Abstract
Experimental models of Parkinson’s disease (PD) are of great importance for improving the design of future clinical trials. Various neurotoxic models are available, including 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat and rotenone. However, no model is considered perfect; each has its own limitations. Based on epidemiological data, a new trend of using environmental toxins in PD modeling seems attractive and has dominated public discussions of the disease etiology. A search for new environmental toxin-based models would improve our knowledge of the pathology of the condition. Here, we discuss some toxins of natural origin (e.g. cycad-derived toxins, epoxomicin, Nocardia asteroides bacteria, Streptomyces venezuelae bacteria, annonacin and DOPAL) that possibly represent a contributory environmental component to PD.
Keywords: animal models, natural toxins, Parkinson’s disease, therapeutic testing
Introduction
The clinical motor dysfunction in Parkinson’s disease (PD) is primarily linked to the depletion of dopamine in the striatum consecutive to the loss of dopaminergic neurons in the substantia nigra (SNc). Even with the advent of powerful new tools such as genomics, proteomics, brain imaging, gene replacement therapy and knockout animal models, the desired end result of neuroprotection is still beyond our current capability [Arias-Carrión et al. 2009]. An integrated approach towards understanding the pathogenesis of this disorder may help in discovering and developing new therapies capable of improving the disease in addition to controlling its clinical manifestations [Obeso et al. 2010; Arias-Carrión et al. 2007]. In sporadic PD, it is thought that exposure to environmental toxins may be a causative factor, perhaps in individuals rendered susceptible by their genetic profile and/or advancing age [Caldwell et al. 2009]. The NIH Committee to Identify Neuroprotective Agents in Parkinson’s (CINAP) published a systematic assessment of currently available pharmacologic neuroprotective agents. However, the CINAP members stated that using multiple PD models and testing drugs on a variety of animals would represent a problem in comparing different therapeutic modalities [Emborg, 2004]. Despite a broad spectrum of models, none is considered a perfect model. This led to an obvious question; what model can be used to verify new neuroprotective drugs? In fact, failure of the available animal models to recapitulate the pathology of Parkinsonism may herald the application of therapeutic approaches in clinical cases [Lohle and Reichmann, 2010; Lane and Dunnett, 2008].
In PD, the primary pathology is the degeneration of dopaminergic neurons in the SNc, resulting in loss of the nigrostriatal pathway and a reduction of dopamine levels in the striatum [Obeso et al. 2010; Arias-Carrion et al. 2009, 2007]. However, neuronal death also occurs in other brain regions, including the locus coeruleus (LC), dorsal motor nucleus of the vagus (DMN) and nucleus basalis of Meynert (NMB), and this can be more severe than neuronal death in the SNc. At these and other pathological sites, protein-rich structures known as Lewy body inclusions appear in the cytoplasm of some remaining neurons [Dawson et al. 2010]. In this context, finding a natural agent that can induce the complete pathological picture of the disease would represent a breakthrough in the field of PD research [Caldwell et al. 2009; McNaught et al. 2004]. Given the extent of knowledge in the study of PD modeling, it is ambitious to describe all models. However, we have reviewed the limitations of available neurotoxic models of PD and some natural toxins implicated as triggering the disorder.
Classic neurotoxin-induced animal models of Parkinson’s disease
Whereas recent genetic discoveries have led to a number of different genetic models of PD, they failed to reproduce the broad extranigral pathology and other pathological landmarks of PD such as Lewy bodies [Hardy, 2010; Hisahara and Shimohama, 2010; Gasser, 2009; Lim and Ng, 2009; Meredith et al. 2008]. Progress will rely on understanding genetic mutations or susceptibility factors that lead to PD, better translation between preclinical animal models and clinical research, and improving the design of future clinical trials. In this regard, the neurotoxic models remained the cornerstone in simulating PD [Bove et al. 2006] and several lines of evidence point to environmental exposure being the contributory factor in the pathogenesis of this disorder.
6-hydroxydopamine
6-hydroxydopamine (6-OHDA) is a selective catecholaminergic neurotoxin that is widely used to study mechanisms of cell death in dopaminergic neurons [Schober, 2004; Ungerstedt, 1968] since it is thought to be taken up by the dopamine transporter (DAT). When injected into the striatum of rats, 6-OHDA produces a protracted retrograde degeneration of nigrostriatal neurons over several weeks and leads to a stable and permanent depletion of tyrosine hydroxylase (TH)-positive nigral neurons [Schober, 2004]. Systemically administered 6-OHDA fails to cross the blood–brain barrier, so it has to be injected locally using stereotaxic procedures to obtain a unilateral lesion [Betarbet et al. 2002; Ungerstedt, 1968]. Usually, there are three sites where injections are made; into the medial forebrain bundle, the SNc or the striatum [Schober, 2004; Betarbet et al. 2002]. Bilateral 6-OHDA injections do not represent the most frequently used model, since bilaterally affected animals require intensive care [Schober, 2004; Betarbet et al. 2002]. Therefore, it represents a model for end-stage PD, which is considered to be a limiting factor of this model. To overcome this limitation, Richter and colleagues developed a model with multiple low-dose injections to better replace the single-injection model [Richter et al. 2008]; however, this model needs multiple injections or special techniques for implanting a pump which carry the risk of obstruction or failure, the extent of the neuronal loss is moderate and the technical expense limits the utility as a subchronic model [Richter et al. 2008; Schober, 2004].
Returning to the standard model of PD, it has many limitations: it is unilateral, does not produce the entire neuropathology of PD and carries the risk of mechanical damage due to the invasive procedure used in its administration [Richter et al. 2008; Schober, 2004; Ungerstedt, 1968].
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
The potent neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), produces many of PD pathological findings. Humans andmany other animal species including nonhuman primates, guinea pigs, mice and cats are susceptible to this neurotoxin [Haobam et al. 2005]. After its systemic administration, MPTP (being highly lipophilic) rapidly crosses the blood–brain barrier. Once in the brain, it is metabolized to 1-methyl-4-phenyl-2,3-dihydropyridinium (MPDP+) by the enzyme monoamine oxidase B (MAO-B) and then (probably by spontaneous oxidation) to 1-methyl-4-phenylpyridinium (MPP+), which is considered the active toxin (MPTP →MPDP+ →MPP+) [Speciale, 2002].
