Abstract
Numerous studies have now documented a link between the immune infiltrate in several human carcinoma types and prognosis and response to therapy. The most comprehensive of these studies were in colorectal cancer with similar conclusions by numerous groups. Analyses of immune infiltrate of several other carcinoma types also showed general correlations between immune infiltrate and prognosis, but with some conflicting results. This review will attempt to summarize the current state of this field and point out what factors may be responsible for some of the conflicting findings. Nonetheless, the breadth of reports drawing similar conclusions for some cancer cell types leads one to more seriously consider the link between immune cell infiltrate and tumor prognosis and/or response to therapy, and the potential for combining conventional cancer therapy with active immunotherapy employing therapeutic cancer vaccines.
Keywords: tumor immune infiltrate, T-cells, cancer prognosis, colon carcinoma, carcinoma
Introduction
Conventional cancer therapeutic modalities, such as chemotherapy and radiation of tumor, and small molecule targeted therapies have been thought of as agents acting directly on tumor cells and/or the tumor vasculature. Patients are believed to respond to these therapies based on the inherent susceptibility of their tumor to the specific therapeutic modality vs. dose-limiting toxicities. Little attention has been given to the host immune system in terms of prognosis and/or potential response to therapy. On the other hand, the role of the immune system in cancer has long been known. For example, patients with immunosuppressive disorders such as HIV/AIDS, or undergoing immunosuppressive regimens for transplantation, have been shown to have a higher incidence of certain cancers.
In recent years, numerous studies have reported a link between prognosis and/or response to conventional therapy, and tumor immune infiltrate in several different solid tumor types. This article will review many of these studies on a cancer type basis and attempt to explain some of the phenomena reported. The studies reported here not only point out the potential importance of evaluating the immune infiltrate of tumor in making decisions of patient prognosis and thus patient management, but also direct one to more seriously consider the role of active immunotherapy (e.g., therapeutic vaccines) in combination with more conventional therapies in order to enhance patients’ clinical responses. Clinical trials combining therapeutic cancer vaccines and chemotherapy, radiation of tumor, and the use of small molecule targeted therapeutics are in progress and may well be an important new frontier in cancer management.
While some the results reported below are quite impressive in terms of similar conclusions being reached for some cancer types by different groups employing different reagents, there are also some conflicting results reported. These can potentially be explained by differences in methodologies used, such as immunohistochemistry vs. light microscopy vs. PCR, and/or by differences in reagents used, i.e., the use of different monoclonal antibodies to target a specific cell type. Older studies used light microscopy, and in some cases no distinction was made between different cell types. The advancement of technologies such as PCR, flow cytometry and microarray techniques has enabled one to study tumor-infiltrating immune cell populations in more detail. The use of computer-assistance quantification software to analyze histological sections may also be valuable since the computer evaluates the whole slide, thus reducing the risk of observer bias in choosing sections to evaluate.1 Another interesting new approach is epigenetic immunophenotyping of tumor-infiltrating immune cells.2 Therefore, the definition of the cell types varies between some studies, and this has been addressed in the tables and text in this review.
The evaluation of immune infiltrates is even more complex due not only to the numerous cell types that can be found in tumors, but also to the possibility that a given immune cell type can vary in terms of state of maturation and/or activation, and the fact that many diverse cell types can share similar markers. For example, a CD4 T-cell found in a tumor can be anergic, activated, or regulatory. The same can be said for several other immune lineages. Nonetheless, the breadth of reports drawing similar conclusions for some cancer cell types leads one to more seriously consider the link between immune cell infiltrate and tumor prognosis and/or response to therapy, and the potential for combining conventional cancer therapy with active immunotherapy employing therapeutic cancer vaccines.
Colorectal cancer: Tumor-infiltrating immune cells as independent predictors of prognosis
In 1987, Jass et al.3 presented a new prognostic tool for classification of rectal cancer. It comprised four variables that were found to be independent predictors of long-term survival, and included the number of lymph nodes with metastatic tumor, the character of the invasive margin, local spread and peritumoral lymphocytic invasion. Using this tool, the authors were able to more accurately predict clinical outcome than when using only Dukes’ staging (n=710, P<0.001). Since then, immune cell infiltration into tumor has been investigated in numerous studies (see Table 1).
Table 1.
Studies of tumor-infiltrating immune cells and prognosis in colorectal carcinoma
| Reference | Principal findings Correlation with better prognosis |
n | P value | Stage of carcinoma |
Methods | Definition of immune cells | Follow- up period (years) |
|---|---|---|---|---|---|---|---|
| 7 | Positive correlation for CD8+ and CD45RO+ T-cells. | 602 | <0.0001 | I-II | RT-PCR, TMA, IHC |
CD8+ and CD45RO+ lymphoid infiltrates in tumors / invasive margin. |
5 |
| 4 | Positive correlation for CD45RO+ T-cells. | 490 | <0.05 | I-IV | RT-PCR, TMA, IHC |
CD3+, CD8+, GrB+, and CD45RO+ lymphoid infiltrates in tumors / invasive margin. |
>13 |
| 5 | Positive correlation for CD8+ and CD45RO+ T-cells. | 490 | <0.05 | I-IV | RT-PCR, TMA, IHC |
CD3+, CD8+, GrB+, and CD45RO+ lymphoid infiltrates in tumors / invasive margin. |
>13 |
| 6 | Positive correlation for CD8+ and CD45RO+ T-cells. | 142 | <0.05 | Metastatic/ non- metastatic |
IHC, RT-PCR, FACS |
CD3+, CD5+, CD8+, TCR+, CD1a+, Ki67+, CD68+, FoxP3+, and cytoDEATH+ tumor infiltrating cells. |
11 |
| 3 | Positive correlation for lymphocytes. | 710 | <0.0001 | I-IV | H, LM | Lymphocytic infiltration. | >10 |
| 8 | Positive correlation for lymphocytes. | 843 | <0.01 | I-IV | H, LM, RT-PCR | Lymphocytes on top of tumor cells. | >4 |
| 9 | Positive correlation for lymphocytes. | 276 | <0.001 | I-IV | H, LM | Lymphocytic infiltration in the center and periphery of tumors. |
>13 |
| 10 | Positive correlation for CD8+ T-cells. | 109 | <0.001 | II-III | H, IHC | CD3+, CD8+, and GrB+ tumor infiltrating cells. | 5 |
| 12 | Positive correlation for CD8+ T-cells. | 119 | <0.001 | I-IV | TMA, IHC | CD8+ cells in tumor tissue. | >10 |
| 13 | Positive correlation for CD8+ and CD57+ cells. | 93 | <0.05 | II-III | H, IHC | CD4+, CD8+, CD56+, and CD57+ intraepithelial cells. |
18 |
| 14 | Positive correlation for CD8+ T-cells, negative for CD4+ T-cells. |
41 | <0.05 | I-IV | FACS | CD3+, CD8+, and CD4+ tumor infiltrating cells. | 5 |
| 15 | Positive correlation for CD8+ T-cells, negative for CD4+ T-cells. |
162 | <0.001 | IV | H, IHC, TMA | CD3+, CD4+, CD8+ and CD45RO+ tumor infiltrating cells. |
>10 |
| 17 | Positive correlation for T-regs in tumor, negative in normal mucosa. |
967 | <0.001 | II-III | IHC, TMA | CD8+, CD45RO+, and FoxP3+ tumor infiltrating cells. |
6 |
| 18 | Positive correlation for T-regs. | 57 | <0.001 | IV | IHC | CD4+, CD8+, and FoxP3+ T-cells in stroma adjacent to neoplastic glands. |
1.25 |
| 19 | Negative correlation for T-regs, positive for CD3+ T- cells. |
160 | <0.05 | II-III | IHC, LM | CD4+, CD8+, CD25+, and FoxP3+ T-cells. | 8 |
| 20 | Negative correlation for dendritic cells. | 104 | <0.05 | II-III | H, IHC | Tumor infiltrating S-100+, HLA class II+, CD208+, and CD1a+ dendritic cells. |
15 |
| 22 | Positive correlation for lymphocytes. | 361 | <0.001 | I-III | H, LM | Lymphocytic infiltration. | 10 |
| 23 | Positive correlation for CD8+ T-cells. | 131 | 0.016 | I-IV | H, IHC | CD8+ and GrB+ tumor infiltrating cells. | 5 |
| 24 | Positive correlation for CD8+ T-cells. | 371 | <0.0001 | I-IV | H, IHC | CD8+ T-cells within cancer cell nests. | 10 |
| 27 | Positive correlation for CD3+ T-cells in node- negative CRC. |
286 | <0.01 | III | IHC | CD3+ cells at the invasive margin. | 6 |
CRC: colorectal cancer; FACS: flow cytometry; H: histopathological analysis; IHC: mmunohistochemistry; LM: light microscopy; TMA: tissue microarray; T-regs: regulatory T-cells.
