Abstract
We review likely neurobiological substrates of cognitions related to fear and anxiety. Cognitive processes are linked to abnormal early activity reflecting hypervigilance in subcortical networks involving the amygdala, hippocampus, and insular cortex, and later recruitment of cortical regulatory resources, including activation of the anterior cingulate cortex and prefrontal cortex to implement avoidant response strategies. Based on this evidence, we present a cognitive-neurobiological information processing model of fear and anxiety, linking distinct brain structures to specific stages of information processing of perceived threat.
With the emergence of neuroscience and brain imaging techniques, researchers have begun to reexamine the nature of anxious cognitions and their relationships to emotions such as anxiety and fear from a new perspective. As we will describe in the coming sections, applying neuroscience methodologies to cognitive models of fear and anxiety has provided important new insights into the biological underpinnings of psychological treatments and is already suggesting new treatment approaches and treatment targets.
For the purposes of this review we define cognition as it is conceptualized in cognitive behavioral therapy (CBT), including both explicit thought processes and less effortful processes involved in perceiving, recognizing, conceiving, and judging the relevance and salience of stimuli along with processes associated with executive control and problem solving such as constructing plans of action. Complex processes such as appraisal involving the recognition of features and their relation to episodic memories and action plans are considered integrations of the basic substrates of cognition.
We follow Ekman’s (1992) definition of fear as a basic emotion associated with reaction to threat useful for mobilizing quick and adaptive reactions in response to threatening situations. Accordingly, fear is assumed to have properties common to all basic emotions including (1) quick onset; (2) brief duration; (3) involuntary onset; (4) yielding almost instant recognition of the fearful stimulus as a function of autonomic arousal; (5) universal antecedent events (i.e., not specific to one particular culture); (6) accompanying physiological symptoms; and (7) distinctive associated facial expressions and behaviors (Ekman, 1992).
In contrast to fear, anxiety is conceptualized as a future-focused cognitive association that connects basic emotions (such as fear) to events, meanings and responses (Izard, 1992). These cognitive associations are less ‘hardwired’ than basic emotions and, therefore, vary widely depending on the individual and the situation. Although fear and anxiety are different, both are adaptive emotional responses to threat. If these emotions become maladaptive (e.g. excessive in intensity, frequency or duration), they may develop into emotional disorders, or anxiety disorders (Barlow, 2002).
This conceptualization is consistent with research suggesting that fear and anxiety are related, but different phenomena that can be distinguished on the neurophysiological level (e.g. Davis, 1998; Grillon et al., 2006). At the same time, there is evidence to suggest that both fear and anxiety are related to all anxiety disorders to varying degrees (Barlow, 2002; Etkin & Wager, 2007).
The objective of the present review is to provide bridges from recent discoveries in neuroscience to information processing models of fear and anxiety. Thus, the specific goal of this review is to integrate the emerging literature on neurobiological correlates of cognitive processes related to fear and anxiety. Our review builds on recent work recognizing the enormous potential of integrating neuroscience in understanding the psychology of clinical disorders, particularly as they apply to understanding response to psychological treatments (Beck, 2008; Clark & Beck, 2010; DeRubeis, Siegle, & Hollon, 2008). These reviews consider possible neurobiological mechanisms underlying the development and maintenance of negative cognitive biases and dysfunctional beliefs with the general suggestion that individuals vulnerable to, or who currently suffer from depression and anxiety show increased reactivity to negative, stress-related, or threat-related information, manifested neurally as a hyper-reactivity in brain regions associated with emotion recognition and generation of emotional reactions such as the amygdala and hypo-reactivity in regulatory circuits including the prefrontal cortex. These biological correlates of depression and anxiety are assumed to be associated with maladaptive cognitive appraisals and information processing biases, which in turn may lead to the occurrence and maintenance of disorder. This model is consistent with the notion that biological and cognitive processes are ‘different sides of the same coin’ (Beck, 2008). Here we augment this model to account for a broader network of brain regions involved in a wide spectrum of anxiety-related phenomena and to formally relate this work to emerging trends in treatment refinement by appealing to likely neural substrates of a hypervigilance-avoidance model common in understanding anxiety from a cognitive perspective.
Hypervigilance, Avoidance, and the Need for Attending to Time in Understanding Anxious Cognitions
To understand brain mechanisms of anxiety, it is essential to account for differential neural-system recruitment across the time course of information processing, from preparation and early reactivity to fear stimuli through later-developing processes such as worry. Recognition of apparent stages of threat processing stems from a now-well-developed cognitive literature. Early theorists hypothesized that anxious individuals are generally hypersensitive towards threatening information, which facilitates the processing of danger (Beck et al., 1985). Therefore, it was assumed that anxious individuals would show a bias towards threatening information. This idea has become known as the hypervigilance hypothesis. In contrast, other authors proposed an avoidance hypothesis, which states that anxious individuals tend to inhibit or even completely avoid deep processing of threatening information leading to cognitive avoidance of threatening stimuli (Foa & Kozak, 1986; Mogg, Matthews, & Weinman, 1987; Mansell, Clark, Ehlers, & Chen, 1999; Mogg, Philippot, & Bradley, 2004; Sposari & Rapee, 2007). Indeed, avoidance behavior is a diagnostic criterion for multiple anxiety spectrum disorders such as specific phobias, social anxiety disorder, generalized anxiety disorder, and post-traumatic stress disorder. Key symptoms of anxiety, such as worry, are hypothesized to represent avoidance processes (Borkovec, Alcaine, & Behar, 2004). For example, although individuals with generalized anxiety disorder may initially experience increased emotional reactions immediately after threat (McLaughlin, Mennin, & Farach, 2006; Mennin, Heimberg, Turk, & Fresco, 2005), worry shifts attention away from emotional experiences to internal processes that control emotion. Similarly, individuals with social anxiety appear to display pronounced behavioral biases towards interoceptive cues compared to external cues (Pineles & Mineka, 2005). These vigilance and avoidance perspectives have been integrated into a two-stage model of information processing, which has become known as the hypervigilance-avoidance hypothesis (Amir, Foa & Coles, 1998; Mogg, Bradley, Bono, & Painter, 1997; Williams, Watts, MacLeod, & Matthews, 1988). This model suggests that anxious individuals, who are hypervigilant to threatening information in the initial stage of its processing, avoid this information in a later stage. Behavioral experimental support for this hypothesis comes from studies using eye-tracking (e.g., Garner, Mogg, & Bradley, 2006), homographs (e.g., Amir et al. 1998), the dot-probe paradigm with varying stimulus-onset asynchronies (e.g., Vassilopoulos, 2005), and paradigms allowing volitional avoidance (Heuer, Rinck, & Becker, 2007). Event-related potential data also suggest that, despite increased early sensory engagement or attention to threat-stimuli (described in detail in the next section), indices of later elaborative processing are decreased in anxiety (Holmes, Nielsen, & Green, 2008; Weinberg & Hajcak, in press) and via peripheral psychophysiology, in individuals with chronic worry (Oathes, Siegle, & Ray, 2011).