MPP+ (which is far less lipophilic than MPTP) depends on specialized carriers to enter into adjacent neurons [Speciale, 2002]. Once in the mitochondria, MPP+ inhibits cellular respiration through the blockade of the electron transport enzyme NADH: ubiquinone oxido-reductase (complex I). Moreover, studies have shown that MPP+ can also directly inhibit complexes III (ubiquinol: ferrocytochrome c oxidoreductase) and IV (ferrocytochrome c:oxygen oxidoreductase or cytochrome c oxidase) of the electron transport chain [Speciale, 2002]. This is not the complete story, as many cofactors seem to contribute to MPTP-induced toxicity, e.g. iron and neuromelanin, vesicular monoamine transporter (VMAT2) levels, reactive oxygen species (ROS) production, apoptosis and changes in pre-mRNA transcripts splicing [Potashkin and Meredith, 2006; Blum et al. 2001; Lotharius and O’Malley, 2000].
One interesting feature of the MPTP mouse model of PD is the transient nature of the striatal damage in young mice. In contrast, administration of the drug to older mice results in a permanent loss of nigrostriatal terminals, as well as cell bodies. This recovery potential in young mice allows for modeling of recovery stages [Jakowec and Petzinger, 2004; Tillerson and Miller, 2003]. Various techniques can be used to modulate the action of MPTP to induce the pathological findings needed to reach the desired PD model. One of these techniques is to give probenecid with MPTP to prolong its persistence in the tissues [Petroske et al. 2001].
Several MPTP dosing regimens have been used. The acute regimen consists of multiple systemic administration of MPTP (usually four doses at 2-h intervals per day). The subacute regimen consists of a single systemic administration per day for several consecutive days (usually 5 days) and the chronic regimen through several weeks [Meredith et al. 2008]. The comparison of these different models indicated clearly that different schedules of administration of MPTP mimic distinct stages of the disease and might induce different mechanisms of neuronal death [Schober, 2004].
Despite being one of the most popular toxic PD models, MPTP has many limitations, the most important of which is the absence of inclusion bodies in DA neurons. Also MPTP models lack the behavioral deficits apparent on standard motor mouse tests [Kim et al. 2010].
Paraquat
The herbicide 1,1-dimethyl-4,4-bipyridium (paraquat, PQ) has been suggested as a possible PD inducing agent in the mid 1980s following the observation that its chemical structure closely resembles that of MPP+ (1-methyl-4-phenylpyridinium ion) [McCormack et al. 2002]. Exposure of mice to paraquat leads degeneration of dopaminergic nigrostriatal neurons. Moreover, some authors report appearance of inclusion bodies containing α-synuclein in the paraquat model which makes it similar to PD pathology [Peng et al. 2005; McCormack et al. 2002].
Despite the similarity with MPTP, there are certainly toxicokinetic and toxicodynamic differences which give paraquat a unique pattern of neurotoxicity [Prasad et al. 2009]. An improvement in modeling of paraquat induced PD can be achieved through adding maneb (another neurotoxic herbicide) [Uversky, 2004]. However, paraquat has a higher systemic toxicity profile which may lead to higher mortalities in the experimental animals [Prasad et al. 2009].
Rotenone
Rotenone is a naturally occurring pesticide derived from the roots of Derris elliptica species and it is known to be an inhibitor of mitochondrial complex I. Chronic systemic exposure to rotenone reproduces many features of PD, including nigrostriatal dopaminergic degeneration and the formation in nigral DA neurons of cytoplasmic inclusions. Rats show bradykinesia, postural instability, unsteady gait and some evidence of tremors [Shimohama et al. 2003]. However, Höglinger and colleagues collected several arguments against suitability of the rotenone model, e.g. lack of recapitulation of PD symptoms, degeneration of striatal neurons, accumulation of tau protein which is more abundant than α-synuclein aggregation and the high mortality rate of the examined animals with subsequent less accurate results obtained [Höglinger et al. 2006]. In Table 1 we list the classical toxins used for inducing PD in animals.
Table 1.
Characteristics and limitations of available toxic Parkinson’s disease (PD) models.
| Toxic model | Characteristics | Limitations |
|---|---|---|
| 6-OHDA |
|
|
| MPTP |
|
|
| Rotenone |
|
|
| Paraquat |
|
|
6-OHDA, 6-hydroxydopamine; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
PD models – where do we stand?
Emborg [2004] stated that no single model is perfect for PD. The same conclusion was obtained by Shimohama and colleagues suggesting further work to reach ’the optimal PD model’ [Shimohama et al. 2003]. Manning-Bog and Langston advised using a model fusion to overcome the single model limitations through combining genetic with toxic models [Manning-Bog and Langston, 2007]. There is no question that using a combined toxic–genetic model is a promising strategy. However, choosing the perfect toxic substance which mimics ‘natural’ conditions is mandatory. Here we have tried to draw together the possible candidate toxins that can be used for this purpose.
Natural PD inducing toxins
While use of pesticides has been suggested to be partially responsible for PD in rural areas, this is not correlated to disease prevalence, as the odds ratio for farming itself cannot be accounted for by pesticide exposure alone [Caldwell et al. 2009]. Associated with rural living, humans have a strong relation with the surrounding environment, and both individual exposure (e.g. occupation) or group exposure (e.g. drinking well water) are present. As such, exposure to environmental agents may contribute to the onset or progression of PD [Pereira and Garrett, 2010].
The success of new environmental toxins to develop mitochondrial complex I inhibition and degeneration of dopaminergic neurons in vitro [Abdulwahid Arif and Ahmad Khan, 2010], coupled with epidemiological data suggesting natural environmental toxin involvement in Parkinsonism pathogenesis would invite us to think of these natural toxins as new candidates for developing models [Tanner, 2010].
The choice of certain natural toxins for PD modeling needs some guidelines as supposed by Shaw and Höglinger [2008]. Based on many works dealing with natural toxins some properties must be fulfilled to make them suitable candidates for toxic models. These characteristics include:
The agent must be of natural origin.