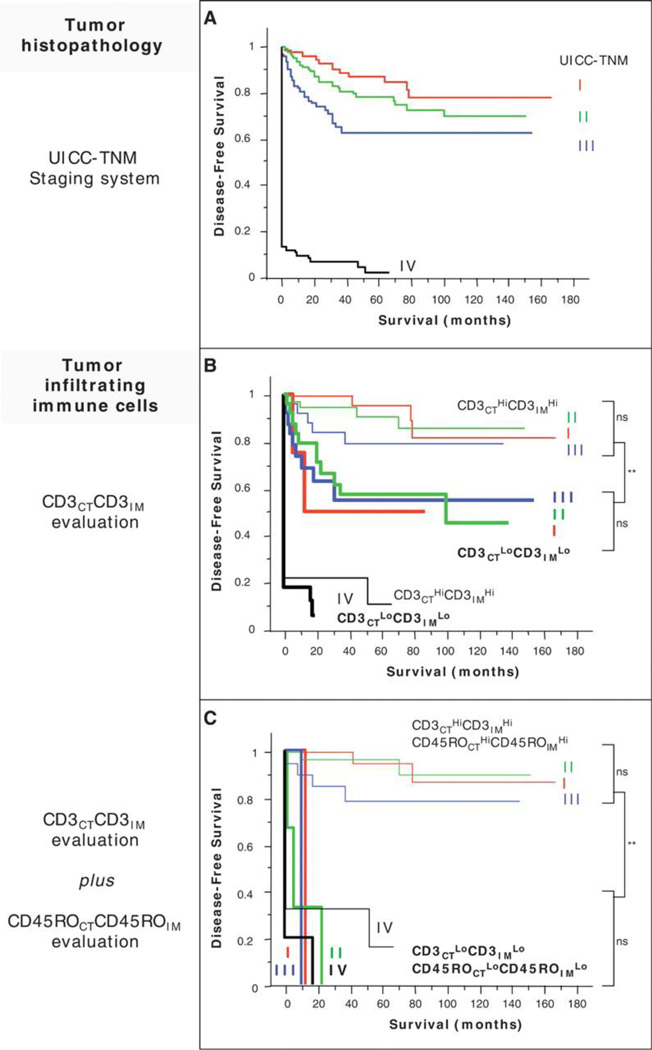
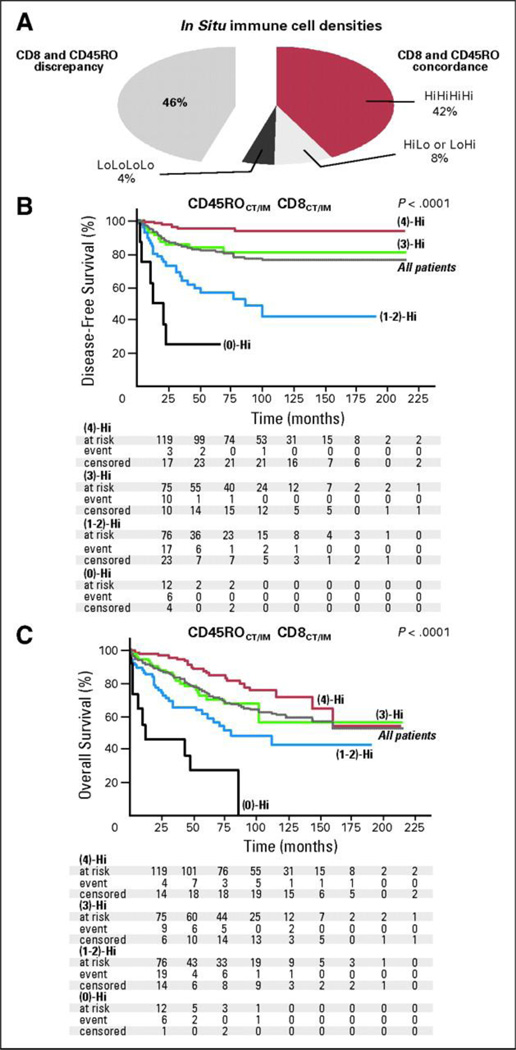
The most comprehensive studies of immune cell infiltrate and prognosis have been carried out by the Fridman group. Pages et al.4 showed that signs of an immune response within colorectal cancers were associated with the absence of pathological evidence of early metastatic invasion and that an increased density of CD45RO+ memory T-cells was an independent predictor of increased overall survival (n=490, P<0.05). Five-year survival and disease-free survival were 46.3% and 43.1%, respectively, for tumors with a high density of CD45RO+ cells compared to 23.7% and 21.5% for tumors with a low density. Tumors without signs of early metastatic invasion had increased infiltrates of immune cells and increased levels of mRNA for products of TH1 effector cells, but not increased levels of inflammatory mediators or immunosuppressive molecules. Markers of T-cell migration, activation and differentiation were also increased, as well as the numbers of CD8+ T-cells. In addition, Galon et al.5 further characterized the tumor-infiltrating immune cells in the same cohort of colorectal cancer patients, and found that the type, density and location of the immune cells were a predictor of survival superior to the histopathological methods currently used, and independent of the tumor node metastasis (TNM) system (Figure 1). By conducting genomic and in situ immunostaining on resected tumors from patients with colorectal cancer, they found that TH1 adaptive immunity had a beneficial effect on clinical outcome. Tumors from patients without recurrence had significantly higher immune cell densities within both the center of the tumor (CT) and the invasive margin (IM). There was a statistically significant correlation between the immune cell density and patient outcome (n=490, P<0.05). They looked at all T-lymphocytes (CD3+), CD8+ T-cell effectors and memory T-cells (CD45RO+). Further investigation of the primary tumor microenvironment showed a correlation between the absence of metastasis to lymph nodes or distant organs and markers of innate immune cells (macrophages, dendritic cells, natural killer (NK) cells and NK T cells) and activated T-cells.6 The co-expression of genes for cytotoxicity and TH1 predicted patient survival independently of metastatic status (n=142, P<0.05). Pages, Galon et al.7 recently published a study in which they classified early-stage colorectal cancer patients (TNM I-II) into four different prognostic groups based on the density of CD45RO+ and CD8+ cells in different tumor regions. Six hundred and two tumors from two independent cohorts were investigated, and they found dramatic differences in disease-free, disease-specific and overall survival (Figure 2). Five-year survival in patients with high densities of both CD8+ and CD45RO+ cells was 86.2%, and only 4.8% of the patients had tumor recurrence, whereas in the group with low densities of these cells 75% had tumor recurrence, and only 27.5% survived. The immune criteria were found to be independent prognostic factors in multivariate analysis (n=602, P<0.0001).