A recent review of the experimental psychology literature on anxiety disorders suggest that facilitated attention, difficulty in disengagement, and attentional avoidance comprise the three components of attentional biases underlying observed hypervigilance/avoidance phenomena (Cisler & Koster, 2010). The authors propose a threat detection mechanism that underlies facilitated attention and further suggest that attentional control ability underlies difficulty in disengagement, whereas emotion regulation goals underlie attentional avoidance. This model is consistent with the experimental psychopathology literature pointing to three components of attention bias, namely hypervigilance, difficulty in disengagement, and attentional avoidance, aside from memory biases (e.g., Fox, Russo, & Dutton, 2002; Koster, Crombez, Verschuere, & DeHouwer, 2004; Mathews, & McLeod, 2005).
The remainder of our review will separately consider brain processes primarily associated with preparatory and early information processing potentially subserving vigilance, and later-developing processes, such as worry, more associated with regulatory control of vigilant responses such as disengagement and, failing that, avoidance.
Neurobiological Correlates of Anxious Cognitions
Early Reactivity to Emotional Stimuli Subserving Vigilance
In order to prepare an appropriate behavioral response to a stimulus, the organism must be able to extract critical information in the environment in order to discriminate threatening from non-threatening stimuli. This discrimination must occur within milliseconds of stimulus presentation in order for the individual to begin to formulate an adaptive behavioral response (Öhman, Flykt, & Lundqvist, 2000). This process requires attention, which may be defined as the mental ability to select behaviorally relevant stimuli, responses, memories, or thoughts, among many other non-relevant stimuli (Corbetta, 1998).Vigilance can thus be considered a process of increased preparatory priming or hyper-attention to threat stimuli.
Attentional processes subserving such hypervigilant reactions are thought to begin within a few milliseconds of the onset of a stimulus. For example, event-related-brain potential (ERP) studies reveal signatures of reactivity to visual stimuli within 50 milliseconds with modulation by attention (e.g., attending to the location of a visual stimulus), and within 100 milliseconds (the ‘P1’ component) in visual processing regions such as occipital sites (Mangun & Hillyard, 1991), both in conditions of overt (conscious processing, e.g., with a saccade) and covert (preconscious, without a saccade) attention (Hillyard, Luck, & Mangun, 1994). These earliest signals of visual processing, which occur even in the absence of conscious processing, differentiate emotional from neutral stimuli (Schupp, Junghöfer, Weike, & Hamm, 2003), are particularly large for negative and threatening facial expressions (Klucharev & Sams, 2004; Pourtois et al., 2005; Streit et al. 2003), and occur faster in the context of threatening information (Laretzaki, Plainis, Argyropoulos, Pallikaris, & Bitsios, 2010) consistent with interplay of emotion and early visual detection mechanisms. Similarly, increased levels of anxiety (Kolassa & Miltner, 2006) and threat sensitivity (Dennis & Chen, 2007) as well as anxiety diagnoses (Holmes, et al., 2008) are associated with potentiation of these early responses to threat stimuli. Thus, aspects of anxiety associated with attention begin extremely quickly following emotional stimuli, consistent with rapid spatial orienting to threat. These responses appear to originate from generators associated with visual information processing (e.g., fusiform gyrus; Pourtois et al. 2005) and have, therefore, been assumed to indicate increased visual attention to threat (Vuilleumier & Pourtois, 2007). It has further been proposed that a set of neural signals in the parietal and frontal cortices (the frontoparietal network) mediates covert visual orienting response processes (Corbetta, 1998).
More formally, a network of subcortical brain structures subserving both sensory processing and affective reactivity are likely involved in early processing associated with hypervigilance, including the amygdala, the basal forebrain, and the pulvinar (Hars, Maho, Edeline, & Hennevin, 1993; LeDoux, Sakaguchi, & Reis, 1984; Morris, Friston, & Dolan, 1997; Morris, Öhman, & Dolan, 1998; Robinson & Peterson, 1992; Taylor, Liberzon, & Koeppe, 2000). Activation of these structures can facilitate selective processing in the visual cortex via direct projections (Emery & Amaral, 2000) or ascending neuromodulatory systems (Briand, Gritton, Howe, Young, & Sarter, 2007; Derryberry & Tucker, 1991). Moreover, several subcortical structures (especially the amygdala) also project to the anterior cingulate cortex, which is part of the anterior attentional system organizing explicit selective attention (Posner & Petersen, 1990). Amygdala activity in anxious individuals is hypothesized to translate into increased dorsal-cingulate-mediated monitoring of emotionally relevant behavioral responses, such as errors, which may be perceived as threatening in anxious individuals (Holroyd & Coles, 2002; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Feedback between the rostral division of the cingulated, which is associated with emotion regulation and more dorsal subdivisions associated with conflict detection and selective attention, has been associated with integrating top-down and bottom-up aspects of reactivity (Bush, Luu, & Posner, 2000).
A review of the literature (Barrett & Wager, 2006; Phan, Wager, Taylor, & Liberzon, 2002; Wager, Phan, Liberzon, & Taylor, 2003) suggests that the right and left amygdalae were preferentially activated with fear and anxiety as compared to other emotional states. Directly supporting the role of the amygdala in a hypervigilance/avoidance framework using forward models of fMRI data, we have shown that in response to spider stimuli, individuals with spider phobia appear to recruit amygdala activity immediately following stimulus presentation more strongly than controls consistent with hypervigilance, but within 500ms appear to display decreased amygdala reactivity compared to controls, consistent with later initiation of an avoidance response (Larson et al., 2006).
Another brain region that has been discussed in the context of internally-focused processing of emotional responses is the insular cortex (Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004; Damasio, 1999; Paulus & Stein, 2006). The insula receives input from receptors in the skin and internal organs and is apparently involved in the perception of the physiological state of the entire body and the subjective feelings that can bring about actions. Information from the insula is relayed to other brain structures that appear to be involved in decision-making, especially the anterior cingulate and prefrontal cortices. Damasio (1999) and others (Critchley et al., 2004; Paulus & Stein, 2006) have proposed that this region plays a crucial role in mapping visceral states that are associated with emotional experiences, giving rise to conscious feelings.