The agent must be available worldwide to contribute to the wide prevalence of PD in the whole world.
The agent must recapitulate PD pathology in experimental animals.
The following natural agents represent promising candidates for application in experimental PD models. Although they do not show the criteria of an ideal PD model, we must put in consideration the fact that they need further research regarding the dosing regimen and species vulnerability before comparing their value to the classical toxins of PD models.
Cycads
The Cycadaceae (cycad) family contains about 45 primitive species of seed-bearing plants that probably dominated the world’s vegetation during the Jurassic and early Cretaceous periods [Barceloux, 2009]. Cycads, like all plants, produce a variety of secondary substances. The most important of such allelochemicals are, in addition to dimeric flavones, the nitrogen-containing methylazoglucosides cycasin, macrozamin and several neocycasins. Moreover, there is a minor cycad toxin which is an unusual, nonprotein amino acid, a derivative of alanine (beta-methylamino alanine [BMAA]), which in higher concentrations has been found to be neurotoxic to mammals and chickens [Schneider et al. 2002].
The linkage between cycads and neurodegenerative diseases began in 1945. In that year Zimmerman reported cases of motor neuron disease among Chamorros, the local citizens of Guam. Later, it was confirmed that the disease was clinically and pathologically similar to amyotrophic lateral sclerosis (ALS), although it was familial and confined to local Chamorros (it was 100 times more prevalent than elsewhere in the world) [Spencer et al. 2009].
Another atypical Parkinson-like syndrome was identified with onset in middle life and accompanied by mental slowness. This syndrome was noticed in the same families who developed the ALS syndrome. In 1961, Hirano and colleagues introduced the term Parkinsonism–dementia complex (PDC) of Guam, and they also noted the widespread presence of Alzheimer-type neurofibrillary tangles (NFTs) in those with the syndrome. It seemed that the ALS and PDC syndromes represented the spectrum of a single disease. This hypothesis was based on the observation of NFTs in both clinical entities [Konagaya et al. 2003].
Two additional links between the Guam syndrome and other neurodegenerative diseases appeared. The first is the transactive response (TAR) DNA-binding protein 43 (TDP-43) and the second is the leucine-rich repeat kinase 2 (Lrrk2). TDP-43 functions as a transcriptional repressor and splicing regulator where it is present in ubiquitin-positive, tau and α-synuclein negative inclusions in frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U) and in ALS. Moreover, it has been shown to occur in some cases of Lewy body disorders alone or in association with AD. Although the role of TDP-43 in these proteinopathies is unknown, it may provide a common pathologic link [Hasegawa et al. 2007].
LRRK-2 (which is strongly linked to autosomal dominant Parkinson disease) may be central to the pathogenesis of several major neurodegenerative disorders associated with Parkinsonism [Wood-Kaczmar et al. 2006]. Pathologic studies of both ALS/PDC and nonsymptomatic Chamorro cases reveal that they are more prone to NFT development. Immunohistochemical studies reveal that the tau isoform distribution is similar to AD; however, the cortical laminar distribution is similar to progressive supranuclear palsy (PSP). In these lesions, immunostaining with antibodies to TDP-43 and LRRK-2 shows a commonality with PD, ALS, and some other neurodegenerative disorders [Spencer et al. 2009].
Failure to find any genetic abnormalities in Guam cases led scientists to think about an environmental component and more specifically Cycas micronesica which is the source of flour of fadang, a traditional food in this locality [Spencer et al. 2005].
The first incriminated toxic component of C. micronesica was the nitrogen-containing methylazoglucosides cycasin. In rat neuronal cultures, the aglycone of cycasin methylazoxymethanol (MAM) can damage neuronal DNA, disrupt glutamate transmission, and promote the accumulation of tau protein and neuronal degeneration [Morris et al. 2001]. However, failure to induce neuropathology in experimental animals using cycasin excluded it from being the causative toxin [Schneider et al. 2002].
Another attractive toxic compound was BMAA. The possible role of BMAA in ALS/PDC replaced the old cycasin theory; however, it was not before 1987 when Spencer and colleagues managed to show the capacity of BMAA to induce pathological changes in primates’ brain. The interest toward BMAA as a putative toxic agent damaging DAergic cells has grown considerably due to the discovery that this toxin is not only present in the Guamanian islands but appears to be ubiquitous throughout the world [Jonasson et al. 2010; Murch et al. 2004; Cox et al. 2003]. This natural amino acid deposits in human tissue proteins. This protein binding represents a source of chronic exposure through protein catabolism [Murch et al. 2004]. BMAA is distributed worldwide with the chance of frequent repetitive exposures.
For this reason, recent studies properly quantified the concentration of BMAA in postmortem brain tissues of patients [Pablo et al. 2009]. The deleterious mechanisms of the toxin could underlie neurological conditions such as ALS–PDC and, potentially, idiopathic PD. Some studies have investigated the effects of BMAA on nigrostriatal DAergic neurons either indirectly (by measuring the ability of these cells to uptake or release dopamine) or directly (through studying the effects of BMAA on single DAergic neurons of the SNpc in an in vitro slice preparation). It was found that brief BMAA applications, at a concentration similar to that found in ALS–PDC brains [Pablo et al. 2009; Murch et al. 2004], induced reversible and reproducible membrane depolarizations/inward currents, firing increase, and Ca2+ accumulation. In contrast, prolonged exposures (12 min) caused functional impairment, cellular shrinkage, calcium overload, cyt-c release, and ROS production that did not return to control values for up to 20 min or several hours after BMAA washout.
Cucchiaroni and colleagues found that the action of BMAA may be due to activation of TRPC-like channels, through mGluR1. Interestingly, GABAergic SNpc cells respond to BMAA with an AMPA-mediated inward current that is not associated with an intracellular calcium increase [Cucchiaroni et al. 2010]. BMAA has been previously reported to activate mGluRs in hippocampal, striatal, and cerebellar neurons [Lobner et al. 2007]. Alternatively, it predominantly activates NMDA receptors in cortical neurons and AMPA/kainate receptors in spinal motoneurons [Rao et al. 2006].