Figure 1.
(A) Kaplan-Meier curves illustrate the duration of disease-free survival according to the UICC-TNM stages [stage I, red line (n = 75 patients); stage II, green line (n = 137); stage III, blue line (n = 99), and stage IV, black line (n = 95)] in patients with CRCs. (B) Kaplan-Meier curves illustrate the duration of disease-free survival according to the UICC-TNM stages [as in (A)] and according to the density of CD3+ cells in combined tumor regions (CD3CTLoCD3IMLo, thick lines, n = 93 patients; CD3CTHiCD3IMHi, thin lines, n = 109). The subgroup of patients that did not appear to have a coordinated in situ immune reaction in tumor regions (Hi/Lo or Lo/Hi for CD3+ cell densities) presented Kaplan-Meier curves similar to those in the entire cohort. (C) Kaplan-Meier curves illustrate the duration of disease-free survival according to the UICC-TNM stages and to the density of CD3+ and CD45RO+ cells in combined tumor regions (CD3CTLoCD3IMLo plus CD45ROCTLoCD45ROIMLo, thick lines, n = 16 patients; CD3CTHiCD3IMHi plus CD45ROCTHiCD45ROIMHi, thin lines, n = 88). Cutoff values were 250, 640, 60, and 190 for CD3CT, CD3IM, CD45ROCT, and CD45ROIM, respectively. In (B) and (C), log-rank statistical test, ** P< 10−4; ns, not significant. CT, center of the tumor; IM, invasive margin. From Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795), 2006:1960–4.
Figure 2.
(A)The pie chart illustrates the repartition of the patients according to the presence of concordance (right part) or discrepancy (left part) between CD8+ and CD45RO+ cell densities in combined tumor regions of stages I and II colorectal tumors. Fifty-four percent of the patients presented with similar high (4)-Hi, low (0)-Hi, or heterogeneous (2)-Hi immune infiltration for markers, whereas 46% presented with distinct patterns. (B, C) Kaplan-Meier curves for the duration of disease-free survival and overall survival according to a combined analysis of CD8+ and CD45RO+ densities in tumor regions (center of the tumor [CT] and invasive margin [IM]), in patients with stage I or II colorectal cancer. Patients are stratified according to an immune score ranging from 0 to 4, depending on the total number of high densities observed (two markers assessed in CT, two markers assessed in IM). For example, (4)-Hi refers to a tumor with high densities of CD8+ and CD45RO+ cells in CT and IM regions of the tumor (red line). (3)-Hi refers to tumors with three high densities (green line). (0)-Hi represents tumors with low densities of CD8+ and CD45RO+ cells in both tumor regions (black line). Patients with an immune score of 1 or 2 [(1)-Hi, (2)-Hi] experiencing similar outcome were therefore regrouped [1–2-Hi; blue line]. Log-rank statistical test, P < .001 for all comparisons. Duration of disease-free survival and overall survival of the entire cohort of patients is also represented (gray line). From Pages F, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 27(35), 2009:5944–51.
Recently, Ogino et al.8 investigated specimens from colorectal cancer patients in two independent prospective cohorts. They found tumor-infiltrating immune cells and a high overall lymphocytic reaction score (comprised of Crohn’s-like reaction, peritumoral reaction, intratumoral periglandular reaction and tumor-infiltrating lymphocytes) to be independent prognostic factors for increased colorectal cancer specific and overall survival (n=843, P<0.01). Tumor-infiltrating immune cells were also found to be an independent prognostic factor of survival and recurrence-free survival in patients with colorectal cancer by Ropponen et al.9, and the authors suggested using immune cell infiltrates in evaluating patients for adjuvant therapy (n=276, P<0.001). Guidoboni et al.10 also found that high numbers of activated cytotoxic lymphocytes in colon cancer independently correlated with improved overall and disease-free survival, particularly in patients with stage III tumors (n=109, P<0.001). A high frequency of microsatellite instability was also correlated to survival, and multivariate analysis revealed that patients with both features had a lower risk than those with either factor separately. The same group had previously shown that tumors with microsatellite instability have a high prevalence of activated cytotoxic T-lymphocytes and increased tumor cell apoptosis.11 Similarly, Zlobec et al.12 found that an infiltrative tumor margin and the absence of CD8+ tumor-infiltrating T-cells were independent predictors of local recurrence in node-reactive colon cancer with microsatellite instability (n=119, P<0.001). This could potentially help identify high-risk patients who could benefit from adjuvant chemotherapy.
In other studies of colorectal cancer, Menon et al.13 found that a lower tumor stage and marked infiltration of CD8+ and CD57+ (expressed on late stage effector CD8+ T-cells and NK-cells) cells at the advancing tumor margin were independent prognostic factors for a longer disease-free survival (n=93, P<0.05). In addition, Diederichsen et al.14 showed a significantly higher 5-year survival in patients with a low CD4+/CD8+ ratio in the tumor-infiltrating lymphocytes. This effect was independent of the Dukes’ stage and patient age (n=41, P<0.05). In accordance with these results, Katz et al.15 recently investigated the presence of tumor-infiltrating lymphocytes in resected colorectal cancer liver metastases, and found that independent correlates of 10-year survival included a high number of CD8+ T-cells and a low number of CD4+ T-cells, and that the combination of CD8+ and CD4+ cell counts was a more powerful predictor of survival than either marker alone (n=162, P<0.001). The authors concluded that analysis of tumor-infiltrating lymphocytes represents a potentially powerful prognostic tool that needs to be further validated. Halama et al.16 recently reported that the presence of tumor-infiltrating CD8+ lymphocytes in the tumor margin of liver metastases of colorectal cancer predicted response to chemotherapy (n=33, P<0.001). Thus, evaluation of tumor-infiltrating lymphocytes may have an impact on the choice of subsequent therapy.
Tumor-infiltrating FoxP3+ regulatory T-cells have also been shown to have a strong prognostic significance in colorectal cancer. In this study, Salama et al.17 found the regulatory T-cell density in normal and tumor tissue to be independent prognostic indicators, but not the density of CD8+ T-cells. High regulatory T-cell density in normal mucosa was associated with worse prognosis. In contrast, a high density of regulatory T-cells in tumor tissue was associated with improved survival (n=967, P<0.001). Similarly, regulatory T-cells were found to be a favorable prognostic factor in advanced colon cancer patients undergoing chemotherapy or chemo-immunotherapy in a study by Correale et al.18 They used immunohistochemistry to evaluate the infiltration of regulatory T-cells in primary tumors from colorectal cancer patients enrolled in a phase III clinical immunotherapy trial, and found that higher scores were associated with a better prognosis (mean overall survival 43.2 months for patients with a high score vs. 28.6 months for patients with a low score), and with better outcome after treatment (progression free survival, 15.8 months for high score vs 8.8 months for low score) (n=57, P<0.001). In this study the presence of regulatory T-cells was evaluated in stroma adjacent to neoplastic glands. In contrast, Sinicrope et al.19 found that an increase in intraepithelial FoxP3+ cells was associated with poor tumor differentiation and advanced patient age, and that a low epithelial CD3+ to FoxP3+ cell ratio was a predictor of shorter disease free survival (n=160 colon carcinomas, 25 normal, P<0.05). By multivariate analysis a low CD3+/FoxP3+ ratio and low numbers of CD3+ T-cells were found to be stronger prognostic variables than tumor stage or number of lymph node metastases. Camus et al.6 did not find regulatory T-cells to be a predictive factor in colorectal carcinoma. Thus, the impact of the presence of regulatory T-cells in colorectal carcinoma is still not quite clear, and the location of the infiltrate would seem to play an important role.