In a recent meta-analysis, individuals suffering from post-traumatic stress disorder, social anxiety disorder and specific phobia evidenced greater activity than controls in the amygdala and insula in response to negative emotional stimuli, similar to patterns found in healthy subjects during fear conditioning (Etkin & Wager, 2007). Hyperactivation in the amygdala and insula were more common in social anxiety disorder and specific phobia than in post-traumatic stress disorder, whereas only patients with post-traumatic stress disorder showed hypoactivation in the dorsal and rostral anterior cingulate cortices and the ventromedial prefrontal cortex. These data are consistent with other literature (Bishop, 2007; Monk, 2008; Shin & Liberzon, 2010; van den Heuvel et al., 2005) and point to differences between anxiety disorders in the processing of fear-relevant material, which is an important area for future research.
Together, these data suggest that early reactivity to emotional stimuli happens before conscious awareness and is associated with activity in brain regions associated with sensory perception (e.g., visual cortex), emotional labeling (e.g., amygdala), and interoception (insula). Thus, to the extent that hypervigilance reflects increased early reactivity in these structures, it could be considered a phenomenon of increased recognition of environmental, internal, and introceptive cues.
Later Regulatory Processing Subserving Avoidance
Preliminary evidence suggests that changes in cognitions and conscious self-regulation of emotions are associated with other changes in brain dynamics likely to occur 500ms to seconds or minutes following the onset of emotional stimuli (e.g., Beauregard, Levesque, Bourgouin, 2001; Blair et al., 2007; Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; LeDoux, 1996; Leutgeb, Schäfer & Schienle, 2009; Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner et al., 2004). A review of studies employing explicit emotion regulation instructions (Ochsner & Gross, 2008) implicates a wide network of cortical regions in dowregulating early reactivity to emotional stimuli, as could occur in avoidance.
The prefrontal cortex (PFC), including all of the ventrolateral, ventromedial, dorsolateral, and dorsomedial regions appear to represent key brain regions involved in the cognitive regulation of emotions (e.g., Urry et al., 2006). The lateral PFC (LPFC) regions appear to be associated with conscious emotion regulation and executive processes (Smith & Jonides, 1999), likely more associated with voluntary regulatory strategies, such as conscious avoidance (Phillips, Ladouceur, & Drevets, 2008). But inhibitory pathways from LPFC regions to subcortical structures associated with vigilance are weak – rather, LPFC regions appear to project to ventromedial prefrontal mid-line structures including orbitofrontal regions (OFC), which proximally inhibit the amygdala. The VMPFC/OFC have bi-directional connections to the amygdala and have been implicated in regulation and adaptation of social behaviors (e.g., Damasio, 1995; Dolan, 1999; Grafman and Litvan, 1999; Kringlebach, 2005). For example, we have shown that functional inhibition of the amygdala by the dorsolateral prefrontal cortex (DLPFC) is mediated by ventromedial regions (Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). Similarly, supporting the functional inhibitory role of these structures, clinical neuropsychological studies have shown that damage to the OFC leads to pseudopsychopathic syndrome that is characterized by impulsivity, emotional outbursts, aggressiveness, shallowness, argumentativeness, hypersexuality, risky decision-making, and failure to observe social and moral rules (Damasio, Tranel, & Damasio, 1990). Thus, the data suggest fundamental ongoing dynamic interactions between brain mechanisms associated with early reactivity such as vigilance and later regulatory control processes as might be involved in avoidance. These later regulatory control processes include the conscious reappraisal of negative emotional stimuli that has been shown to be associated with increased activity in the dorsal and ventral regions of the left LPFC and the dorsal medial PFC (DMPFC) and decreased activity in the amygdala and OFC. These patterns of activation are in turn associated with decreased subjective endorsement of negative affect (Ochsner et al., 2002; 2004). In contrast, attending to negative stimuli in the absence of conscious reappraisal is associated with greater activation of medial orbitofrontal cortex (MOFC) and increased subjective endorsement of negative affect. These findings suggest that lateral and dorsal medial PFC prefrontal regions engaged through reappraisal techniques may modulate the emotional processes implemented in the amygdala and MOFC and involved in the evaluation of the emotional significance and contextual relevance of the stimulus. The data further suggest that subcortical and ventromedial prefrontal structures are associated with negative affect in response to certain stimuli, whereas dorsal prefrontal regions may be engaged during conscious reappraisal to dampen this outflow from more ventral structures.
Further studies of late regulatory processes suggest unique recruitment of PFC regions may be dependent upon regulatory goals. Down-regulation of emotion through cognitive reappraisal has been found to uniquely recruit regions of right lateral and orbital PFC implicated in behavioral inhibition, whereas up-regulation uniquely recruited regions of left rostromedial PFC, which is implicated in the retrieval of emotion knowledge (Ochsner et al., 2004). Further, self-focused regulation has been found to recruit medial prefrontal regions implicated in internally focused processing, whereas situation-focused regulation recruited lateral prefrontal regions implicated in externally focused processing (Ochsner et al., 2004). Thus, it appears that there are both common and distinct neural systems that support various forms of reappraisal. It further appears that the specific prefrontal systems that modulate the amygdala are contingent on the regulatory goal and on the strategy that is used.
Recent evidence suggests that differential recruitment of the DLPFC in service of down-regulating emotional responses may distinguish individuals with anxiety disorders and healthy controls, particularly in response to disorder-relevant threat stimuli. In a study examining individuals diagnosed with social anxiety disorder and healthy controls, showed comparable recruitment of DMPFC during conscious reappraisal of both physical and social threat in both groups (Goldin, Manber, Hakimi, Canli, & Gross, 2009). However, when reappraising socially threatening stimuli, healthy controls demonstrated greater neural responses in the DLPFC, pointing to an enhanced coordination of cognitive control circuitry not shown in social anxiety disorder. By contrast, individuals with social anxiety disorder evidenced greater right mid-DLPFC activation relative to healthy controls in response to physical threat. In fact, the differences in recruitment of DLPFC structures occurred only in response to social threat but not physical threat. This may suggest that there are strategic differences in how or when these structures are recruited. Alternatively, it is possible that individuals with SAD show hyperreactivity in subcortical structures, including the amygdala, in response to social threat, which functionally inhibits the recruitment of prefrontal regulatory control.