The BMAA hypothesis, however, faced criticism because of the high doses used in the experiment which cannot be true to real life. This criticism led to fading of the BMAA theory for a while. The enthusiasm towards BMAA was regained with recent findings of the possible exposure to high doses (in contrast to what was expected before) through the bound fraction of the toxin rather than the free amino acid, the biomagnification of BMAA exposure through eating animals previously fed on the cycad plants, e.g. flying fox, and the transfer of this cyanotoxin through aquatic ecosystems suggesting further human exposure [Jonasson et al. 2010; Chen et al. 2002; Cox and Sacks, 2002].
BMAA as an inducer of PD faces a lot of debate;first, there is no detected animal model for such a toxin; second, it lacks specificity towards SN. In other words, BMAA cannot be considered a specific PD-inducing toxin [Karamyan and Speth, 2008]. The previous research points out that cycads are not an attractive research tool in the field of PD modeling. However, a recent finding of the possible role of cycads was based on another constituent (sterol glucosides).
Cycad’s sterol glucosides
Recently a new direction rose proposing a new component in cycads as the agent responsible for neurodegeneration. These sterol b-D-glucosides have been isolated by Schulz and colleagues, who found that these glucosides induce the excitotoxic release of glutamate [Schulz et al. 2006].
Based on this hypothesis, Shen and colleagues used washed cycad seeds to make flour pellets (the washed seeds are devoid of BMAA) and fed them to outbred Sprague-Dawley rats [Shen et al. 2010]. The experiments were performed with the intention of developing a model of ALS–PDC. However, the rats showed Parkinsonism pathology without ALS syndrome. Also the cycad-fed rats showed the gradual development of multiple PD motor abnormalities after 2–3 months of feeding, including spontaneous unilateral rotation, shuffling gait, and stereotypy. Histological and biochemical examination of brains from cycad-fed rats revealed an initial decrease in the levels of dopamine and its metabolites in the striatum, followed by neurodegeneration of dopaminergic cell bodies in the SNc. Alpha-synuclein and ubiquitin aggregates were found in the DAergic neurons of the SNc and neurites in the striatum (STR). In addition, α-synuclein aggregates were found in the neurons of the locus coeruleus and cingulate cortex. In an organotypic slice culture of the rat SN and the striatum, an organic extract of cycad causes a selective loss of dopamine neurons and α-synuclein aggregates in the SN.
Unlike genetic models or toxicant-based end-stage models of Parkinsonism that may have a great deal of phenotypic similarity among animals, there is considerable variability in the response of individual animals to cycad ingestion. However, one of the strengths of this cycad-fed rat model is that it recapitulates the variability and progression of symptoms and neuropathology seen in human Parkinsonism. Moreover, this model also allows examination of the early stages of the disorder based on the chronic progressive nature of the induced disease.
This new trend of using the seeds without attempting to extract a single toxic component may prove useful. In fact, it seems that this approach is more representative of real life exposure. Despite the debate about which toxic component in cycads is responsible for neurodegeneration, they represent a possible new substance for PD modeling with proven capacity to induce progressive and chronic neurodegeneration in a fashion similar to reality [Bradley and Mash, 2009]. To sum up, we can see that cycad offers a possible natural toxin candidate. It has been linked epidemiologically to a neurodegenerative condition with pathological similarity to PD: cycads are available worldwide and their use in in vivo studies could recapitulate the pathological features of PD [Shen et al. 2010].
Although cycad seeds have been linked to ALS/PDC syndrome, the recent findings of Shen and colleagues introduce them as promising tool for modeling PD experimentally [Shen et al. 2010].
Epoximicin
Epoximicin is a natural proteasome inhibitor (PI). It is a product of actinomycetes bacteria which live in moist soil. When the bacteria release this toxic metabolite it can pass into the underground water store providing a route for human exposure [Ensign et al. 1993].
PIs are small, bioavailable molecules that attack the 20S proteasomal subunit, the primary site responsible for the cleavage of peptide bonds. By covalently binding to the 20S subunit, PIs successfully block proteolysis and degradation of proteins. Defects in the ubiquitin–proteasome system (UPS) and degradation of unwanted proteins are considered a common feature in PD. Moreover, in hereditary forms of PD, mutations in the UPS (parkin and ubiquitin C-terminal hydrolase L1) are responsible for the development of the disease. Mayer used the Cre-recombinase/loxP genetic approach to ablate the proteasomal Psmc1 ATPase gene and deplete 26S proteasomes in neurons in different regions of the brain to mimic neurodegeneration [Mayer, 2003]. This approach generates dopaminergic neurodegeneration accompanied by Lewy pathology. Based on the previous data, proteasome inhibition can be used to induce PD [Bedford et al. 2009].
The original idea of using epoximicin as a systemic PI in PD modeling was presented by McNaught and colleagues who showed that systemic exposure of rats to epoximicin can induce a model of PD. After a latency of 1–2 weeks, treated animals develop a gradually progressive, L-dopa/apomorphine-responsive, Parkinsonian syndrome with a decrease in striatal 11C-CFT binding on positron emission tomography (PET). Pathologically, there is neurodegeneration in the SNc, as well as in the locus coeruleus (LC), dorsal motor nucleus of the vagus (DMN), and nucleus basalis of Meynert (NMB), a pattern of neuronal loss similar to that found in PD. Furthermore, neurodegeneration is accompanied by intracytoplasmic Lewy body-like inclusions that stain positively for α-synuclein, ubiquitin, and other proteins [McNaught et al. 2004]. Thus, PIs can induce a Parkinsonian syndrome in rats that more closely recapitulates the features of the illness in humans than other models.
The same model, however, has not been reproducible in many trials in rodents in spite of using the same doses as used initially. This raised concerns about the possible application of epoximicin in animal models of PD [Bove et al. 2006; Kordower et al. 2006]. Matsui and colleagues, however, demonstrated that the administration of this natural PI to medaka fish via the cerebrospinal fluid (CSF) induces PD-like symptoms in the form of reduced spontaneous movement. Fish treated with epoximicin developed inclusion bodies similar to Lewy bodies throughout the central nervous system, which are associated with PD. Treatment with epoximicin also induced selective loss of dopaminergic and noradrenergic neurons. So, in this model epoximicin replicated the cardinal signs of PD in the form of locomotor dysfunction, selective dopaminergic cell loss, and inclusion body formation. The reason for this controversy between models is unknown, but in medaka the TH-positive neurons have relatively large cell bodies with well-developed fibers. These large neurons may require more optimal conditions for survival and this may explain the increased vulnerability of these cells. The problems with animal models of epoximicin can be solved by increasing the dose to overcome the lesser vulnerability of animal neurons compared with the medaka fish [Matsui et al. 2010].