Dendritic cells have also been investigated in colorectal carcinoma. Sandel et al.20–21 showed that patients with high numbers of mature CD208+ infiltrating dendritic cells in tumor epithelium had shorter overall survival than patients with low numbers (n=104, P<0.01). In addition, patients with high numbers of CD1a+ infiltrating dendritic cells at the tumor margin had shorter disease-free survival (P<0.05).
Di Giorgio et al.22 also found that the presence of lymphocytic infiltration in the tumor was related to a better prognosis by multivariate analysis in colorectal cancer patients resected between 1960 and 1978 (n=361, P<0.001). Some studies have also emphasized the location of immune infiltrate in tumor. Naito et al.23 divided the tumor-infiltrating lymphocytes into three locations, those within cancer nests, those distributed in the cancer stroma, and those present along the invasive margin. CD8+ T-cells within cancer nests were found to be most significantly associated with survival of the patients, both by monovariate and multivariate analyses, and to the same extent as Dukes’ staging (n=131, P<0.05). Further analysis by Chiba et al.24 showed that the beneficial effect of intraepithelial CD8+ T-cells became significant only after 2 years’ follow-up, and patients with survival more than 5 years had significantly higher numbers of these cells (n=371, P<0.001).
Michael-Robinson et al.25 found that there were more tumor-infiltrating immune cells in tumors with microsatellite instability, that >75% of these were T-cells, and that these tumors had an increased apoptotic rate (n=102, P<0.001). Their conclusion is that most likely tumor-infiltrating immune cells and apoptosis are independent characteristics of these tumors. A later study in colorectal cancer with and without microsatellite instability by Prall et al.26 suggested combining CD8+ tumor-infiltrating lymphocytes with microsatellite status as prognostic and predictive factors. They found that CD8+ high cases had a trend towards a better clinical course (n=152, P=0.058).
In contrast to the numerous studies above, Laghi et al.27 recently found that an increasing percentage of CD3+ tumor-infiltrating T-cells progressively reduced the risk of metachronous metastasis in node-negative colorectal cancer, but not in node-positive cancer (n=286, P<0.01). The authors concluded that the density of CD3+ T-cells cannot be used as an independent predictor of outcome in patients with stage III colorectal cancer. The main difference in this study compared to the other studies cited is that the authors did not investigate CD4+, CD8+ and regulatory T-cells separately, but rather all CD3+ T-cells together.
In conclusion, the number, type and location of tumor immune infiltrates was shown to be of importance for prediction of outcome. Two studies found tumor-infiltrating regulatory T-cells to be beneficial, whereas one study found them to be a negative predictive factor. This may depend on the location of the regulatory T-cells within the tumor, and the possibility that regulatory T-cells can be confused with newly activated T-cells. Dendritic cells were found to be a negative predictive factor, and to our knowledge Th17-cells have not yet been investigated in colorectal carcinoma. In summary, a large number of studies to date have shown infiltrating CD8+ T-cells to be a positive predictive factor in colorectal carcinoma, and in some studies high CD8+ T-cells combined with low CD4+ T-cells was even better.
Carcinomas (non-colorectal) in which studies have found a strong association between tumor-infiltrating immune cells and prognosis (see Table 2)
Table 2.
Carcinomas (non-colorectal) in which studies have found an association between tumor-infiltrating immune cells and prognosis
| Reference | Principal findings Correlation with better prognosis |
n | P value | Stage of carcinoma |
Methods | Definition of immune cells | Follow-up period (years) |
|---|---|---|---|---|---|---|---|
| Hepatocellular carcinoma | |||||||
| 28 | Positive correlation for cytotoxic T-cells, negative for T-regs. |
302 | <0.001 | I-IV | H, IHC, TMA | CD3+, CD4+, CD8+, GrB+ and FoxP3+ tumor infiltrating cells. | >9 |
| 29 | Negative correlation for T-regs. | 147 | <0.01 | I-III | H, IHC | CD4+, CD8+, and FoxP3+ tumor infiltrating cells. | 4 |
| 30 | Positive correlation for DC and cytotoxic T-cells. |
123 | <0.01 | I-IV | H, IHC | CD3+, CD8+, CD45RO+ T-cells and S-100+ DC in HCC nodules. | 2 |
| 31 | Negative correlation for Th17-cells. | 178 | <0.001 | I-IV | IHC, FACS | CD3+, CD4+, IL-17A+, CD62L-, CD45RO+, CCR4+, and CCR6+cells. | 8 |
| Gallbladder carcinoma | |||||||
| 32 | Positive correlation for CD4+ and CD8+ T-cells and DC. |
110 | <0.01 | I-IV | H, IHC | CD4+, CD8+, CD57+, and S-100 protein+ tumor infiltrating cells. | >5 |
| Pancreatic carcinoma | |||||||
| 33 | Positive correlation for CD4+ and CD8+ T-cells. |
80 | <0.01 | I-IV | H, IHC | CD4+, CD8+, and S-100 protein+ tumor infiltrating cells. | 8 |
| 34 | Negative correlation for T-regs. | 198 | <0.001 | I-IV | H, IHC | CD4+, CD25+, and FoxP3+ tumor infiltrating cells. | 4 |
| Esophageal carcinoma | |||||||
| 35 | Positive correlation for CD4+ and CD8+ T-cells. |
70 | <0.001 | I-IV | H, IHC, RT-PCR | Tumor infiltrating, peritumoral or stromal CD8+, IFNg+ T-cells. | >6 |
| 36 | Positive correlation for CD4+ and CD8+ T-cells. |
122 | <0.0001 | I-IV | H, IHC | Tumor infiltrating CD4+, CD8+, and CD57+ cells. | >9 |
| Ovarian carcinoma | |||||||
| 37 | Positive correlation for T-cells. | 186 | <0.001 | III-IV | H, IHC, FACS, RT-PCR |
CD3+, CD4+, CD8+, CD16+, CD19+ and CD45+ cells. | >11 |
| 38 | Positive correlation for CD3+ T-cells. | 116 | <0.001 | I-IV | H, IHC | CD3+ T-cells in cancer stroma and epithelium. | 10 |
| 39 | Positive correlation for CD8+ T-cells and a high CD8+/T-reg ratio. |
117 | <0.001 | I-IV | H, IHC | CD4+, CD8+, CD25+, and FoxP3+ tumor infiltrating cells. | 10 |
| 40 | Positive correlation for Th17-cells. | 85 | <0.001 | II-IV | H, IHC, FACS | Tumor infiltrating CD3+, CD4+, CD45RO+, CCR4+, CCR6+, IL-17+ Th17-cells. |
4 |
| Endometrial carcinoma | |||||||
| 41 | Positive correlation for CD3+ T-cells. | 65 | <0.05 | I-IV | H, IHC | Tumor infiltrating CD3+, CD4+, CD8+, and CD57+ cells. | >10 |
| 42 | Positive correlation for CD8+ T-cells and a high CD8+/T-reg ratio. |
368 | <0.05 | I-IV | H, IHC, TMA | Tumor infiltrating CD8+, CD45RO+, and FoxP3+ cells. | >5 |
| Cervical carcinoma | |||||||
| 43 | Positive correlation for CD8+ T-cells, and a high CD8+/CD4+ or CD8+/T-reg ratio. |
59 | <0.05 | I-II | H, IHC | Tumor infiltrating CD1a+, CD3+, CD4+, CD8+, CD45RO+, CD57+, FoxP3+, and DC-Lamp+ cells. |
− |
| Bladder carcinoma | |||||||
| 44 | Positive correlation for tumor infiltrating lymphocytes. |
514 | <0.01 | I-IV | H, LM | Tumor infiltrating lymphocytes. | 15 |
| Urothelial carcinoma | |||||||
| 45 | Positive correlation for CD8+ T-cells. | 69 | <0.05 | I-IV | H, IHC | Tumor infiltrating CD8+ T-cells. | >8 |
DC: dendritic cells; FACS: flow cytometry; H: histopathological analysis; IHC: immunohistochemistry; LM: light microscopy; TMA: tissue microarray; T-regs: regulatory T-cells.
