Abstract
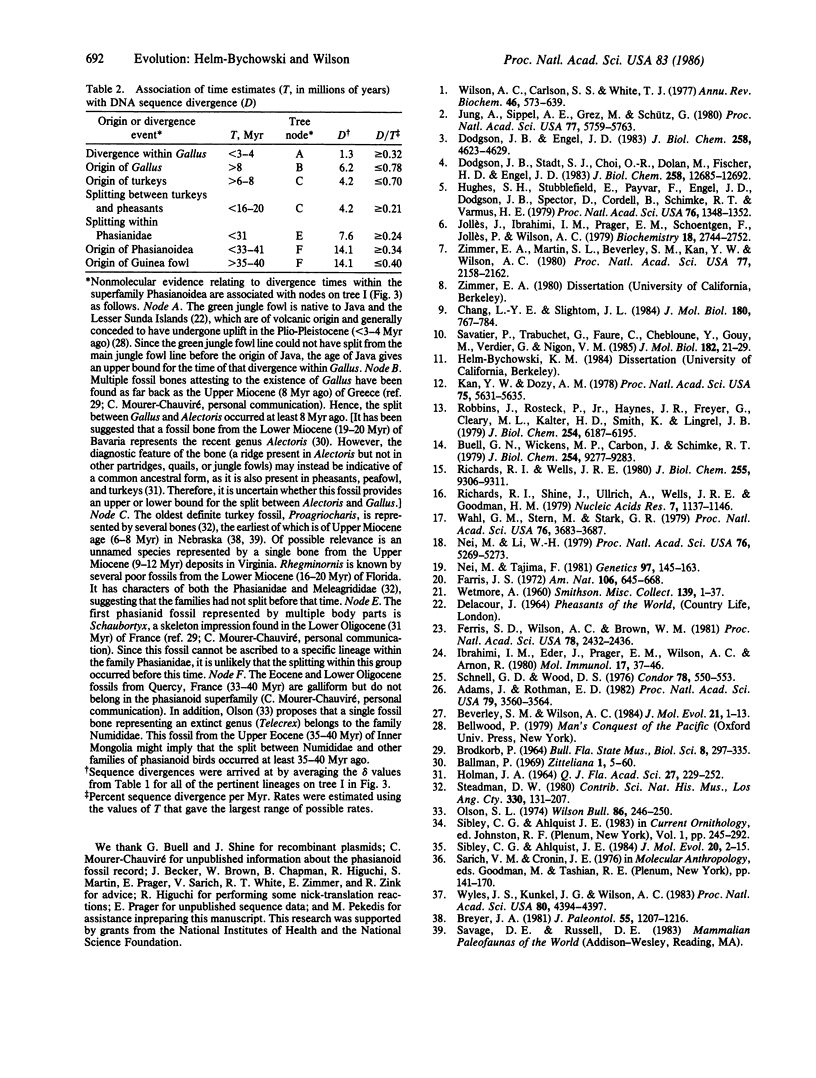
To examine the tempo of genomic evolution in birds, we mapped 161 restriction sites in the nuclear DNA of seven species of birds belonging to the pheasant superfamily Phasianoidea. The three regions mapped lie on different chromosomes and bear eight genes, coding for lysozyme c, three "alpha-like" globins, and four "beta-like" globins. Together, the three regions span about 56 kilobases, most of which is presumably noncoding. The maps differed from one another at a minimum of 77 sites and by 9 length mutations. The extent of sequence divergence due to base substitutions was inferred to be similar for all three regions, even though the three coding regions differ by 5-fold from one another in mean rate of evolution at the amino acid level. A tree relating the maps differs in branching order from that implied by the traditional classification of phasianoid birds and is supported by published protein comparisons. Five of the nodes in the tree were associated with fossil evidence and historical biogeographic information, allowing us to estimate the mean rate of DNA divergence to be 0.34-0.40% per million years. This rate is similar to that estimated for the globin gene regions of higher primates, which validates the concept of an evolutionary clock at the DNA level. Our fossil-based calibration of DNA evolution differs by a factor of almost 2 from that proposed by others on the basis of biogeography. In consequence, published estimates of divergence times for birds and primates that are based on a biogeographically calibrated DNA clock may be too long.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J., Rothman E. D. Estimation of phylogenetic relationships from DNA restriction patterns and selection of endonuclease cleavage sites. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3560–3564. doi: 10.1073/pnas.79.11.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Molecular evolution in Drosophila and the higher Diptera II. A time scale for fly evolution. J Mol Evol. 1984;21(1):1–13. doi: 10.1007/BF02100622. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Carbon J., Schimke R. T. Isolation of recombinant plasmids bearing cDNA to hen ovomucoid and lysozyme mRNAs. J Biol Chem. 1979 Sep 25;254(18):9277–9283. [PubMed] [Google Scholar]
- Chang L. Y., Slightom J. L. Isolation and nucleotide sequence analysis of the beta-type globin pseudogene from human, gorilla and chimpanzee. J Mol Biol. 1984 Dec 25;180(4):767–784. doi: 10.1016/0022-2836(84)90256-0. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Engel J. D. The nucleotide sequence of the adult chicken alpha-globin genes. J Biol Chem. 1983 Apr 10;258(7):4623–4629. [PubMed] [Google Scholar]
- Dodgson J. B., Stadt S. J., Choi O. R., Dolan M., Fischer H. D., Engel J. D. The nucleotide sequence of the embryonic chicken beta-type globin genes. J Biol Chem. 1983 Oct 25;258(20):12685–12692. [PubMed] [Google Scholar]