These findings demonstrate that epoximicin can be considered to be an effective dopaminergic neurotoxin that may offer a good model for PD, as it is widely distributed, linked epidemiologically to PD, and can re-induce the pathological features of PD experimentally.
Nocardia asteroides
The epidemic of postencephalic Parkinsonism was first described by von Economo in 1917. This gave rise to the belief that PD might be associated with an infectious agent such as a virus, more infectious agents such as Borrelia burgdorferi, Mycoplasma pneumoniae and Helicobacter pylori have been postulated as possible inducing agents for Parkinsonism [Tam et al. 2002].
Currently, there is a well-documented animal model for PD in mice, using the GUH-2 strain of Nocardia asteroides. N. asteroides is a bacterial strain found worldwide that is commonly isolated from soil and aquatic sources. It is a Gram-positive aerobic bacterium that primarily affects the lung and skin. Pathogenic strains can cause a variety of pulmonary, skin, systemic and brain lesions in immunocompromised or normal humans. N. asteroides (GUH-2) is neuroinvasive after tail vein injection into mice [Barry and Beaman, 2007].
The first experimental trial of N. asteroides was conducted by Kohbata and Beaman who through multiple injections of this pathogen were able to induce a PD-like condition in mice which was later confirmed by postmortem pathological examination [Kohbata and Beaman, 1991]. They found that after administration of a nonlethal dose of log phase cells of GUH-2 (<200 colony forming units reaching the brain), Nocardiae invaded through the capillary endothelium, crossed the basal lamina, and then grew perivascularly within the brain parenchyma without associated inflammatory responses. These bacteria grew primarily within neurons, along axons, and in astroglia. Approximately 10 days after infection, mice developed one or more features of neurological impairment. Later on, the headshaking, stooped posture, and bradykinesia tended to persist for the life of the mouse. However, administration of levodopa (20 mg/kg i.p.) caused transient reversal of head-shaking, tremors, and hypoactivity. These abnormal movements were confirmed by histopathological analysis of the brains from mice. Histopathological findings showed decrease of neurons in the substantia nigra, a loss of Nissl substance in the remaining neurons, and diminished immunostaining for tyrosine hydroxylase in the substantia nigra pars compacta. The previous data suggest a possible dopaminergic involvement in the mice inoculated with GUH-2. Another interesting finding in this model was the selective reduction in neostriatal DA concentrations at 1 week after GUH-2 inoculation, without a change in NE or 5HT concentrations. Moreover, the selective changes for neostriatal metabolism at 1 week suggest that GUH-2 has specificity for nigral neurons engaged in dopamine synthesis. A significant finding in the case of idiopathic PD is the Lewy body, although no animal model of Parkinsonism has led to their generation. In the case of GUH-2-induced models, hyaline-like intraneuronal inclusions were found in the murine brain, which is considered a major strength of this type of model with regards to its relation to the progression of disease [Hyland et al. 2000].
Based on many studies, we can say that Nocardia induce neurodegeneration in vivo and in vitro similar to that seen with MPTP, supporting this model for the study of Parkinsonism [Khobata and Beaman, 1991; Hyland et al. 2000]. Moreover, another study used N. otitidiscaviarum (GAM-5) which is isolated from a patient with an actinomycetoma. GAM-5 produced signs similar to PD following intravenous administration to NMRI mice. Motor signs included head tremor, akinesia/bradykinesia, flexed posture, deviation of the head, trunk flexion, and postural instability in a manner similar to N. asteroides. In both models (GAM-5 and GUH-2), the loss of bacteria in the brain coincided with the appearance of the motor impairments described above [Diaz-Corrales et al. 2004].
Why the mice developed signs of Parkinsonism after the apparent elimination of the bacteria from the brain is still unknown. A possible explanation could be the release of neurotoxic substances from the nocardial cells as they were killed and cleared from the brain [Beaman and Tam, 2008].
A final strength supporting the use of these bacteria in PD models is the intracytoplasmic hyaline-like inclusion bodies in neurons, mostly in the substantia nigra, that resemble Lewy bodies. Whether or not the chemical composition of these intracytoplasmic hyaline-like inclusion bodies is the same as that found in PD remains to be determined. Nevertheless, Nocardia-induced PD animal models represents a new, different, and promising approach based on the previous findings [Barry and Beaman, 2007].
Other natural agents
Many other agents have been suggested as possible substitutes to the classical PD-inducing agents. Some of them are of bacterial origin, e.g. Streptomyces venezuelae, and others are of plant origin, e.g. annonacin [Höllerhage et al. 2009].
On Guadeloupe island, the consumption of Annona muricata has been linked to sporadic taupathy. The main element in A. muricata (annonacin) can easily cross biological membranes, owing to its high lipophilicity, so is capable of entering nerve cells. After chronic exposure in rats, neuronal cell loss and gliosis were observed in the brain stem and basal ganglia [Caparros-Lefebvre and Lees, 2005].
The aggregation of the microtubule-associated protein (tau protein) characterizes several neurodegenerative diseases known collectively as tauopathies [Trojanowski and Lee, 2005]. While PD, the most frequent cause of Parkinsonism, is the best known of the α-synucleinopathies, tau pathology is also seen in many PD cases. Staining of some Lewy bodies with multiple tau antibodies has been reported, suggesting cellular colocalization of these two pathologies [Ding and Johnson, 2008]. Höllerhage and colleagues found that lipophilic complex I inhibitors of natural or synthetic origin can reproduce in vitro tauopathies. Exposure to annonacin led to phosphorylation and somatodendritic redistribution of tau in a manner similar to MPTP effects. Epidemiological and bioanalytical studies should be undertaken to verify this hypothesis. Moreover, in vivo experiments are needed to combine these findings with the data on absorption, distribution, metabolism, and elimination (ADME) which are still lacking [Höllerhage et al. 2009].
