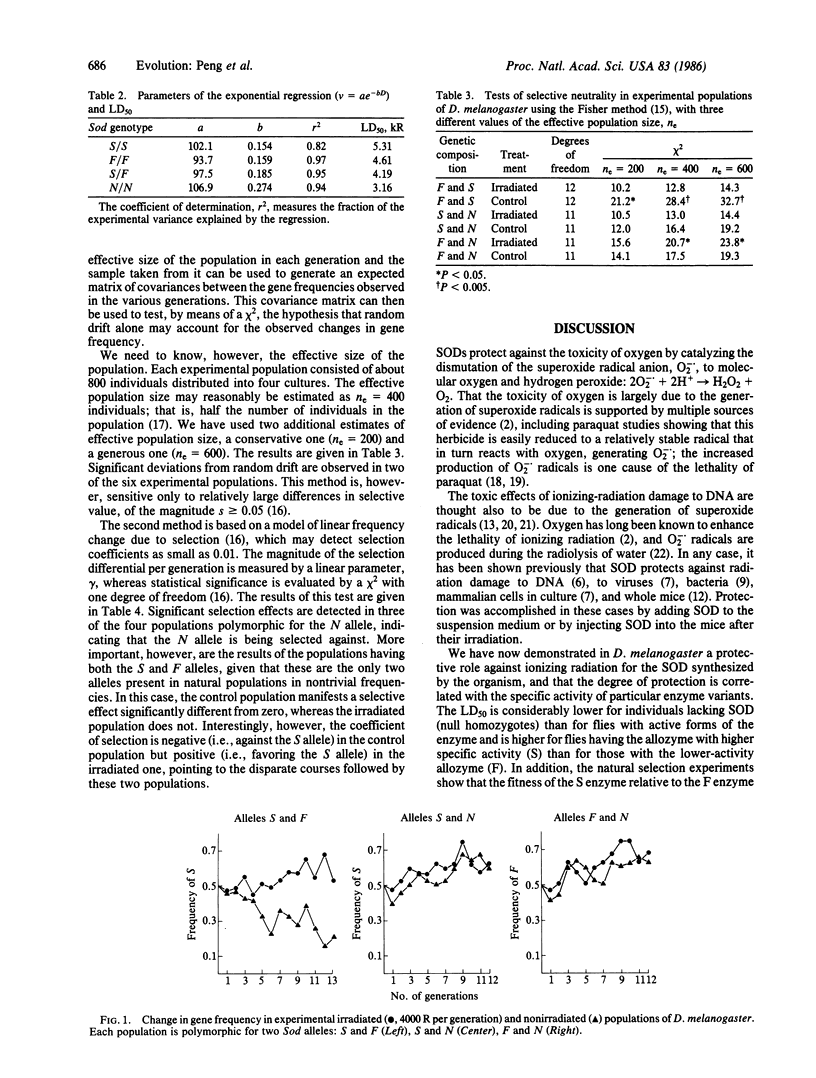
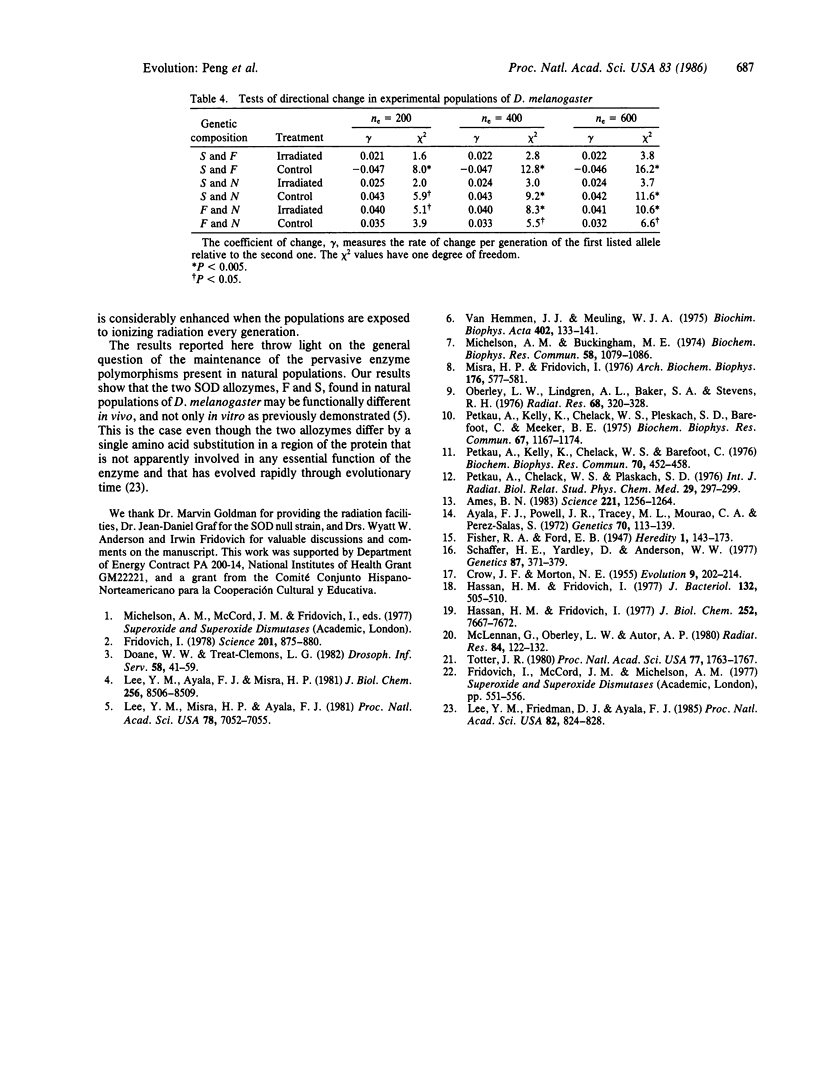
Abstract
The toxic effects of ionizing radiation to DNA are thought to be due to the generation of the superoxide radical, 02-. Superoxide dismutase (SOD), which scavenges 02-., has been invoked as a protecting enzyme against ionizing radiation in viruses, bacteria, mammalian cells in culture, and live mice. We now demonstrate that SOD is involved in the resistance of Drosophila melanogaster against irradiation. The protection is greatest when flies carry the S form of the enzyme (which exhibits highest in vitro specific activity), intermediate when they carry the F form of the enzyme, and lowest when they are homozygous for N, an allele that reduces the amount of the enzyme to 3.5% of the normal level. Natural selection experiments show that the fitness of the high-activity S allele is increased in an irradiated population relative to the nonirradiated control. These results point towards a possible adaptive function of the S/F polymorphism found in natural populations of D. melanogaster.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R., Tracey M. L., Mourão C. A., Pérez-Salas S. Enzyme variability in the Drosophila willistoni group. IV. Genic variation in natural populations of Drosophila willistoni. Genetics. 1972 Jan;70(1):113–139. doi: 10.1093/genetics/70.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Lee Y. M., Ayala F. J., Misra H. P. Purification and properties of superoxide dismutase from Drosophila melanogaster. J Biol Chem. 1981 Aug 25;256(16):8506–8509. [PubMed] [Google Scholar]
- Lee Y. M., Friedman D. J., Ayala F. J. Superoxide dismutase: an evolutionary puzzle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):824–828. doi: 10.1073/pnas.82.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Misra H. P., Ayala F. J. Superoxide dismutase in Drosophila melanogaster: biochemical and structural characterization of allozyme variants. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7052–7055. doi: 10.1073/pnas.78.11.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan G., Oberley L. W., Autor A. P. The role of oxygen-derived free radicals in radiation-induced damage and death of nondividing eucaryotic cells. Radiat Res. 1980 Oct;84(1):122–132. [PubMed] [Google Scholar]