Whereas increased DLPFC activation is associated with the down regulation of emotional responses (Ochsner et al., 2002; 2004), evidence suggests that the ventrolateral PFC (VLPFC) may be implicated in both emotion generation and regulation. Greater activation in the VLPFC has been found to correlate with decreased activation in the amygdala and reduced negative emotional experience during cognitive reappraisal, suggesting that the VLPFC may be associated with conscious and voluntary regulation of emotional processes (Ochsner et al., 2002, 2004; Wager, Davidson, Hughes, Lindquist, & Ochnser, 2008.) However, VLPFC activation has been found to be associated with both decreases and increases in negative emotion through different subcortical pathways. Using a pathway-mapping analysis, Wager and colleagues (2008) identified one pathway from the VLPFC involving the nucleus accumbens predicted greater reductions in negative emotion during reappraisal, while another pathway, linked with the amygdala, predicted reduced reappraisal success and, therefore, an increase in negative emotion. These two separable pathways together explained approximately 50% of the variance in self-reported emotion. These findings suggest that the prefrontal cortex is involved in both creating and mitigating negative emotion, depending on the content of the thoughts.
To relate these data to anxiety, increased VLPFC function may be associated with increased use of regulatory strategies, such as avoidance in response to anxious hyper-reactivity. For example, in adult anxiety disorders, increased amygdala activation to threat-related stimuli is accompanied by altered VLPFC responses (Rauch, Savage, Alpert, Fischman, & Jenike, 1997; Shin et al., 2005; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002). Adolescents with generalized anxiety disorder also display both increased VLPFC activity, which has been implicated in cognitive aspects of emotional information processing, and attentional biases away from angry faces (Monk et al., 2006). Similarly, increased VLPFC activity occurs when generalized anxiety disorder adolescents direct attention towards threat (McClure et al., 2007). Together, these data suggest that the VLPFC plays a regulatory role proportional to hyperreactivity in anxious individuals.
In sum, an integrative review of the neuroscience and information processing literature is consistent with a cognitive-neurobiological information processing model, which will be presented below. This model relates cognitive processing in anxiety to early vigilance processes and later avoidance processes.
An Integrative Model of How Neural Mechanisms of Fear Cognitions Subserve Vigilance/avoidance
Multiple reviews in the past decade describe the mutually inhibitory relationships among subcortical regions involved in emotional reactivity, including the amygdala, and cortical regions involved in emotion regulation (e.g., Davidson, Jackson, & Kalin, 2000; LeDoux, 2000). These relationships can be disrupted by, for example, increased activity in the amygdala and abnormalities in prefrontal control, as supported by a wealth of neuroscience literature (e.g., Beck, 2008; Liberzon & Sripada, 2008; Phillips, et al., 2008) as well as their resolution in CBT (e.g., Clark & Beck, 2010; de Carvalho et al., 2010; DeRubeis, et al., 2008).
To this literature we add the theoretical framework that anxious cognitions subserving avoidance are specifically a product of neural mechanisms of hyperreactivity. Activation of regulatory control regions is increased simply because inputs to these areas are increased by hyper-reactivity. Pathologies, such as depression, have been characterized as resulting from decreased prefrontal function yielding decreased regulatory control (e.g., Rogers et al., 2004). In contrast, we suggest that fear and anxiety involves preserved regulatory control system function in the presence of learned beliefs regarding the appropriateness and utility of avoidant coping strategies
Our model relates cognitive processing in anxiety to early vigilance processes and later avoidance processes. As we have discussed, immediately following a stimulus, the anxious individual perceives the stimulus as potentially threatening due to hyperreactivity of the amygdala. Other cortical centers are proximally recruited, in particular the hippocampus, which makes episodic memory associations with other threat information potentially contributing to further anxiety, and the insular cortex, which provides interoceptive context and in this case is potentially perceived as physiological symptomatology. If the dorsal anterior cingulate cortex is also recruited, the individual may perceive conflict contributing to further anxiety. These areas form a network as the amygdala has extensive connections to other brain areas also associated with emotional and cognitive functions, including the sensory cortices, and the hippocampal complex (Young, Scannell, Burns, & Blakemore, 1994).
The PFC may be involved with the later emotion processing stages associated with regulatory function, which, in the case of anxiety, often includes avoidance. There is evidence to suggest that the LPFC is involved in the selection of the appropriate cognitive operations that are associated with a down-regulation of the emotional response by sending commands to ventromedial regions. If avoidance is a learned regulatory strategy it is likely to recruit the DLPFC, which would begin this regulatory cascade. The VMPFC/OFC, in turn, inhibit the amygdala. These signals can modulate the activity in brain structures involved in autonomic, visceral, and endocrine functioning, such as the hypothalamus and the insular cortex (Beauregard, 2007).
It is notable, however, that decreases in DLPFC activation have been found to correspond with positive treatment outcomes following CBT for specific phobias, and that increased DLPFC activation pre-treatment is associated with increased avoidance and anxious responding (Paquette et al., 2003; Straube et al., 2006). This suggests the DLPFC is implicated in both down-regulating emotional responses and facilitating cognitive avoidance, potentially at the expense of other more adaptive regulatory processes such as extinction learning (e.g. Phelps et al., 2004; Shienle et al., 2007). This would further suggest that teaching adaptive regulation skills through CBT does not necessarily mean bringing the DLPFC ‘online’ as much as increasing the flexible recruitment of the DLPFC in service of more efficient and adaptive regulation (e.g. Johanson et al., 2006).
Thus, the prefrontal cortices, including the ventrolateral, dorsolateral, dorsomedial, and orbitofrontal areas of the prefrontal cortex, are key brain regions involved in the selection and execution of emotion regulation strategies. The lateral PFC regions have been associated with conscious emotion regulation, executive processes, and working memory. The bidirectional relationship between the sub-cortical and cortical brain structures that are involved in the neurocircuitry of emotion processing implies that later stages can influence earlier stages. This inter-relationship explains the hypervigilance-avoidance phenomenon during the processing of certain fearful stimuli.
A simplified schematic of the interplay between these key brain structures and their relationships to relevant aspects of cognitive processing and psychophysiological outputs is displayed in Figure 1. As depicted in this model, some of the output variables also serve as input variables, depending on the aspect of cognitive processing. For example, it is assumed that the physiological arousal associated with emotional responding results in activation of the insula, which is strongly connected to the amygdala, in turn, modulating hippocampal processing, finally resulting in enhanced consolidation or storage for events that elicit an arousal response in the hippocampus.
Figure 1.
