Abstract
This case study series investigated a new treatment for paradoxical insomnia patients as there is no standard treatment for this patient group at this time. Four paradoxical insomnia patients had a polysomnography (PSG) sleep study, an unsuccessful brief course of behavioral treatment for insomnia, and then a novel sleep education treatment comprising review of their PSG with video and exploration of the discrepancy between their reported and observed sleep experience. Two patients responded well to sleep education, mainly with improved self-reported sleep onset latency, total sleep time, and Insomnia Severity Index scores; and the other two, who exhibited sleep architecture anomalies, were unresponsive. These findings suggest that sleep education holds promise for some paradoxical insomnia patients. Suggestions for future studies are given.
Paradoxical insomnia is a complaint of severe insomnia disproportional to the presence of objective sleep disturbance or daytime impairment (American Academy of Sleep Medicine [AASM], 2005). Previously known as sleep state misperception, paradoxical insomnia has been a difficult subtype of insomnia to assess and treat (Edinger & Krystal, 2003). Hypervigilance occurs while the patient is trying to sleep, suggesting paradoxical insomnia has a hyperarousal component, and there may be physiological or perceptual deficits that affect sleep/wake discrimination causing sleep time underestimation (Bonnet & Arand, 1997). Improving sleep/wake discriminations in people with paradoxical insomnia may lead to reductions in sleep complaints (Downey & Bonnet, 1992).
Using various criteria to establish a diagnosis of paradoxical insomnia, studies with small sample sizes placed prevalence estimates among people presenting with insomnia in the range of 9.2% to 40.3% (BaHammam, 2004; Coleman et al., 1982; Dorsey & Bootzin, 1997; Edinger et al., 2000). Although paradoxical insomnia appears to be a prevalent subtype of chronic insomnia, little is known about its etiology, the course of the disorder, or its treatment responsivity. No standard treatment has been identified for patients with paradoxical insomnia.
In a recent case study (Geyer, Lichstein, Carney, & Dillard, 2008), a method to improve sleep/wake discriminations was applied to a patient with paradoxical insomnia. This patient had failed to show any improvement after abbreviated behavioral treatment for insomnia and, hence, was given the opportunity to review the polysomnography(PSG) waveforms and video portions of her sleep study. The patient was asked to indicate when in the video portion of the study sleep onset occurred. The discrepancy between the self-reported sleep onset latency (SOL) and the SOL as identified by the sleep study was discussed. This discussion was accompanied by explanations of the PSG waveforms and the diagnosis of paradoxical insomnia. The patient’s SOL had improved at both 2 weeks and 2 months follow-up. In this case series, we replicate our case report.
METHOD
Patients
All patients at an accredited sleep disorders center who had both a complaint of insomnia and PSG evaluation over a 6-month period were considered for inclusion in this study. Although most insomnia patients seen at the sleep center are not evaluated with PSG, several of these patients were studied with PSG because of lack of response to conventional therapies.
Patients met diagnostic criteria for paradoxical insomnia: met criteria for insomnia, symptoms present for at least 6 months, chronic pattern of reported little sleep, mismatch between subjective reports of insomnia and PSG findings, and symptoms were not attributable to substance abuse or solely to another sleep disorder (AASM, 2005). All patients reported they do not sleep at all some nights. Excluded were children <19 years of age and patients with unstable, sleep intrusive psychiatric/medical problems as determined by clinical interview. Prospective participants were disqualified if the PSG was positive for other sleep disorders.
Measures
At the initial visit, PSG was recorded on digital PSG equipment (Grass Telefactor, PA) using standard recording and scoring procedures (Iber et al., 2007). The results were interpreted by a physician board certified in sleep medicine. Total sleep time (TST), sleep efficiency (SE), SOL, number of awakenings (NWAK), and wake time after sleep onset (WASO) were computed, as well as standard sleep architecture variables.
Sleep diaries obtained these same sleep pattern variables at two posttreatment points, specified later. A second self-report measure, the Insomnia Severity Index (ISI), was also administered (Morin, 1993). The ISI is a valid and reliable instrument in measuring perceived insomnia severity and its daytime consequences (Bastien, Vallieres, & Morin, 2001).