Another agent suggested by Panneton and colleagues was the dopamine metabolite DOPAL. They demonstrated that injections of DOPAL selectively kill SN DA neurons and trigger a behavior consistent with other PD animal models. This study supports the ‘catecholaldehyde hypothesis’ as an important link for the etiology of sporadic PD [Panneton et al. 2010]. However, future validation of DOPAL in PD modeling is needed to justify its use in experimental studies.
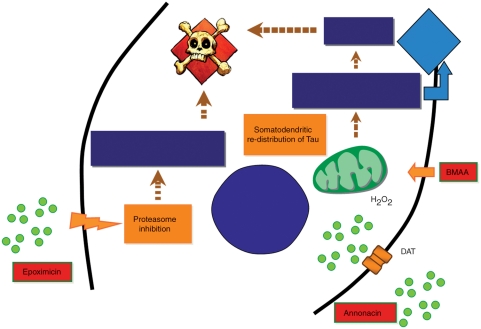
Table 2 shows a list of suggested natural agents that can be used in toxic PD models, while Figure 1 represents different mechanisms exerted by some natural toxins to induce PD.
Table 2.
Natural toxins that can be used in Parkinson’s disease models.
| Agent | Source |
|---|---|
| Sterol glucosides | Cycads |
| Epoximicin | Actinomycetes bacteria |
| N. asteroides | Nocardia asteroides bacteria |
| S. venezuelae | Streptomyces venezuelae bacteria |
| Annonacin | Annonacea plant |
| DOPAL | Dopamine metabolite |
Figure 1.
A diagram showing different pathways of some natural agents that can induce PD experimentally. Epoximicin through proteasome inhibition leads to the accumulation of unwanted scaffold proteins which will further lead to dopaminergic degeneration and inclusion body formation. Beta-methylamino alanine (BMAA) through an increase in reactive oxygen species (ROS) and increasing calcium intake will lead to cellular degeneration. Annonacin is working as a mitochondrial complex I inhibitor which affects oxidative process and aids in dopaminergic degeneration.
Conclusion
Owing to the limitations of the available PD models, a search for new modeling techniques seems mandatory to improve the PD research and evaluation of new promising therapies. Recently, natural toxins have seemed more appropriate to be used in PD models, depending on the epidemiological data that suggest the role of exposure to such natural agents in inducing PD. In our review we have explored some natural agents that can be used as targets for future research to replace classical toxic PD models. Based on previous research, some of the natural agents induce a chronic progressive PD model in rats with pathological findings recapitulating the pathology of this disease (cycad seeds, N. asteroides). Other agents prove efficient in inducing dopaminergic degeneration in cell cultures (annonacin) or medaka fish brains (epoximicin). However, most of the suggested natural agents need further optimization both on the levels of exposure dose and the precise pathological findings predicted from such natural toxins. We consider this review an invitation to conduct more research to validate possible natural agents inducing PD models. Only after appropriate design of PD models based on natural toxins can new therapeutic modalities be challenged more confidently for neuroprotection against PD.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Abdulwahid Arif I., Ahmad Khan H. (2010) Environmental toxins and Parkinson’s disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health 26: 121–128 [DOI] [PubMed] [Google Scholar]
- Arias-Carrión O., Freundlieb N., Oertel W.H., Höglinger G.U. (2007) Adult neurogenesis and Parkinson’s disease. CNS Neurol Disord Drug Targets 6: 326–335 [DOI] [PubMed] [Google Scholar]
- Arias-Carrión O., Yamada E., Freundlieb N., Djufri M., Maurer L., Hermanns G., et al. (2009) Neurogenesis in substantia nigra of Parkinsonian brains?. J Neural Transm Suppl 73: 279–285 [DOI] [PubMed] [Google Scholar]
- Barceloux D.G. (2009) Cycad seeds and chronic neurologic disease (Cycas species). Dis Mon 55: 353–360 [DOI] [PubMed] [Google Scholar]
- Barry D.P., Beaman B.L. (2007) Nocardia asteroides strain GUH-2 induces proteasome inhibition and apoptotic death of cultured cells. Res Microbiol 158: 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B.L., Tam S. (2008) An unusual murine behavior following infection with log-phase Nocardia asteroides type 6 strain GUH-2 (Nocardia cyriacigeorgica GUH-2). Microbes Infect 10: 840–843 [DOI] [PubMed] [Google Scholar]
- Bedford L., Paine S., Rezvani N., Mee M., Lowe J., Mayer R.J. (2009) The UPS and autophagy in chronic neurodegenerative disease: six of one and half a dozen of the other—or not?. Autophagy 5: 224–227 [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T.B., Greenamyre J.T. (2002) Animal models of Parkinson’s disease. Bioessays 24: 308–318 [DOI] [PubMed] [Google Scholar]
- Blum D., Torch S., Lambeng N., Nissou M., Benabid A.L., Sadoul R., et al. (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65: 135–172 [DOI] [PubMed] [Google Scholar]
- Bove J., Zhou C., Jackson-Lewis V., Taylor J., Chu Y., Rideout H.J., et al. (2006) Proteasome inhibition and Parkinson’s disease modeling. Ann Neurol 60: 260–264 [DOI] [PubMed] [Google Scholar]
- Bradley W.G., Mash D.C. (2009) Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler 10(Suppl. 2): 7–20 [DOI] [PubMed] [Google Scholar]
- Caldwell K.A., Tucci M.L., Armagost J., Hodges T.W., Chen J., Memon S.B., et al. (2009) Investigating bacterial sources of toxicity as an environmental contributor to dopaminergic neurodegeneration. PLoS One 4:e7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros-Lefebvre D., Lees A.J. (2005) Atypical unclassifiable Parkinsonism on Guadeloupe: an environmental toxic hypothesis. Mov Disord 20(Suppl. 12): S114–S118 [DOI] [PubMed] [Google Scholar]
- Chen K.M., Craig U.K., Lee C.T., Haddock R. (2002) Cycad neurotoxin, consumption of flying foxes, and ALS/PDC disease in Guam. Neurology 59: 1664, author reply 1664-1665 [DOI] [PubMed] [Google Scholar]