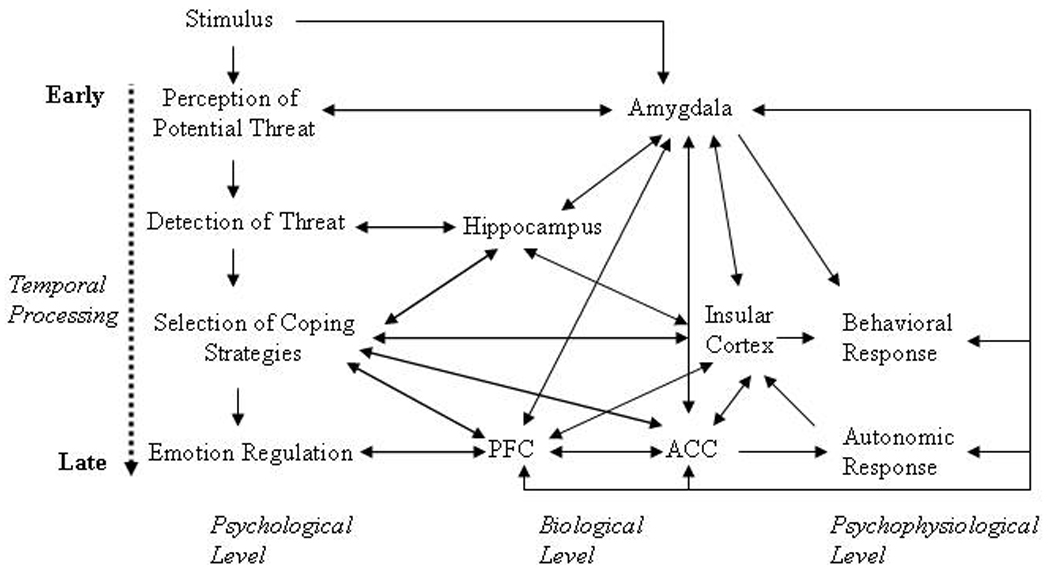
A Cognitive-Neurobiological Information Processing Model of Fear and Anxiety.
Note: The Figure depicts cognitive processing of threat on the psychological, biological, and psychophysiological level from earlier to later processing stages. Note: PFC: prefrontal cortex. ACC: anterior cingulate cortex. Depending on the threat assessment, the errors can depict excitatory or inhibitory influences.
We believe this illustrative figure not only summarizes many of the findings reported in the literature, but it also provides a heuristically useful model of the interplay and relationship between different brain structures and information processing stages on the psychological, biological, and psychophysiological levels. Based on our review, anxious cognitions may be understood, in part, as decision points during the processing of threat information. After a potential threat is perceived and detected, subsequent decision points involve selecting adequate coping strategies and then to applying coping strategies with the goal of regulating a fear response. These decision points are associated with the activation of brain structures such as the amygdala at the very early stage, followed by the hippocampus and the insular cortex, and later the anterior cingulate and prefrontal cortices.
Model Implications: Basic and Clinical
Based on these model assumptions, we will outline some research perspectives that give rise to some high-risk hypotheses related to treatment.
Defining Cognitions Based on Timing of the Emotional Process
The implication of our model for neuroscience is that the temporal dynamics of brain activation must be considered, especially when examining the biological correlates of cognitive processes. Such temporal analyses are increasingly conducted in affective neuroimaging studies. For example, Goldin, McRae, Ramel, and Gross (2008) examined 17 women who viewed a series of films while undergoing fMRI scanning after receiving instructions to reappraise or suppress their emotional response. Compared to suppression, reappraisal resulted in early (0–4.5 sec) PFC activation, decreased amygdala response, decreased insular response, and also a less negative emotion response, whereas suppression produced late (10.5–15 sec) PFC responses, and a less negative emotional experience, but increased amygdala and insular activation.
Consistent with these findings, our model suggests that cognitions are associated with different neurobiological correlates, depending on the processing stage and the timing of the information processing. At an early, subconscious, fast-acting, and automatic stage, cognitions are expressed in the form of attention biases that are primarily associated with activation of the amygdala. The thalamo-amygdala projections contribute to the processing of the affective significance of sensory cues, whereas the cortico-amygdala projections are involved with the processing of complex stimuli. The later, slower-acting and deliberate cognitive processes occur when the threat is detected. In this stage of cognitive processing, the organism chooses coping strategies, and then finally applies emotion regulation strategies to cope with the fearful situation. The hippocampus, insular cortex, anterior cingulate, and prefrontal cortices play a crucial role in these later cognitive processes.
Improving Symptoms by Changing Anxious Cognitions during CBT
CBT is an effective psychotherapy for a variety of psychological conditions including anxiety disorders (Butler, Chapman, Forman, & Beck, 2006; Hofmann & Smits, 2008). Framing how symptoms of anxiety may change in this intervention within the proposed model can help to generalize the model’s ultimate utility. CBT teaches anxious individuals to replace automatic responses to emotional stimuli with more controlled responses such as reducing behavioral avoidance and altering their automatic thoughts, biases, cognitive distortions, and physiological responses (Kendall, 1993). Thus, CBT may facilitate use of adaptive emotion regulation strategies, helping to regulate automatic limbic reactivity in place of maladaptive coping mechanisms, such as avoidance. An in-depth review if this literature on neurobiological changes associated with treatment is outside the scope of this article (for a review, see Frewen Dozois, & Lanius, 2008; Linden 2006; Porto et al., 2009; Roffman, Marci, Glick, Dougherty, & Rauch, 2005). In summary, studies collectively support the notion that response to CBT for anxiety disorders is associated with changes in recruitment of brain regions that subserve affective and self-regulation including the prefrontal cortices (in particular, DLPFC and medial and lateral OFC), the anterior cingulate, and the insular cortices (Davidson, 2000; Ochsner & Gross, 2008). For example, following CBT, individuals with spider phobia display decreased DLPFC (Paquette et al, 2003; Straube et al, 2006) and ventromedial (Schienle et al, 2007) activity consistent with either less need for regulation or more efficient recruitment of these regulatory regions. Thus, CBT appears to be associated with less consuming affective reactivity and more effective use of neural mechanisms of problem-solving, affect regulation, and self-referential and relational processing. Anxious individuals with the highest pre-treatment anterior cingulate reactivity to emotional stimuli respond best to this treatment (Nitschke et al., 2009), suggesting that they most need what it has to offer. In this way, CBT may improve coping by increasing regulation of negative affect. Decreased DLPFC activation seen at post-CBT may also reflect changes in avoidant processing as a result of changes in threat contingencies. Successful reappraisal during treatment (as evidenced by initial increases in DLPFC) may facilitate an increase in approach behavior and extinction learning, thereby reducing the need for effortful regulation post-treatment (as evidenced by decreased DLPFC activation). Moreover, CBT may enhance executive functioning and deployment of working memory in problem-solving during difficult emotional and stressful situations, as suggested by the changes in activation in the DLPFC. Future studies examining changes in DLPFC across the course of CBT treatment would clarify the role of DLPFC activation in symptom improvement.