Hepatocellular and gallbladder carcinoma
Gao et al.28 investigated the association between intratumoral regulatory T-cells and cytotoxic T-cells, and overall survival in patients with hepatocellular carcinoma. They found that the number of either cell type alone was an independent predictor for overall survival, and that the combination of high cytotoxic T-cells with low regulatory T-cells was an independent predictor of overall survival and disease-free survival (n=302, P<0.001). Similarly, Kobayashi et al.29 found a high prevalence of regulatory T-cells to be an independent prognostic factor for decreased overall survival and disease-free survival (n=147, P<0.01), whereas the prevalence of tumor-infiltrating CD8+ T-cells was not. Disease-free survival was 36.2 months in patients with a low prevalence of regulatory T-cells, compared to 27.3 months in patients with a high prevalence, and overall survival was 60.3 versus 45.1 months, respectively. In an additional study, Cai et al.30 found that high numbers of tumor-infiltrating dendritic cells were an independent positive predictor of tumor-free survival (n=123, P<0.01), and the numbers of CD45+ memory cells, CD3+ T-cells and CD8+ cells also strongly correlated to tumor-free survival. Zhang et al.31 investigated intratumoral Th17-cells in hepatocellular carcinoma patients, and found that a high prevalence of these cells was an independent predictor of decreased disease-free survival (7 months versus 16 months), and overall survival (34 months versus 49 months) (n=178, P<0.001).
In summary, high numbers of CD8+ T-cells and dendritic cells were positive predictive factors in hepatocellular carcinoma, whereas both regulatory T-cells and Th17-cells were shown to be negative predictive factors.
In gallbladder adenocarcinoma, Nakakubo et al.32 found a correlation between prolonged survival and a high degree of CD4+ T-cell, CD8+ T-cell and dendritic cell infiltration (n=110, P<0.01).
Pancreatic carcinoma
Fukunaga et al.33 found a high degree of infiltration of both CD4+ and CD8+ T-cells to be an independent favorable prognostic factor for overall survival in patients with pancreatic adenocarcinoma (n=80, P<0.01) 33. In addition, Hiraoka et al.34 investigated the prevalence of regulatory T-cells in pancreatic adenocarcinoma, and found that a high prevalence of regulatory T-cells was an independent negative predictive factor for survival (n=198, P<0.001).
Esophageal carcinoma
Schumacher et al.35 found that the presence of intratumoral CD8+ T-cells, more than peritumoral infiltration, was an independent prognostic factor indicating favorable outcome in both squamous cell and adenocarcinomas of the esophagus (n=70, P<0.001). Cho et al.36 also found that esophageal carcinoma patients high in both CD4+ and CD8+ tumor-infiltrating T-cells had a significantly higher survival rate than patients with low levels of either one or both subsets of T-cells. A high level of both CD4+ and CD8+ tumor-infiltrating T-cells was an independent prognostic factor for survival (n=122, P<0.0001).
Ovarian cancer
Zhang et al.37 evaluated the presence of immune cells in tumor-cell islets in ovarian cancer. They showed that for patients with advanced-stage ovarian cancer the five-year survival rate among patients with tumor-infiltrating T-cells was 38%, compared to 4.5% among patients with no tumor-infiltrating lymphocytes (n=186, P<0.001). They also examined 74 patients with a complete clinical response after debulking and platinum-based chemotherapy. The five-year overall survival rate was 74% in patients with T-cells in tumor cell islets, compared to 12% in patients with no T-cells in the islets. The presence of intratumoral T-cells independently correlated with delayed recurrence and delayed death in multivariate analysis (n=74, P<0.001).
In accordance with these results, Tomsova et al.38 showed that intraepithelial CD3+ T-cells were a significant independent positive predictor of overall survival in patients with ovarian carcinoma (n=116, P<0.001). Sato et al.39 also found that patients with higher frequencies of intraepithelial CD8+ T-cells demonstrated longer survival compared to patients with lower frequencies (55 versus 26 months) (n=117, P<0.001), independently of response to chemotherapy. They also found that a high CD8+/regulatory T-cell ratio was associated with a favorable prognosis, with a median survival of 74 months compared to 25 months in patients with a low ratio (P<0.001). Interestingly, Kryczek et al.40 recently showed that a high level of IL-17 in ascites fluid produced by tumor-infiltrating Th17-cells was a positive predictor for survival in patients with grade II-IV ovarian cancer (n=85, P<0.001). They showed that the tumor-infiltrating Th17-cells were the sole producer of IL-17, which together with IFN-γ acted to recruit CD8+ T-cells and natural killer cells into the tumor microenvironment. The median survival in patients with high levels of IL-17 was 78 months, compared to 27 months in patients with low levels. In summary, high numbers of tumor CD8+ T-cells, Th17-cells, and a high CD8+ T-cell: regulatory T-cell ratio were found to be positive predictive factors in ovarian carcinoma.
Endometrial and cervical cancer
Ino et al.41 investigated 65 cases of endometrial cancer, and found that a low number of tumor-infiltrating CD3+ T-cells was an independent prognostic factor for impaired progression-free survival (n=65, P<0.05). In addition, de Jong et al.42 found that high numbers of CD8+ T-lymphocytes were an independent positive predictive factor for overall survival in all cohorts of endometrial cancer 42. A high CD8+/FoxP3+ ratio was associated with improved survival in type I endometrial cancer, and CD45RO+ lymphocytes were also associated with improved overall survival (n=368, P<0.05).
Piersma et al.43 investigated human papilloma virus (HPV) induced cervical cancer and found a significantly stronger CD8+ T-cell infiltration, a higher CD8+/CD4+ T-cell ratio, and a higher CD8+/regulatory T-cell ratio in patients with no metastases to the draining lymph nodes, which is associated with better prognosis (n=59, P<0.05). Interestingly, the highest numbers of tumor-infiltrating CD8+ T-cells were found in patients with no lymph node metastases and displaying a concomitant systemic anti-tumor immune response assessed by determination of the HPV-specific T-cell response in blood.
Bladder cancer and urothelial cancer
Lipponen et al.44 found that dense tumor-infiltrating lymphocytes were a highly significant predictor of favorable prognosis in invasive transitional cell bladder cancer (n=514, P<0.01). In accordance with these results, patients with advanced urothelial carcinoma with higher numbers of CD8+ T-cells in the tumor had better disease-free survival (n=69, P<0.001) and overall survival (P<0.05) in a recent study by Sharma et al.45
Carcinomas in which studies have found a mixed or weak association between tumor-infiltrating immune cells and prognosis (see Table 3)
Table 3.