- Michelson A. M., Buckingham M. E. Effects of superoxide radicals on myoblast growth and differentiation. Biochem Biophys Res Commun. 1974 Jun 18;58(4):1079–1086. doi: 10.1016/s0006-291x(74)80254-8. [DOI] [PubMed] [Google Scholar]
- Misra H., Fridovich I. Superoxide dismutase and the oxygen enhancement of radiation lethality. Arch Biochem Biophys. 1976 Oct;176(2):577–581. doi: 10.1016/0003-9861(76)90201-0. [DOI] [PubMed] [Google Scholar]
- Moustafa Hassan H., Fridovich I. Regulation of superoxide dismutase synthesis in Escherichia coli: glucose effect. J Bacteriol. 1977 Nov;132(2):505–510. doi: 10.1128/jb.132.2.505-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley L. W., Lindgren L. A., Baker S. A., Stevens R. H. Superoxide lon as the cause of the oxygen effect. Radiat Res. 1976 Nov;68(2):320–328. [PubMed] [Google Scholar]
- Petkau A., Chelack W. S., Pleskach S. D. Letter: Protection of post-irradiated mice by superoxide dismutase. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Mar;29(3):297–299. doi: 10.1080/09553007614550341. [DOI] [PubMed] [Google Scholar]
- Petkau A., Kelly K., Chelack W. S., Barefoot C. Protective effect of superoxide dismustase on erythrocytes of x-irradiated mice. Biochem Biophys Res Commun. 1976 May 17;70(2):452–458. doi: 10.1016/0006-291x(76)91067-6. [DOI] [PubMed] [Google Scholar]
- Petkau A., Kelly K., Chelack W. S., Pleskach S. D., Barefoot C., Meeker B. E. Radioprotection of bone marrow stem cells by superoxide dismutase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1167–1174. doi: 10.1016/0006-291x(75)90796-2. [DOI] [PubMed] [Google Scholar]
- Schaffer H. E., Yardley D., Anderson W. W. Drift or Selection: A Statistical Test of Gene Frequency Variation over Generations. Genetics. 1977 Oct;87(2):371–379. doi: 10.1093/genetics/87.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totter J. R. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1763–1767. doi: 10.1073/pnas.77.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hemmen J. J., Meuling W. J. Inactivation of biologically active DNA by gamma-ray-induced superoxide radicals and their dismutation products singlet molecular oxygen and hydrogen peroxide. Biochim Biophys Acta. 1975 Aug 21;402(2):133–141. doi: 10.1016/0005-2787(75)90031-3. [DOI] [PubMed] [Google Scholar]