Improving Symptoms by Correcting Early Cognitive Biases
Symptom improvement has been linked to changes in attentional biases (Mogg & Bradley, 1998). Supporting evidence comes from studies reporting a reduction in attention biases during the course of treatment in both uncontrolled (Mattia, Heimberg & Hope, 1993) and waitlist-controlled studies (Mathews, Mogg, Kentish, & Eysenck, 1995). More importantly, this model further makes the high-risk prediction that changes in cognitive biases at an early information processing stage can lead to later improvements in psychopathology.
Consistent with this prediction is a prospective study by MacLeod and Hagan (1992) that showed that attention bias correlated with affective responses to stressful real-life events. However, the only way to definitively answer the question of causality is with experimental designs in which attention is manipulated (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). This issue was examined by two experimental studies manipulating attentional allocation directly through a process of attention modification (MacLeod et al., 2002). Using a dot-probe discrimination task, two words (one threat and one neutral word) were presented on a computer monitor. The words appeared for a brief interval (20 or 480 ms) followed by a visual probe. Participants were instructed to indicate the location of the probe. Conditions were designed to create a strong contingency between neutral or threat words and probe location (i.e., the probe replaced the threat word in the attend negative condition). The results showed that attention shifted in the 480 ms condition, such that participants in the attend negative condition exhibited a tendency to orient toward the threat words, whereas participants in the attend neutral condition tended to orient toward neutral words. In addition, participants in the attend negative condition reported significantly more negative mood in response to a stressful task than participants in the attend neutral condition, and also experienced higher levels of stress during an impossible anagram stressor.
Building on these studies, investigators recently modified the dot probe paradigm such that participants were asked to detect a probe by identifying letters replacing one member of a pair of words. One of the words was threatening, the other word nonthreatening. Participants’ attention was trained by including a contingency between the location of the probe and the non-threat word. For the control group, the probe was equally likely to appear after the threat word and the non-threat word. Participants in the training group showed a change in attention bias and a decrease in distress as indicated by self-report and interviewer measures. This paradigm was successfully applied for treating generalized anxiety disorder (Amir, Beard, Burns, & Bomyea, 2009) and public speaking anxiety (Amir, Weber, Beard, Bomyea, & Taylor, 2008).
As noted earlier, it is possible that attention retraining acts on the sensory gating mechanism of the ventral pathway that serves a modulatory function via afferent connections to the amygdala (e.g., Bishop, 2007; Davis & Whalen, 2001). More specifically, it is possible that attention retraining changes the sensory input that reaches the amygdala via projections from the anterior thalamus, which could produce motor and autonomic activity via projections to motor areas, brain stem nuclei, and other brain areas (Amunts et al., 2005; Rauch, Shin & Wright, 2003).
Improving Symptoms by Specifically Targeting Neurobiological Structures
The model predicts that changes in activation of specific brain structures are associated with subjective experience of anxiety and behaviors (and vice versa). Real-time functional brain imaging provides the possibility of directly increasing or decreasing activation of specific brain areas using neurofeedback techniques (e.g., deCharms, 2008). This new technology has been successfully applied to change activation in the somatosensory cortex (e.g., Posse et al., 2001), the amygdala (Posse et al., 2003), the insular cortex (Caria et al., 2007), and the anterior cingulate cortex (e.g., Yoo et al., 2004). For example, the study by Posse et al. (2003) trained subjects to self-regulate their amygdala activation through a strategy of self-induced sadness. The results showed that participants’ emotion ratings correlated with the activity in the amygdala. Despite these promising early results, the correlational nature of these studies should cause them to be interpreted with caution. Our model predicts that neurofeedback training may also be beneficial for regulating the fear response when targeting the insular cortex, the anterior cingulate cortex, and the prefrontal cortex.
Enhancing Treatment Efficacy by Targeting Multiple Levels of Processing
An important assumption of the model is the bi-directionality between cognitive processes, brain activation, behaviors, and emotions. This implies that targeting multiple levels in treatment will result in enhanced efficacy. One possible strategy to potentiate the efficacy of CBT is the enhancement of extinction learning at the subcortical level in the context of CBT. Animal research has shown that intra-amygdala infusions of an NMDA receptor antagonist shortly before extinction training dose-dependently block extinction (Falls, Miserendino, & Davis, 1992). Moreover, d-cycloserine (DCS), a partial NMDA agonist, dose-dependently enhances extinction in rats (Walker, Ressler, Lu, & Davis, 2002; Ledgerwood, Richardson, & Cranney, 2003, 2004). Similarly, DCS has been shown to enhance exposure therapy of height phobia (Ressler et al., 2004), social anxiety disorder (Hofmann et al., 2006; Guastella et al., 2008), and obsessive-compulsive disorder (Kushner et al., 2007; Wilhelm et al., 2008). In other words, it is apparently possible to facilitate the efficacy of CBT with a cognitive enhancer. However, the precise action is not entirely understood. Animal research suggests that DCS specifically acts upon NMDA receptor in the amygdala (Walker et al., 2002). An fMRI study with spider-phobic people suggests that DCS may enhance PFC, ACC, and the insular cortices when participants were asked to view pictures of spiders (Aupperle et al., 2009). Future research on this exciting area might answer important questions about the biological correlates of fear and anxiety processing and may further lead to new treatment strategies.
Concluding Comments
Our review has suggested that anxious and fearful cognitions may be defined as temporally specific features of information processing of emotional material that are associated with activation in specific brain networks. These processes include both structures associated with early hyperreactivity and later recruitment of prefrontal resources associated with avoidant coping. The same neurocircuitry is also hypothesized to underlie anxious behaviors and associated psychophysiological arousal. Our model predicts that anxious cognitive processes and relevant aspects of brain activation are closely and bi-directionally connected. Therefore, terms such as cognition and cognitive process are inherently intertwined with brain processes subserving the temporal processing of threat information.