- Cox P.A., Banack S.A., Murch S.J. (2003) Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci U S A 100: 13380–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P.A., Sacks O.W. (2002) Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 58: 956–959 [DOI] [PubMed] [Google Scholar]
- Cucchiaroni M.L., Viscomi M.T., Bernardi G., Molinari M., Guatteo E., Mercuri N.B. (2010) Metabotropic glutamate receptor 1 mediates the electrophysiological and toxic actions of the cycad derivative beta-N-Methylamino-L-alanine on substantia nigra pars compacta DAergic neurons. J Neurosci 30: 5176–5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T.M., Ko H.S., Dawson V.L. (2010) Genetic animal models of Parkinson’s disease. Neuron 66: 646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Corrales F.J., Colasante C., Contreras Q., Puig M., Serrano J.A., Hernandez L., et al. (2004) Nocardia otitidiscaviarum (GAM-5) induces Parkinsonian-like alterations in mouse. Braz J Med Biol Res 37: 539–548 [DOI] [PubMed] [Google Scholar]
- Ding H., Johnson G.V. (2008) The last tangle of tau. J Alzheimers Dis 14: 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo C.V. (1917) Encephalitis lethargica. Wiener Klinische Wochenschrift 30: 581–585 [Google Scholar]
- Emborg M.E. (2004) Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J Neurosci Methods 139: 121–143 [DOI] [PubMed] [Google Scholar]
- Ensign J.C., Normand P., Burden J.P., Yallop C.A. (1993) Physiology of some actinomycete genera. Res Microbiol 144: 657–660 [DOI] [PubMed] [Google Scholar]
- Gasser T. (2009) Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med 11: e22. [DOI] [PubMed] [Google Scholar]
- Haobam R., Sindhu K.M., Chandra G., Mohanakumar K.P. (2005) Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav Brain Res 163: 159–167 [DOI] [PubMed] [Google Scholar]
- Hardy J. (2010) Genetic analysis of pathways to Parkinson disease. Neuron 68: 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Arai T., Akiyama H., Nonaka T., Mori H., Hashimoto T., et al. (2007) TDP-43 is deposited in the Guam Parkinsonism-dementia complex brains. Brain 130: 1386–1394 [DOI] [PubMed] [Google Scholar]
- Hirano A., Kurland L.T., Krooth R.S., Lessel S. (1961) Parkinsonism-dementia complex, an endemic disease on the island of Guam: 1. Clinical features. Brain 84: 642–661 [DOI] [PubMed] [Google Scholar]
- Hisahara S., Shimohama S. (2010) Toxin-induced and genetic animal models of Parkinson’s disease. Parkinsons Dis 2011: 951709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G.U., Oertel W.H., Hirsch E.C. (2006) The rotenone model of Parkinsonism—the five years inspection. J Neural Transm Suppl 70: 269–272 [DOI] [PubMed] [Google Scholar]
- Höllerhage M., Matusch A., Champy P., Lombes A., Ruberg M., Oertel W.H., et al. (2009) Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies. Exp Neurol 220: 133–142 [DOI] [PubMed] [Google Scholar]
- Hyland K., Beaman B.L., LeWitt P.A., DeMaggio A.J. (2000) Monoamine changes in the brain of BALB/c mice following sub-lethal infection with Nocardia asteroides (GUH-2). Neurochem Res 25: 443–448 [DOI] [PubMed] [Google Scholar]
- Jakowec M.W., Petzinger G.M. (2004) 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of Parkinson’s disease, with emphasis on mice and nonhuman primates. Comp Med 54: 497–513 [PubMed] [Google Scholar]
- Jonasson S., Eriksson J., Berntzon L., Spacil Z., Ilag L.L., Ronnevi L.O., et al. (2010) Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc Natl Acad Sci U S A 107: 9252–9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamyan V.T., Speth R.C. (2008) Animal models of BMAA neurotoxicity: a critical review. Life Sci 82: 233–246 [DOI] [PubMed] [Google Scholar]
- Kim S.T., Son H.J., Choi J.H., Ji I.J., Hwang O. (2010) Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain Res 1306: 176–183 [DOI] [PubMed] [Google Scholar]
- Kohbata S., Beaman B.L. (1991) L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun 59: 181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konagaya M., Kato T., Sakai M., Kuru S., Matsuoka Y., Konagaya Y., et al. (2003) A clinical and pathological study of a Japanese case of Amyotrophic Lateral Sclerosis/Parkinsonism-Dementia Complex with family history. J Neurol 250: 164–170 [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Kanaan N.M., Chu Y., Suresh Babu R., Stansell J., III, Terpstra B.T., et al. (2006) Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Ann Neurol 60: 264–268 [DOI] [PubMed] [Google Scholar]
- Lane E., Dunnett S. (2008) Animal models of Parkinson’s disease and L-dopa induced dyskinesia: how close are we to the clinic?. Psychopharmacology (Berl) 199: 303–312 [DOI] [PubMed] [Google Scholar]
- Lim K.L., Ng C.H. (2009) Genetic models of Parkinson disease. Biochim Biophys Acta 1792: 604–615 [DOI] [PubMed] [Google Scholar]
- Lobner D., Piana P.M., Salous A.K., Peoples R.W. (2007) Beta-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol Dis 25: 360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohle M., Reichmann H. (2010) Clinical neuroprotection in Parkinson’s disease - still waiting for the breakthrough. J Neurol Sci 289: 104–114 [DOI] [PubMed] [Google Scholar]
- Lotharius J., O’Malley K.L. (2000) The Parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem 275: 38581–38588 [DOI] [PubMed] [Google Scholar]
- Manning-Bog A.B., Langston J.W. (2007) Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotox Res 11: 219–240 [DOI] [PubMed] [Google Scholar]
- Matsui H., Ito H., Taniguchi Y., Inoue H., Takeda S., Takahashi R. (2010) Proteasome inhibition in medaka brain induces the features of Parkinson’s disease. J Neurochem 115: 178–187 [DOI] [PubMed] [Google Scholar]