Carcinomas in which studies have found a mixed, weak or negative association between tumor-infiltrating immune cells and prognosis
| Reference | Principal findings Correlation with better prognosis |
n | P value | Stage of carcinoma | Methods | Definition of immune cells | Follow-up period (years) |
|---|---|---|---|---|---|---|---|
| Lung carcinomas | |||||||
| 46 | Positive correlation for T-cells and B-cells in squamous cell carcinoma. |
1290 | <0.05 | I-III | H, IHC | CD3+, CD4+, CD8+, and CD20+ cells in cancer cell nests. |
>10 |
| 47 | Positive correlation for CD4+ and CD8+ T-cells in NSCLC. | 109 | <0.01 | I-III | H, IHC | CD4+ and CD8+ T-cells in cancer cell nests and stroma. | >7 |
| 48 | Positive correlation for macrophages and CD8+ T-cells in NSCLC. | 199 | <0.001 | IV | H, IHC | CD8+, CD68+, c-kit+ cells in cancer cell nests and stroma. |
>5 |
| 49 | No correlation with prognosis in NSCLC. | 128 | − | I-IV | H, IHC | CD8+ T-cells in cancer cell nests, invasive margin, or cancer stroma. |
>5 |
| Breast carcinoma | |||||||
| 50 | Positive correlation for lymphoid infiltration in patients <40 years of age. |
1919 | <0.001 | I-III | H, LM | Tumor infiltrating lymphocytes. | >14 |
| 51 | Positive or negative correlation depending on estrogen receptor status. |
155 | <0.001 | I-IV | H, TMA | Lymphocyte marker genes CCL5, CD19, CD37, CD3D, CD3E, CD3G, CD3Z, CD79A, CD79B, CD8A, CD8B1, IGHG3, IGJ, IGLC1, CD14, LCK, LTB and MS4A1. |
>20 |
| 52 | No correlation with prognosis. | 75 | <0.05 | I-IV | H, IHC | Tumor infiltrating CD3+ T-cells. | − |
| 53 | Negative correlation for T-regs. | 237 | <0.05 | I-III | TMA, IHC | FoxP3+ lymphocytes in invasive tumor. | 11 |
| 54 | Negative correlation for FoxP3+ cells. | 1445 | <0.05 | I-III | TMA, IHC | FoxP3+ cells in tumor cell nests and stroma. | 11 |
| Prostate carcinoma | |||||||
| 56 | Positive correlation for high numbers of tumor infiltrating lymphocytes. |
325 | <0.05 | I-IV | H, LM | Tumor infiltrating lymphocytes. | >13 |
| 57 | Negative correlation for tumor infiltrating T-cells and B-cells. | 188 | <0.05 | I-III | H, IHC, TMA | Tumor infiltrating CD4+, CD8+, and CD20+ cells. | >18 |
| 58 | Negative correlation for tumor infiltrating mast cells. | 104 | <0.01 | I-IV | H, IHC | Tumor infiltrating tryptase-positive mast cells. | 8 |
| Head and Neck carcinoma | |||||||
| 59 | Positive correlation for CD8+ and CD20+ cells in early disease, negative for inoperable disease. |
115 | <0.05 | I-IV | H, IHC, TMA | Tumor infiltrating CD3+, CD4+, CD8+, CD20+, CD68+, FoxP3+, and GrB+ cells. |
6 |
| 60 | No correlation with prognosis. | 33 | <0.05 | I-IV | H, IHC, TMA | Tumor infiltrating CD3+, CD8+, CD20+, CD68+, FoxP3+, and GrB+ cells. |
6 |
| 61 | Positive correlation for T-regs. | 84 | <0.05 | I-IV | IHC | Tumor infiltrating CD3+, CD4+, CD25+, CD69+, and FoxP3+ cells. |
2 |
| Renal cell carcinoma | |||||||
| 62 | Negative correlation for neutrophils. | 121 | <0.001 | I-IV | H, IHC | Tumor infiltrating CD8+, CD34+, CD57+, and CD66b+ cells. |
16 |
| 63 | Negative correlation for neutrophils, positive for CD57+ natural killer cells. |
85 | <0.01 | I-IV | H, IHC | Tumor infiltrating CD4+, CD8+, CD20+, CD56+, CD57+, and CD66b+ cells. |
>6 |
| 64 | Negative correlation for mononuclear cells. | 306 | <0.05 | I-IV | H, LM | Tumor infiltrating lymphocytes. | >11 |
| 65 | Negative correlation for CD8+ T-cells, positive for CD4+ T-cells. | 79 | <0.01 | I-IV | H, FACS | Tumor infiltrating CD3+, CD4+, CD8+, CD11+, CD16+, CD19+, CD20+, CD45RA+, and CD57+ cells. |
>5 |
| 66 | Negative correlation for CD4+ T-cells. | 73 | <0.001 | I-IV | H, IHC | Tumor infiltrating CD4+ and CD8+ T-cells. | >3 |
FACS: flow cytometry; H: histopathological analysis; IHC: immunohistochemistry; LM: light microscopy; TMA: tissue microarray; T-regs: regulatory T-cells; NSCLC, non-small-cell lung cancer.
Lung cancer
Ruffini et al.46 evaluated the prevalence of tumor-infiltrating T-cells and B-cells in tumor nests in 1,290 patients operated for primary lung neoplasms 46. They found lymphocytic infiltrates in 23% of the patients, and an association between the presence of immune cells and improved survival in early stage squamous cell carcinoma, but no other lung neoplasms (n=1,290, P<0.05). They observed overall better survival in patients with tumor-infiltrating lymphocytes, but this was not significant.
Tumor infiltration by CD8+ and CD4+ T-cells was found to be an independent favorable prognostic factor in non-small cell lung cancer (NSCLC) by Hiraoka et al.47. In this study, the number of CD8+ T-cells alone had no prognostic significance, but the survival rate for patients with high levels of both CD8+ and CD4+ T-cells was significantly higher than for all other groups (n=109, P<0.01).
Kawai et al.48 found that more CD8+ T-cells or macrophages in cancer nests than in stroma were independent positive prognostic predictors for survival in NSCLC (n=199, P<0.001). In contrast to these results, Mori et al.49 investigated 128 cases of NSCLC, and found that the number of CD8+ T-cells in cancer nests was related to the histological subtype and the differentiation of the tumor, but not to patient survival (n=128).
Breast cancer
Menard et al.50 found a strong positive correlation between survival rates and the presence of lymphocytic infiltrates in patients less than 40 years old with primary ductal and lobular infiltrating breast cancer. Lymphoid infiltration was an independent predictive factor for 5-, 10-, and 15-year survival in women under 40 years of age, but not in older patients (n=1919, P<0.001).
Calabro et al.51 recently showed that for estrogen receptor positive patients with breast cancer a high degree of lymphocyte infiltration was associated with shorter survival, whereas for estrogen receptor negative patients a high degree of lymphocyte infiltration was associated with longer survival (n=155, P<0.001). Lucin et al.52 investigated the presence of CD3+ T-cell infiltrates in ductal invasive carcinomas, and found that tumors with a high degree of infiltrate had predominantly negative lymph nodes, and vice versa (n=75, P<0.05), but they did not find any correlation with prognosis.