Recognizing the importance of biological substrates of cognitions in emotional processing offers new possibilities for intervention research. As suggested by our review, neuroscience is likely to specifically clarify and enrich CBT for anxiety disorders. Moreover, understanding cognitive and emotional processes on the biological level opens up new windows of opportunity for intervention research.
Acknowledgments
Dr. Hofmann is supported by NIMH grants MH-078308 and MH-081116. He is also a paid consultant of Merck/Schering-Plough for work unrelated to this study. Dr. Siegle’s contributions were supported by NIMH grants MH082998 and P50 MH080215. He is an unpaid consultant for TrialIQ for work unrelated to this study. Ms. Ellard is supported by an NIMH Ruth L. Kirschstein National Research Service Award (NRSA) grant 1F31MH084422.
References
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:26–35. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Foa EB, Coles ME. Negative interpretation bias in social phobia. Behaviour Research and Therapy. 1998;36:945–957. doi: 10.1016/s0005-7967(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117:860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Hale LR, Chambers RJ, Cain SE, Barth FX, Shrp SC, Denney DR, Savage CR. An fMRI study examining effects of acute d-cycloserine during symptom provocation in spider phobia. CNS Spectrum. 2009;14:556–571. doi: 10.1017/s1092852900024044. [DOI] [PubMed] [Google Scholar]
- Bandura A. Clinical functioning. In: Bandura A, editor. Self-efficacy: The exercise of control. New York: Freeman and Company; 1997. pp. 319–368. [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2nd Edition. New York: Guilford Press; 2002. [Google Scholar]
- Barrett LF, Wager TD. The structure of emotion: Evidence from neuroimaging studies. Current Directions in Psychological Science. 2006;15:79–83. [Google Scholar]
- Beauregard M. Mind does really matter: Evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Progress in Neurobiology. 2007;81:218–236. doi: 10.1016/j.pneurobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Cogntive therapy and the emotional disorders. New York: Penguin; 1991. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G, Greenberg RC. Anxiety Disorders and Phobias: A Cognitive Perspective. New York, NY: Basic Books; 1985. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Science. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine O, Behar E. In: Avoidance theory of worry and generalized anxiety disorder. Heimberg RG, Turk CL, Mennin DS, editors. New York: Guilford Press; 2004. pp. 77–108. Generalized anxiety disorder: Advances in research and practice. [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Progress in Neurobiology. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clinical Psychology Review. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends in Cognitive Science. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Hackman A, Wells A, Ludgate J, Gelder M. Brief cognitive therapy for panic disorder: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 1999;67:583–589. doi: 10.1037//0022-006x.67.4.583. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, assessment and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Science. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. New York: Harcourt; 1999. [Google Scholar]
- Damasio AR. On some functions of the human prefrontal cortex. Annals of the New York Academy of Sciences. 1995;769:241–251. doi: 10.1111/j.1749-6632.1995.tb38142.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychologicalical Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Carvalho MR, Dias GP, Cosci F, de-Melo-Neto VL, Bevilaqua MC, Gardino PF, et al. Current findings of fMRI in panic disorder: contributions for the fear neurocircuitry and CBT effects. Expert Review of Neurotherapeutics. 2010;10:291–303. doi: 10.1586/ern.09.161. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nature Review Neuroscience. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Chen CC. Neurophysiological mechanisms in the emotional modulation of attention: the interplay between threat sensitivity and attentional control. Biologial Psychology. 2007;76:1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Tucker DM. The adaptive base of the neural hierarchy: Elementary motivational controls on network function. In: Dienstbier RA, editor. Nebraska Symposium on Motivation, 1990: Perspectives on motivation. Lincoln: University of Nebraska Press; 1991. pp. 289–342. [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Review Neuroscience. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. On the neurology of morals. Nature Neuroscience. 1999;11:927–929. doi: 10.1038/14707. [DOI] [PubMed] [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Emery NJ, Amaral DG. The role of the amygdala in primate social cognition. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 156–191. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in post-traumatic stress disorder, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition and Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Lanius RA. Neuroimaging studies of psychological interventions for mood and anxiety disorders: Empirical and methodological review. Clinical Psychology Review. 2008;28:229–247. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115:760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural basis of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Litvan I. Importance of deficits in executive functions. Lancet. 1999;354:1921–1923. doi: 10.1016/S0140-6736(99)90438-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of d-cycloserine enhancement of exposure therapy for social anxiety disorder. Biological Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hannesdottir DK, Ollendick TH. The role of emotion regulation in the treatment for child anxiety disorders. Clinical Child and Family Psychology Review. 2006;10:275–293. doi: 10.1007/s10567-007-0024-6. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56:61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behaviour Research and Therapy. 2007;45:2990–3001. doi: 10.1016/j.brat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Luck SJ, Mangun GR. The cuing of spatial attention to visual field locations: Analysis with ERP recordings. In: Heinze HJ, Munte TF, Mangun GR, editors. Cognitive electrophysiology. Boston, MA: Birkhauser; 1994. pp. 1–25. [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ. Cognitive-Behavioral Therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Green S. Effects of anxiety on the processing of fearful and happy faces: an event-related potential study. Biological Psychology. 2008;77:159–173. doi: 10.1016/j.biopsycho.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The Neural Basis of Human Error Processing: Reinforcement Learning, Dopamine, and the Error-Related Negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Izard CE. Basic emotions, relations among emotions, and emotion-cognition relations. Psychological Review. 1992;99:561–565. doi: 10.1037/0033-295x.99.3.561. [DOI] [PubMed] [Google Scholar]
- Johanson A, Risberg J, Tucker DM, Gustafson L. Changes in frontal lobe activity with cognitive therapy for spider phobia. Applied Neuropsychology. 2006;13:34–41. doi: 10.1207/s15324826an1301_5. [DOI] [PubMed] [Google Scholar]