- Mayer R.J. (2003) From neurodegeneration to neurohomeostasis: the role of ubiquitin. Drug News Perspect 16: 103–108 [DOI] [PubMed] [Google Scholar]
- McCormack A.L., Thiruchelvam M., Manning-Bog A.B., Thiffault C., Langston J.W., Cory-Slechta D.A., et al. (2002) Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10: 119–127 [DOI] [PubMed] [Google Scholar]
- McNaught K.S., Perl D.P., Brownell A.L., Olanow C.W. (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56: 149–162 [DOI] [PubMed] [Google Scholar]
- Meredith G.E., Totterdell S., Potashkin J.A., Surmeier D.J. (2008) Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord 14(Suppl. 2): S112–S115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H.R., Al-Sarraj S., Schwab C., Gwinn-Hardy K., Perez-Tur J., Wood N.W., et al. (2001) A clinical and pathological study of motor neurone disease on Guam. Brain 124: 2215–2222 [DOI] [PubMed] [Google Scholar]
- Murch S.J., Cox P.A., Banack S.A. (2004) A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc Natl Acad Sci U S A 101: 12228–12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J.A., Rodriguez-Oroz M.C., Goetz C.G., Marin C., Kordower J.H., Rodriguez M., et al. (2010) Missing pieces in the Parkinson’s disease puzzle. Nat Med 16: 653–661 [DOI] [PubMed] [Google Scholar]
- Pablo J., Banack S.A., Cox P.A., Johnson T.E., Papapetropoulos S., Bradley W.G., et al. (2009) Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol Scand 120: 216–225 [DOI] [PubMed] [Google Scholar]
- Panneton W.M., Kumar V.B., Gan Q., Burke W.J., Galvin J.E. (2010) The neurotoxicity of DOPAL:behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One 5: e15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Stevenson F.F., Doctrow S.R., Andersen J.K. (2005) Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem 280: 29194–29198 [DOI] [PubMed] [Google Scholar]
- Pereira D., Garrett C. (2010) [Risk factors for Parkinson disease: an epidemiologic study]. Acta Med Port 23: 15–24 [PubMed] [Google Scholar]
- Petroske E., Meredith G.E., Callen S., Totterdell S., Lau Y.S. (2001) Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 106: 589–601 [DOI] [PubMed] [Google Scholar]
- Potashkin J.A., Meredith G.E. (2006) The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid Redox Signal 8: 144–151 [DOI] [PubMed] [Google Scholar]
- Prasad K., Tarasewicz E., Mathew J., Strickland P.A., Buckley B., Richardson J.R., et al. (2009) Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain. Exp Neurol 215: 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.D., Banack S.A., Cox P.A., Weiss J.H. (2006) BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp Neurol 201: 244–252 [DOI] [PubMed] [Google Scholar]
- Richter F., Hamann M., Richter A. (2008) Moderate degeneration of nigral neurons after repeated but not after single intrastriatal injections of low doses of 6-hydroxydopamine in mice. Brain Res 1188: 148–156 [DOI] [PubMed] [Google Scholar]
- Schneider D., Wink M., Sporer F., Lounibos P. (2002) Cycads: their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften 89: 281–294 [DOI] [PubMed] [Google Scholar]
- Schober A. (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318: 215–224 [DOI] [PubMed] [Google Scholar]
- Schulz J.D., Hawkes E.L., Shaw C.A. (2006) Cycad toxins, Helicobacter pylori and Parkinsonism: cholesterol glucosides as the common denomenator. Med Hypotheses 66: 1222–1226 [DOI] [PubMed] [Google Scholar]
- Shaw C.A., Hoglinger G.U. (2008) Neurodegenerative diseases: neurotoxins as sufficient etiologic agents?. Neuromolecular Med 10: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.B., McDowell K.A., Siebert A.A., Clark S.M., Dugger N.V., Valentino K.M., et al. (2010) Environmental neurotoxin-induced progressive model of Parkinsonism in rats. Ann Neurol 68: 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S., Sawada H., Kitamura Y., Taniguchi T. (2003) Disease model: Parkinson’s disease. Trends Mol Med 9: 360–365 [DOI] [PubMed] [Google Scholar]
- Speciale S.G. (2002) MPTP: insights into Parkinsonian neurodegeneration. Neurotoxicol Teratol 24: 607–620 [DOI] [PubMed] [Google Scholar]
- Spencer P.S., Nunn P.B., Hugon J., Ludolph A.C., Ross S.M., Roy D.N., et al. (1987) Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science 237(4814): 517–522 [DOI] [PubMed] [Google Scholar]
- Spencer P.S., Palmer V., Kisby G. (2009) The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology 72: 474–476, author reply 475-476 [PubMed] [Google Scholar]
- Spencer P.S., Palmer V.S., Ludolph A.C. (2005) On the decline and etiology of high-incidence motor system disease in West Papua (southwest New Guinea). Mov Disord 20(Suppl. 12): S119–S126 [DOI] [PubMed] [Google Scholar]
- Tam S., Barry D.P., Beaman L., Beaman B.L. (2002) Neuroinvasive Nocardia asteroides GUH-2 induces apoptosis in the substantia nigra in vivo and dopaminergic cells in vitro. Exp Neurol 177: 453–460 [DOI] [PubMed] [Google Scholar]
- Tanner C.M. (2010) Advances in environmental epidemiology. Mov Disord 25(Suppl. 1): S58–S62 [DOI] [PubMed] [Google Scholar]
- Tillerson J.L., Miller G.W. (2003) Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods 123: 189–200 [DOI] [PubMed] [Google Scholar]
- Trojanowski J.Q., Lee V.M. (2005) Pathological tau: a loss of normal function or a gain in toxicity?. Nat Neurosci 8: 1136–1137 [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5: 107–110 [DOI] [PubMed] [Google Scholar]
- Uversky V.N. (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318: 225–241 [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A., Gandhi S., Wood N.W. (2006) Understanding the molecular causes of Parkinson’s disease. Trends Mol Med 12: 521–528 [DOI] [PubMed] [Google Scholar]