Bates et al.53 found high numbers of tumor-infiltrating regulatory T-cells to be an independent prognostic factor for shorter recurrence-free survival and overall survival in breast cancer (n=237, P<0.05). In addition, Mahmoud et al.54 found intratumoral and tumor-adjacent FoxP3+ T-cells to be associated with worse prognosis in breast carcinoma (n=1445, P<0.05), but the number of FoxP3+ T-cells was not found to be an independent prognostic factor in multivariate analysis. They therefore suggested that other inflammatory cell subsets may be more critical variables in breast carcinoma. One concern with this paper is that they looked at no markers other than FoxP3, which is not only expressed in regulatory T-cells, but also in activated T-cells.
Interestingly, Ono et al.55 recently reported that the pathological complete response rate to neoadjuvant chemotherapy in breast cancer was significantly higher in patients with tumor-infiltrating lymphocytes (n=68, P<0.01).
Prostate cancer
Vesalainen et al.56 found that the density of tumor-infiltrating lymphocytes in primary prostatic adenocarcinoma was independent of the tumor differentiation, and low numbers of tumor-infiltrating lymphocytes were a sign of high risk of tumor progression and fatal disease when they investigated 325 cases with long-term follow-up. Low numbers of tumor-infiltrating lymphocytes were an independent negative predictor of survival (n=325, P<0.05). In contrast, Kärjä et al.57 investigated the prognostic significance of tumor-infiltrating T-cells and B-cells in 188 radical prostatectomy specimens from patients with local prostate cancer and found that a strong expression of intratumoral T-cells and B-cells was an independent predictor of shortened PSA recurrence-free survival (n=188, P<0.05). Nonomura et al.58 found that higher mast cell counts infiltrating the tumor tissue was a significant negative prognostic marker of PSA-free survival (n=104, P<0.01).
Head and neck cancer
Distel et al.59 recently investigated 115 patients with squamous cell carcinomas of the oropharynx and hypopharynx. They found that higher numbers of intraepithelial CD8+ T-cells and CD20+ B-cells led to improved survival in the low-risk group (early disease), whereas in the high-risk group (inoperable disease) high CD20+ counts indicated shorter survival (n=115, P<0.05). In contrast, Pretscher et al.60 did not observe an association between tumor-infiltrating immune cells at the primary site and clinical outcome. However, increased numbers of CD8+ T-cells in metastatic tumors and large numbers of B-cells in lymph node metastases were associated with favorable outcome (n=33, P<0.05). In addition, Badoual et al.61 found infiltration of regulatory T-cells to be an independent positive prognostic factor for locoregional control and overall survival in head and neck cancer (n=84, P<0.05).
Renal cell carcinoma (see Table 3)
Studies have found a negative association between tumor-infiltrating immune cells and prognosis
In contrast to all other carcinomas described above, in renal carcinoma tumor-infiltrating immune cells have been described as having a negative effect. Jensen et al.62 reported that the presence of intratumoral neutrophils in renal cell carcinoma was an independent prognostic factor associated with short recurrence-free survival (n=121, P<0.0001). The 5-year recurrence-free survival was 53% in patients with intratumoral neutrophils compared to 87% in patients without. Donskov et al.63 also found the presence of intratumoral neutrophils to be an independent poor prognostic factor (n=85, P<0.001). In contrast, low intratumoral CD57+ natural killer cells were found to be an independent poor prognostic factor (P<0.01).
In accordance with these results, Webster et al.64 found that patients with a mononuclear cell infiltration had a significantly increased likelihood of dying from renal cell carcinoma (n=306, P<0.05). Igarashi et al.65 found that in patients with stage III-IV disease, an increased infiltration of CD4+ T-cells, but a decreased infiltration of CD8+ T-cells, constituted a good prognostic factor (n=79, P<0.01). In contrast, Bromwich et al.66 also found that increased numbers of intratumoral CD4+ T-cells were associated with poor survival (n=73, P<0.001). Clearly there is a need for further study of tumor-infiltrating immune cells in renal cell carcinoma, since previous studies have not been conclusive.
Discussion
In conclusion, in many solid tumors the presence of tumor-infiltrating immune cells correlates with better overall survival. However, it is necessary to know the phenotype of the immune cells; for example, in colorectal cancer the number of CD8+ T-cells, and a high ratio between CD8+ and CD4+ T-cells have been correlated with increased survival, whereas high numbers of mature dendritic cells predicted shorter survival (Table 1). Regulatory T-cells have been shown to have both a positive and a negative impact in colorectal carcinoma, possibly depending on the location of the infiltrate and on the definition of “regulatory T-cells” used. These cells were also shown to be a positive predictive factor in head and neck cancer, but a negative predictor in hepatocellular, pancreatic, ovarian, endometrial, cervical and breast carcinomas (Tables 1 and 2). However, as mentioned above, one must be extremely careful in distinguishing true regulatory T-cells with suppressive functions from activated T-cells.
In addition to phenotype, the location of the immune-cell infiltrate has also been shown to be important. The tumor microenvironment in different regions of the tumor has been shown to influence the immune system to promote either anti-tumor immunity, or tumor progression. CD8+ T-cells within cancer nests were shown to be better predictors of outcome than the same cells found in other areas of the tumor in both colorectal cancer and NSCLC. Macrophages in different areas of human tumors exhibit distinct activation patterns, influenced by soluble factors derived from tumors, such as IL-10,67 TGF-β,68 and hyaluronan,69 and by tumor-associated hypoxia.70 These tumor-associated macrophages (TAMs) therefore acquire an M1 tumor-killing phenotype, or an M2 tumor-promoting phenotype.
In contrast to most other carcinomas, in renal cell carcinoma increased numbers of intratumoral CD8+ T-cells and neutrophils were predictors of poor survival. The reasons behind these discrepancies need to be further studied to increase our understanding of the biology of tumor immunology and the potential for treatment of the individual carcinomas. Infiltration of IL-17–producing Th17-cells was shown to be a positive predictive factor in ovarian carcinoma, and a negative factor in hepatocellular carcinoma (Table 2). To our knowledge, Th17-cells have not yet been investigated in other carcinomas.
Factors influencing the immunogenicity of tumors
Several factors can be responsible for the link observed between immune cell infiltrate of some tumor types, such as that observed in colorectal cancer, and patient prognosis and/or response to therapy. First is the inherent immunogenicity of a specific tumor. This can be due to the expression of a point mutated oncogene or suppressor gene product, a gene product involved in the epithelial-to-mesenchymal transition (EMT) process, or the overexpression of oncofetal, tissue lineage and/or tissue differentiation protein gene products71. Peptides from each of these gene products have been shown to be coupled with MHC on the surface of the tumor cells for T-cell receptor recognition. Consequently, examples of all of the above gene products have been shown to be immunogenic in terms of enhancing human T-cell responses both in vitro and clinical studies.72 Another factor that can influence the immunogenicity of a given tumor is the level of expression of MHC Class I and Class II molecules. While it has been shown that some tumors downregulate these molecules, others do not. Still another factor that can influence the inherent immunogenicity of a tumor is the level of secretion by the tumor of immunosuppressive factors such as TGF-β. Interestingly, in breast cancer the impact of lymphocyte infiltration was shown to depend on the estrogen receptor (ER) status of the tumor. In ER+ patients a high degree of lymphocyte infiltration was associated with shorter survival, whereas in ER- patients it was associated with longer survival.
