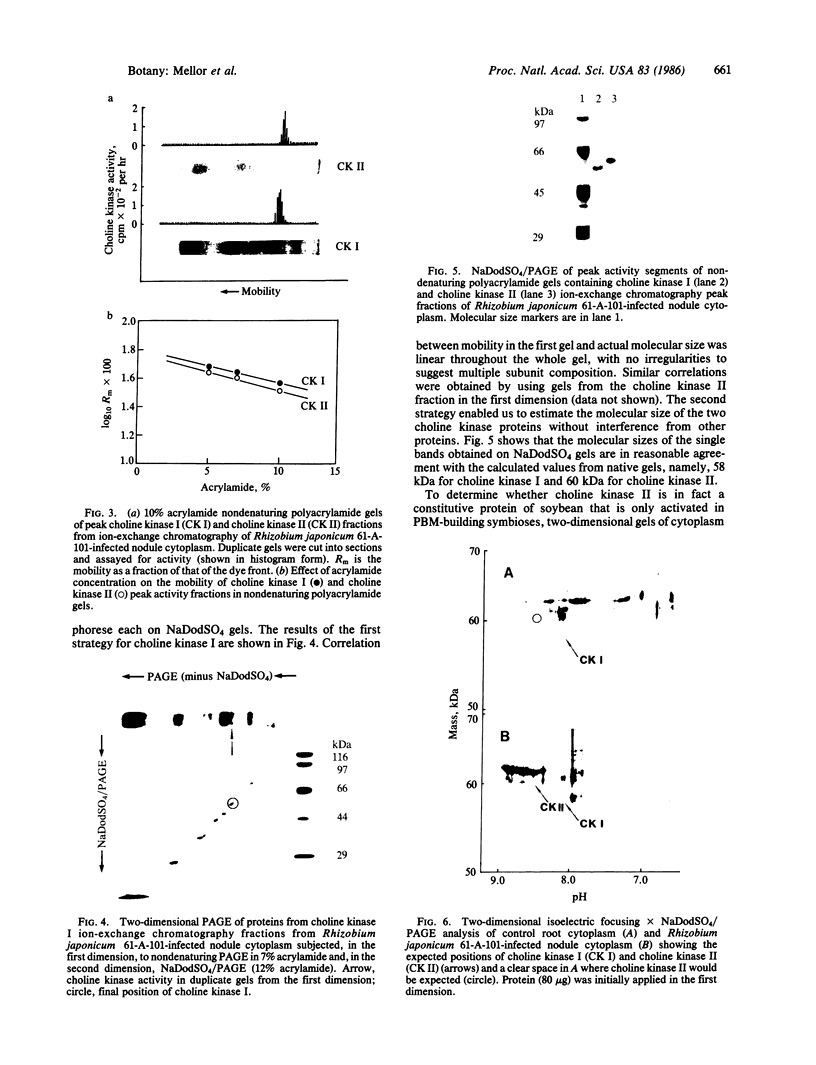
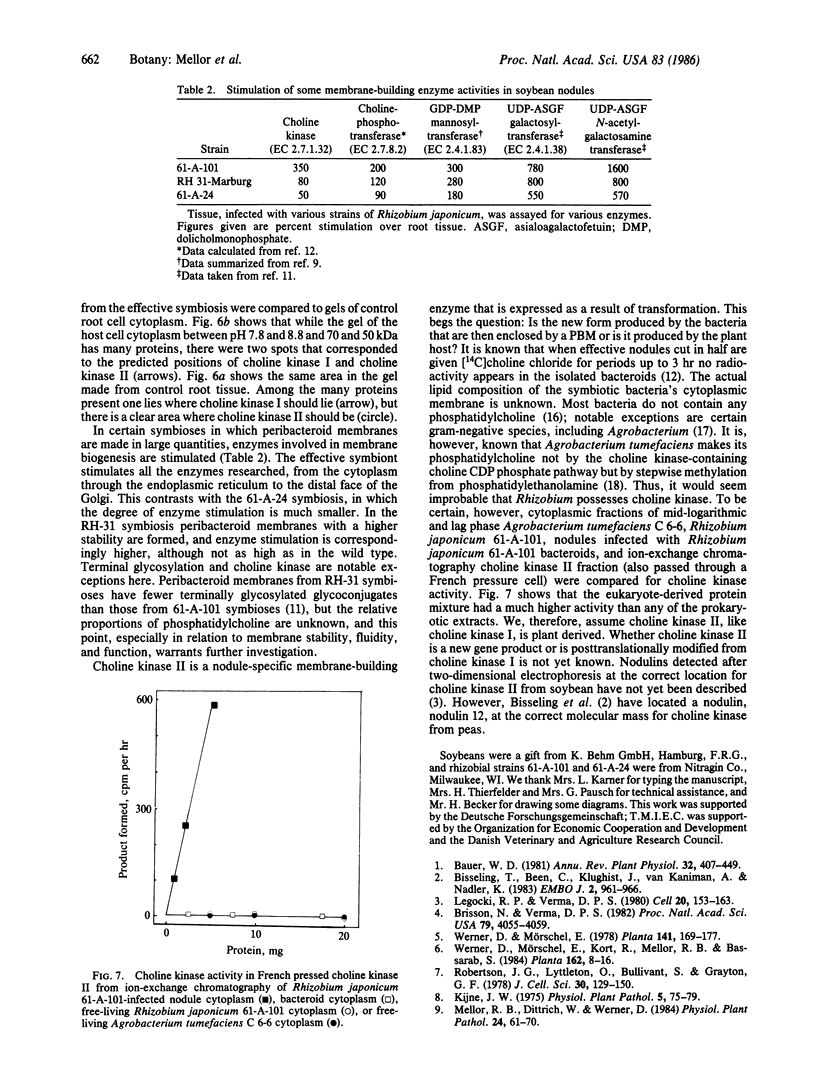
Host-cell cytoplasm from soybean plants infected with the peribacteroid membrane (PBM)-building Rhizobium japonicum strain 61-A-101 (effective, N2-fixing) had much higher choline kinase activity than cytoplasm from either uninfected tissue or tissue infected with the non-PBM-building (ineffective, non-N2-fixing) strain 61-A-24. Ion-exchange chromatography showed that both types of nodule and root tissue possessed constitutive choline kinase I activity that had a Km for choline of ≈150 μM. The nodules of the effective symbiosis had another activity, choline kinase II (Km = 81 μM). Nondenaturing and NaDodSO4 electrophoresis revealed no multimeric subunit structure of the two enzyme forms but did show the molecular sizes for choline kinase I, 58-59 kDa, and choline kinase II, 60 kDa. Choline kinase I and II and pI values of 8.1 and 8.5, respectively, and two-dimensional gel electrophoresis of whole cytoplasm from control and infected tissue showed a spot corresponding to choline kinase II only in the case of the effective symbiosis, whereas both tissue types had spots corresponding to choline kinase I. Choline kinase II is presumed to be encoded by the plant as neither free-living nor symbiotic (bacteroid) forms of the prokaryote showed any choline kinase activity.
Keywords: nodulin-like protein, Rhizobium japonicum, Glycine max, symbiosis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P., Bullivant S., Grayston G. F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J Cell Sci. 1978 Apr;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]