Treatments
Behavioral treatment
The treatment package included sleep education, sleep hygiene instructions, progressive muscle relaxation (practice before bedtime), and stimulus control.
Sleep education
Each patient was scheduled to view their baseline diagnostic study, including the PSG output and bedroom video. The viewing lasted 30 to 60 min.
The EEG waveforms were reviewed in detail with the patient and accompanied by an explanation of sleep onset, wake time during the night, and depth of sleep. Special attention was given to SOL because this was the primary complaint in this sample. The patients were asked to identify when sleep onset occurred using the video recording of their study. Once the patients identified the onset of sleep, there was a discussion of whether the PSG waveforms supported his or her beliefs about the video portion of the study. Physician and patient discussed the discrepancy between the sleep diary assessment of SOL and the identification of PSG SOL. All patients acknowledged this discrepancy. The patients were also counseled regarding the difficulty in accurate self-assessment of SOL.
Procedures
Patients provided a sleep diary the morning after their diagnostic PSG to contrast with the sleep study. Baseline typical self-reported sleep was obtained by clinical interview at intake, along with the ISI (Assessment 1 [A1]). There were two posttreatment assessment points. Assessment 2 (A2) was conducted about 4 months after A1 at the conclusion of behavioral treatment, and comprised 2 weeks of sleep diaries and the ISI. Assessment 3 (A3) occurred about 1 month after A2 following the sleep education treatment session, and repeated the data collection of A2.
The behavioral treatments were administered by James D. Geyer during an approximate 4-month period. About eight treatment sessions were delivered at about 2-week intervals: Two relaxation/sleep hygiene sessions were followed by six stimulus control sessions. Patients were contacted by telephone during off weeks to monitor treatment progress, offer encouragement, and manage obstacles to adherence. The sleep education treatment was also administered by James D. Geyer in the month following the behavioral treatment series.
RESULTS
Baseline Evaluation
Twelve patients were invited to participate, and all agreed. We later decided to disqualify the data of eight patients because they had comorbid sleep apnea treated with continuous positive airway pressure (CPAP). The presence of sleep apnea made it more difficult to isolate the influence of sleep education treatment. We report the results for four patients, all of whom were free of clinically significant sleep disorders aside from their insomnia complaint. See Table 1 for demographic and sleep study measures at A1. PSG results showed greater WASO (average 56 min) than SOL (average 24 min) disturbance, although SOL was the primary complaint of these patients. TST and SE could be construed as mild insomnia in Patients 1 through 3 (average TST = 370 min [6.2 hr]; SE = 83%), and normal sleep characterized Patient 4 (TST = 434 min [7.2 hr]; SE = 91%). Sleep architecture was corrupted in Patients 1 and 3, with respect to elevated Stage 1 Non-REM% (N1%, average 40%) and depressed Stage 3 Non-REM% (N3%, average 2%) and REM% (R%, 0%).
TABLE 1.
Demographics and Polysomnography Results
| Variable | Patient No.
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Gender | Male | Female | Female | Female |
| Age | 53 | 39 | 67 | 50 |
| Stage N2 latency (sleep onset latency) | 16 | 30 | 23 | 27 |
| Stage N1% | 42 | 10 | 37 | 14 |
| Stage N2% | 55 | 53 | 63 | 48 |
| Stage N3% | 3 | 16 | 0 | 11 |
| Stage R% | 0 | 21 | 0 | 27 |
| Number of awakenings | 2 | 1 | 0 | 1 |
| Wake time after sleep onset | 64 | 55 | 67 | 39 |
| Total sleep time | 359 | 396 | 355 | 434 |
| Sleep efficiency | 82 | 86 | 81 | 91 |
Note. N2 = Non-REM Stage 2; N1% = Non-REM Stage 1%; N2% = Non-REM Stage 2%; N3% = Non-REM Stage 3%; R% = REM Stage %.
Treatment Outcome
From baseline (A1) to post-behavioral treatment (A2), none of the patients reported substantial positive change in any of the four self-reported sleep variables (see Table 2). Indeed, two patients got worse. On average, for the four patients, TST decreased 30 min (A1 = 230 min and A2 = 200 min), and SOL increased 3 min (A1 = 105 min and A2 = 108 min).
TABLE 2.
Self-Report Measures
| Assessment | Patient No.