The link between chemotherapy/radiation therapy and immunity
While it has long been known that the effect of multiple regimens of chemotherapy and/or radiation therapy can suppress a patient’s immune system, preclinical data are now demonstrating that certain chemotherapeutic agents, irradiation of tumor, and certain small molecule targeted therapeutics can actually enhance the host immune response when used in conjunction with vaccine therapy, or following vaccine therapy. These studies have shown that when tumor cells are lysed by certain chemotherapeutic agents or radiation, apoptotic and/or necrotic tumor vesicles can be taken up by tumor-infiltrating dendritic cells and present tumor antigen peptides to T-cells, thus enhancing the immune response to tumor.73 Other studies have shown that when tumors are exposed to certain chemotherapeutic agents or external beam radiation, tumor cells alter their phenotype via the upregulation of tumor antigens, death receptors such as FAS, adhesion molecules and/or MHC molecules, and thus render these tumor cells more susceptible to T-cell lysis.74–75 Other studies have shown that certain chemotherapeutic agents and small molecule targeted therapeutics will temporarily deplete immune cell subsets differentially; the subsequent differential homeostatic proliferation of immune cell subsets has been shown to be exploited by delivering vaccine at the time of T-cell expansion.76–77 It is interesting to note that the vast majority of small molecule targeted therapies that reach clinical trials are being evaluated preclinically with human cells in vitro and employing xenograft models, i.e., in a host without an intact immune system. The role of these agents, either positively or negatively, on the host immune system should thus not be neglected.
Conclusions
The studies reported here not only point out the potential importance of evaluating the immune infiltrate in tumor in making decisions of patient prognosis and thus patient management, but also direct one to more seriously consider the role of active immunotherapy employing therapeutic vaccines in combination with more conventional therapies in order to enhance patient responses. Clinical trials combining therapeutic cancer vaccines and chemotherapy, radiation of tumor, and the use of small molecule targeted therapeutics are in progress and may well be an important new frontier in cancer management.
Acknowledgments
The authors thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.
Grant support: Intramural Research Program of the Center for cancer Research, National Cancer Institute, NIH.
Footnotes
Author contributions: Both authors contributed equally to the literature searches involved and in the writing of this review article.
References
- 1.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Sehouli J, Loddenkemper C, Cornu T, Schwachula T, Hoffmuller U, Grutzkau A, Lohneis P, Dickhaus T, Grone J, Kruschewski M, Mustea A, Turbachova I, Baron U, Olek S. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics. 2011;6:236–246. doi: 10.4161/epi.6.2.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 4.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, Trajanoski Z, Fridman WH, Galon J. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 7.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 8.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macri E, Lanza G, Boiocchi M, Dolcetti R. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008;14:3792–3797. doi: 10.1158/1078-0432.CCR-08-0048. [DOI] [PubMed] [Google Scholar]
- 13.Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]
- 14.Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, Jarnagin W, Fong Y, Blumgart L, D’Angelica M, Dematteo RP. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524–2530. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 16.Halama N, Michel S, Kloor M, Zoernig I, Pommerencke T, Schirmacher P, von Knebel-Döberitz M, Weitz J, Grabe N, Jäger D. Immune infiltrates in liver metastases of colorectal cancer and response to chemotherapy. J Clin Oncol. 2009;27 Abstract 15069. [PMC free article] [PubMed] [Google Scholar]
- 17.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 18.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576–2582. doi: 10.1158/1078-0432.CCR-04-1448. [DOI] [PubMed] [Google Scholar]
- 21.Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn C, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother. 2004;53:978–986. doi: 10.1007/s00262-004-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Giorgio A, Botti C, Tocchi A, Mingazzini P, Flammia M. The influence of tumor lymphocytic infiltration on long term survival of surgically treated colorectal cancer patients. Int Surg. 1992;77:256–260. [PubMed] [Google Scholar]
- 23.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 24.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael-Robinson JM, Biemer-Huttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–366. doi: 10.1136/gut.48.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, Mantovani A, Roncalli M, Malesci A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 30.Cai XY, Gao Q, Qiu SJ, Ye SL, Wu ZQ, Fan J, Tang ZY. Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:293–301. doi: 10.1007/s00432-006-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Nakakubo Y, Miyamoto M, Cho Y, Hida Y, Oshikiri T, Suzuoki M, Hiraoka K, Itoh T, Kondo S, Katoh H. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer. 2003;89:1736–1742. doi: 10.1038/sj.bjc.6601331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 36.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 37.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 38.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O, Kikkawa F. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 42.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 44.Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992;29A:69–75. doi: 10.1016/0959-8049(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 45.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macri L, Daniele L, Oliaro A. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 47.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, Kudoh S, Ochiai A. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 49.Mori M, Ohtani H, Naito Y, Sagawa M, Sato M, Fujimura S, Nagura H. Infiltration of CD8+ T cells in non-small cell lung cancer is associated with dedifferentiation of cancer cells, but not with prognosis. Tohoku J Exp Med. 2000;191:113–118. doi: 10.1620/tjem.191.113. [DOI] [PubMed] [Google Scholar]
- 50.Menard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, Salvadori B, Colnaghi MI, Rilke F. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3:817–819. [PubMed] [Google Scholar]
- 51.Calabro A, Beissbarth T, Kuner R, Stojanov M, Benner A, Asslaber M, Ploner F, Zatloukal K, Samonigg H, Poustka A, Sultmann H. Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Res Treat. 2009;116:69–77. doi: 10.1007/s10549-008-0105-3. [DOI] [PubMed] [Google Scholar]
- 52.Lucin K, Iternicka Z, Jonjic N. Prognostic significance of T-cell infiltrates, expression of beta 2-microglobulin and HLA-DR antigens in breast carcinoma. Pathol Res Pract. 1994;190:1134–1140. doi: 10.1016/s0344-0338(11)80439-5. [DOI] [PubMed] [Google Scholar]
- 53.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 54.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3(+) infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127:99–108. doi: 10.1007/s10549-010-0987-8. [DOI] [PubMed] [Google Scholar]
- 55.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Kouno T, Tamura K, Ando M, Katsumata N, KInoshita T, Fujiwara Y. Evaluation of tumor-infiltrating lymphocytes (TIL) and tumor cell apoptosis as predictive markers for response to neoadjuvant chemotherapy in triple-negative breast cancer. J Clin Oncol. 2009;27 Abstract 559. [Google Scholar]
- 56.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 57.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–4438. [PubMed] [Google Scholar]
- 58.Nonomura N, Takayama H, Nishimura K, Oka D, Nakai Y, Shiba M, Tsujimura A, Nakayama M, Aozasa K, Okuyama A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007;97:952–956. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, Nkenke E, Buttner M, Niedobitek G, Grabenbauer GG. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro-and hypopharynx: Prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45:e167–e174. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 60.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 62.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 63.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 64.Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML, Cheville JC, Kwon ED. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107:46–53. doi: 10.1002/cncr.21951. [DOI] [PubMed] [Google Scholar]
- 65.Igarashi T, Takahashi H, Tobe T, Suzuki H, Mizoguchi K, Nakatsu HO, Ito H. Effect of tumor-infiltrating lymphocyte subsets on prognosis and susceptibility to interferon therapy in patients with renal cell carcinoma. Urol Int. 2002;69:51–56. doi: 10.1159/000064361. [DOI] [PubMed] [Google Scholar]
- 66.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 68.Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- 69.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–595. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 70.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gulley JL, Arlen PM, Hodge JW, Schlom J. Vaccines and Immunostimulants. In: Hong W, editor. Cancer Medicine. Vol. 8. Shelton, CT: People’s Medical Publishing House-USA; 2010. pp. 725–736. [Google Scholar]
- 73.Lake RA, van der Most RG. A better way for a cancer cell to die. N Engl J Med. 2006;354:2503–2504. doi: 10.1056/NEJMcibr061443. [DOI] [PubMed] [Google Scholar]
- 74.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui Y, Zhang H, Meadors J, Poon R, Guimond M, Mackall CL. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009;114:3831–3840. doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]