- Kendall PC. Cognitive-behavioral therapies with youth: Guiding theory, current status, and emerging developments. Journal of Consulting and Clinical Psychology. 1993;61:235–247. doi: 10.1037//0022-006x.61.2.235. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Sams M. Interaction of gaze direction and facial expressions processing: ERP study. Neuroreport: For Rapid Communication of Neuroscience Research. 2004;15:621–625. doi: 10.1097/00001756-200403220-00010. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Miltner WHR. Psychophysiological correlates of face processing in social phobia. Brain Research. 2006;1118:130–141. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, DeHouwer J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thurus P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62:835–858. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson D, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Laretzaki G, Plainis S, Argyropoulos S, Pallikaris IG, Bitsios P. Threat and anxiety affect visual contrast perception. Journal of Psychopharmacoogyl. 2010;24:667–675. doi: 10.1177/0269881108098823. [DOI] [PubMed] [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: Amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Behavioral Neuroscience. 2003;117:341–505. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of conditioned fear: Consequences for reinstatement. Behavioral Neuroscience. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Touchstone; 1996. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis D. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned by acoustic stimuli. Journal of Neuroscience. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Linden DEJ. How psychotherapy changes the brain: The contribution of functional neuroimaging. Molecular Psychiatry. 2006;11:528–538. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behavior Research and Therapy. 1992;30:151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulation of sensory-evoked brain potentials provide evidence for changes in perceptual processing during visual-spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A, Chen YP. Social anxiety and attention away from emotional faces. Cognition and Emotion. 1999;13:673–690. [Google Scholar]
- Mathews A, McLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mogg K, Kentish J, Eysenck M. Effect of psychological treatment on cognitive bias in generalized anxiety disorder. Behavior Research and Therapy. 1995;33:293–303. doi: 10.1016/0005-7967(94)e0022-b. [DOI] [PubMed] [Google Scholar]
- Mattia JI, Heimberg RG, Hope DA. The revised Stroop color-naming task in social phobics. Behavior Research and Therapy. 1993;31:305–313. doi: 10.1016/0005-7967(93)90029-t. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair JR, Fromm S, Charney DS, Leibluft E, Ernst M, Pine DS, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Mennin DS, Farach FJ. The contributory role of worry in emotion generation and dysregulation in generalized anxiety disorder. Behaviour Research and Therapy. 2006;45:1735–1752. doi: 10.1016/j.brat.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behavior Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy. 1997;35:297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Weinman J. Memory bias in clinical anxiety. Journal of Abnormal Psychology. 1987;96:94–98. doi: 10.1037//0021-843x.96.2.94. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Neural responses to salient visual stimuli. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1997;264:769–775. doi: 10.1098/rspb.1997.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Murphy N, Brown WS. Did my neurons make me do it? Philosophical and neurobiological perspectives on moral responsibility and free well. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. American Journal of Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes DJ, Siegle GJ, Ray WJ. Chronic worry and the temporal dynamics of emotional processing. Emotion. 2011;11:101–114. doi: 10.1037/a0021781. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural system supporting the cognitive down- and up regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Lundqvist D. Unconscious emotion: Evolutionary perspectives, psychophysiological data and neuropsychological mechanisms. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 296–327. [Google Scholar]
- Öst LG. One-session group treatment of spider phobia. Behaviour Research and Therapy. 1996;34:707–715. doi: 10.1016/0005-7967(96)00022-8. [DOI] [PubMed] [Google Scholar]
- Paquette V, Levesque J, Mensour B, Leroux J-M, Beaudoin G, Bourgouin P, Beauregard M. Change the mind and you change the brain: Effects of cognitive-behavioral therapy on the neutral correlates of spider phobia. NeuroImage. 2003;18:401–409. doi: 10.1016/s1053-8119(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. The insular view of anxiety. Biological Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Mineka S. Attentional biases to internal and external sources of potential threat in social anxiety. Journal of Abnormal Psychology. 2005;114:314–318. doi: 10.1037/0021-843X.114.2.314. [DOI] [PubMed] [Google Scholar]
- Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. Journal of Neuropsychiatry and Clinical Neuroscience. 2009;21:114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald K, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporalimbic regions detects amygdala activation during single-trail self-induced sadness. Neuroimage. 2003;18:760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Posse S, Binkofski F, Schneider F, Gembris D, Frings W, Habel U, Salloun JB, Mathiak S, Kieselev V, et al. A new approach to measure single-event related brain activity using real-time fMRI: Feasibility of sensory, motor, and higher cognitive tasks. Human Brain Mapping. 2001;12:25–41. doi: 10.1002/1097-0193(200101)12:1<25::AID-HBM30>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: Time course and topographic evoked-potentials mapping. Human Brain Mapping. 2005;26:65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging of amygdala function in anxiety disorders. Annals of the New York Academy of Science. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biological Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE. The pulvinar and visual salience. Trends in Neuroscience. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Marci CD, Glick DM, Dougherty DD, Rauch SL. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychological Medicine. 2005;35:1385–1398. doi: 10.1017/S0033291705005064. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The brain and emotion. Oxford, England: Oxford University Press; 1999. [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia: An fMRI study on exposure therapy. European Archives of Clinical Neuroscience. 2007;257:486–493. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Stark R, Vaitl D. Long-term effects of cognitive behavior therapy on brain activation in spider phobia. Psychiatry Research: Neuroimaging. 2009;172:99–102. doi: 10.1016/j.pscychresns.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sposari JA, Rapee RM. Attentional bias toward facial stimuli under conditions of social threat in socially phobic and nonclinical participants. Cognitive Therapy and Research. 2007;31:23–37. [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Streit M, Dammers J, Simsek-Kraues S, Brinkmeyer J, Wolwer W, Ioannides A. Time course of regional brain activations during facial emotion recognition in humans. Neuroscience Letters. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnsone T, Klin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel OA, Veltman DJ, Groenenwegen HJ, Witter MP, Merkelbach J, Cath DC, vand en Balkom AJLM, van Opeen P, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Grados M, Slomine B, Herskovits EH, Thompson RE, Salorio C, et al. Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biological Psychiatry. 2004;55:208–216. doi: 10.1016/s0006-3223(03)00708-x. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos SP. Social anxiety and the vigilance-avoidance pattern of attentional processing. Behavioural and Cognitive Psychotherapy. 2005;33:13–24. [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine assessed with fear-potentiated startle. Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Electrocortical evidence for vigilance-avoidance in Generalized Anxiety Disorder. Psychophysiology. doi: 10.1111/j.1469-8986.2010.01149.x. (in press). [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with d-cycloserine for obsessive-compulsive disorder. American Journal of Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester, England: Wiley; 1988. [Google Scholar]
- Yoo SS, Fairneny T, Chen NK, Choo SE, Panych LP, Park H, Lee SY, Jolez FA. Brain-computer interface using fMRI: Spatial navigation by thoughts. Neuroreport. 2004;15:1591–1995. doi: 10.1097/01.wnr.0000133296.39160.fe. [DOI] [PubMed] [Google Scholar]
- Young MP, Scannell JW, Burns GA, Blakemore C. Analysis of connectivity: Neural systems in the cerebral cortex. Review Neuroscience. 1994;5:227–250. doi: 10.1515/revneuro.1994.5.3.227. [DOI] [PubMed] [Google Scholar]