- Ferris S. D., Wilson A. C., Brown W. M. Evolutionary tree for apes and humans based on cleavage maps of mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2432–2436. doi: 10.1073/pnas.78.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Stubblefield E., Payvar F., Engel J. D., Dodgson J. B., Spector D., Cordell B., Schimke R. T., Varmus H. E. Gene localization by chromosome fractionation: globin genes are on at least two chromosomes and three estrogen-inducible genes are on three chromosomes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1348–1352. doi: 10.1073/pnas.76.3.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi I. M., Eder J., Prager E. M., Wilson A. C., Arnon R. The effect of a single amino acid substitution on the antigenic specificity of the loop region of lysozyme. Mol Immunol. 1980 Jan;17(1):37–46. doi: 10.1016/0161-5890(80)90122-4. [DOI] [PubMed] [Google Scholar]
- Jollès J., Ibrahimi I. M., Prager E. M., Schoentgen F., Jollès P., Wilson A. C. Amino acid sequence of pheasant lysozyme. Evolutionary change affecting processing of prelysozyme. Biochemistry. 1979 Jun 26;18(13):2744–2752. doi: 10.1021/bi00580a009. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981 Jan;97(1):145–163. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Shine J., Ullrich A., Wells J. R., Goodman H. M. Molecular cloning and sequence analysis of adult chicken betal globin cDNA. Nucleic Acids Res. 1979 Nov 10;7(5):1137–1146. doi: 10.1093/nar/7.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Wells J. R. Chicken globin genes. Nucleotide sequence of cDNA clones coding for the alpha-globin expressed during hemolytic anemia. J Biol Chem. 1980 Oct 10;255(19):9306–9311. [PubMed] [Google Scholar]
- Robbins J., Rosteck P., Jr, Haynes J. R., Freyer G., Cleary M. L., Kalter H. D., Smith K., Lingrel J. B. The isolation and partial characterization of recombinant DNA containing genomic globin sequences from the goat. J Biol Chem. 1979 Jul 10;254(13):6187–6195. [PubMed] [Google Scholar]
- Savatier P., Trabuchet G., Faure C., Chebloune Y., Gouy M., Verdier G., Nigon V. M. Evolution of the primate beta-globin gene region. High rate of variation in CpG dinucleotides and in short repeated sequences between man and chimpanzee. J Mol Biol. 1985 Mar 5;182(1):21–29. doi: 10.1016/0022-2836(85)90024-5. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Wyles J. S., Kunkel J. G., Wilson A. C. Birds, behavior, and anatomical evolution. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4394–4397. doi: 10.1073/pnas.80.14.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer E. A., Martin S. L., Beverley S. M., Kan Y. W., Wilson A. C. Rapid duplication and loss of genes coding for the alpha chains of hemoglobin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2158–2162. doi: 10.1073/pnas.77.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]