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Assessment 1: Baseline | ||||
| TST | 240 | 200 | 180 | 300 |
| SOL | 90 | 120 | 120 | 90 |
| NWAK | 3 | 10 | 7 | 2 |
| WASO | 80 | 75 | 90 | 30 |
| ISI | 20 | 21 | 23 | 19 |
| Assessment 2: Post-behavioral treatment | ||||
| TST | 260 | 210 | 90 | 240 |
| SOL | 100 | 120 | 120 | 90 |
| NWAK | 4 | 9 | 9 | 1 |
| WASO | 75 | 60 | 90 | 30 |
| ISI | 20 | 21 | 21 | 20 |
| Assessment 3: Post-sleep education | ||||
| TST | 285 | 300 | 120 | 330 |
| SOL | 80 | 30 | 135 | 30 |
| NWAK | 4 | 5 | 5 | 2 |
| WASO | 60 | 60 | 90 | 45 |
| ISI | 18 | 13 | 21 | 10 |
Note. Baseline sleep data were derived from a clinical interview. The remaining sleep data were from 2 weeks of sleep diaries. TST = total sleep time; SOL = sleep onset latency; NWAK = number of awakenings; WASO = wake after sleep onset; ISI = Insomnia Severity Index.
Comparing the outcome of the sleep education session (A3) with post-behavioral A2, Patients 2 and 4 registered substantial reported gains in SOL. Average A2 SOL for these two patients was 105 min, and this dropped to 30 min at A3. Little change occurred with Patients 1 and 3 from A2 (average SOL = 110 min) to A3 (average SOL = 108 min).
TST also revealed a differential treatment response to sleep education. Both Patients 2 and 4 increased TST 90 min from A2 to A3. During this same period, Patients 1 and 3 averaged 28 min increased TST.
ISI findings paralleled the sleep diaries: no change from A1 to A2 for all four patients (average of 21 at both points) and gains only by Patients 2 and 4 at A3, the two patients averaging an ISI of 12 at A3.
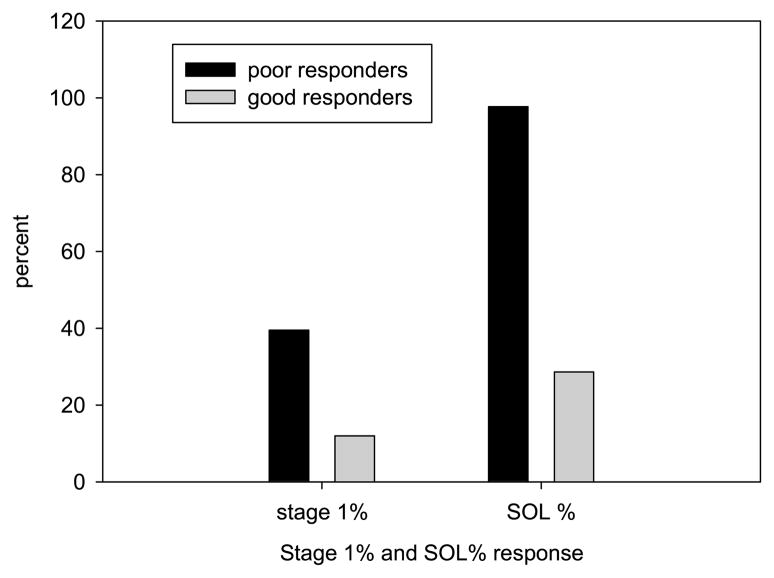
Figure 1 portrays the strong correspondence between normal N1% and sleep education benefits. Patients 1 and 3 exhibited elevated N1% (average 40%) compared to 12% in Patients 2 and 4, and as stated earlier; self-reported SOL improved only in Patients 2 and 4 from A2 to A3. A comparable association existed for SOL with N3% and R%.
FIGURE 1.
Correspondence between Non-REM Stage 1% (N1%) and sleep education self-reported sleep onset latency (SOL) treatment response. Note. N1% is derived from the diagnostic polysomnography. The N1% dark bar is the average N1% of Patients 1 and 3 (the 2 weak responders), and the light bar is the average N1% of Patients 2 and 4 (the 2 good responders). SOL% captures the decrease after sleep education (Assessment 3) compared to the behavioral treatment post assessment (Assessment 2): SOL Assessment 3 ÷ SOL Assessment 2 × 100. The SOL% dark bar indicates Patients’ 1 and 3 average SOL post-sleep education was equal to nearly 100% of behavioral treatment post SOL (no change). The SOL% light bar shows Patients 2 and 4 averaged about 30% of behavioral treatment post SOL (about a 70% SOL reduction following sleep education).
DISCUSSION
Two of four patients receiving sleep education for paradoxical insomnia exhibited meaningful improvement in insomnia based on the ISI and self-reported SOL and TST, replicating and extending the results of our previous case study (Geyer et al., 2008). Sleep architecture anomalies characterized the two unresponsive cases.
Sleep architecture markers of paradoxical insomnia or indicators of receptivity to treatment have not previously been reported, although somewhat lower delta sleep has been observed in a group of individuals diagnosed with subjective insomnia (Krystal, Edinger, Wohlgemuth, & Marsh, 2002). We cannot judge the replicability of our findings.
This study did not address mechanisms of change. We cannot determine if reported sleep simply conformed to a revised conceptualization of the sleep experience, if vigilance relaxed and awareness during sleep subsided, or if there was change in objective sleep.
Obvious limitations with respect to both internal and external validity accrue to this small clinical case series. Chief among these are inability to control placebo and experimenter demand effects, absence of objective posttreatment assessment, minimal evaluation of adherence, and minimal evaluation of daytime functioning. Further, we cannot rule out if a more robust course of cognitive behavior therapy (CBT) would have been effective or if sleep education profited from its sequence position following behavioral treatment.
Even a modicum of treatment success for an insomnia subtype that has baffled clinicians is welcome news. Further research should refine the treatment methodology from the limitations noted earlier. Current diagnostic systems do not provide definitive diagnostic criteria for paradoxical insomnia, and this muddies research in this area. At this point, scientific inquiry of paradoxical insomnia would be nicely advanced by a well-controlled, randomized clinical trial comparing sleep education to CBT.
Contributor Information
James D. Geyer, Alabama Neurology and Sleep Medicine, Tuscaloosa, Neurology and Sleep Medicine, The College of Community Health Sciences, The University of Alabama, Tuscaloosa
Kenneth L. Lichstein, Department of Psychology, The University of Alabama, Tuscaloosa
Megan E. Ruiter, Department of Psychology, The University of Alabama, Tuscaloosa
L. Charles Ward, Veteran’s Administration Medical Center, Tuscaloosa, AL.
Paul R. Carney, Division of Pediatric Neurology, Department of Pediatrics, McKnight Brain Institute, University of Florida College of Medicine, Gainesville
Stephenie C. Dillard, Neurology and Sleep Medicine, The College of Community Health Sciences, The University of Alabama, Tuscaloosa
References
- American Academy of Sleep Medicine. Diagnostic and coding manual. 2. Westchester, IL: Author; 2005. International Classification of Sleep Disorders. [Google Scholar]
- BaHammam A. Polysomnographic characteristics of patients with chronic insomnia. Sleep and Hypnosis. 2004;6:163–168. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Physiological activation in patients with sleep state misperception. Psychosomatic Medicine. 1997;59:533–540. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn M. Sleep–wake disorders based on a polysomnographic diagnosis: A national cooperative study. Journal of the American Medical Association. 1982;247:997–1003. [PubMed] [Google Scholar]
- Dorsey CM, Bootzin RR. Subjective and psychophysiologic insomnia: An examination of sleep tendency and personality. Biological Psychiatry. 1997;41:209–216. doi: 10.1016/0006-3223(95)00659-1. [DOI] [PubMed] [Google Scholar]
- Downey R, III, Bonnet MH. Training subjective insomniacs to accurately perceive sleep onset. Sleep. 1992;15:58–63. doi: 10.1093/sleep/15.1.58. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Glenn M, Sullivan RJ, Bastian LA, Marsh GR, et al. Insomnia and the eye of the beholder: Are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? Journal of Consulting and Clinical Psychology. 2000;68:586–593. [PubMed] [Google Scholar]
- Edinger JD, Krystal AD. Subtyping primary insomnia: Is sleep state misperception a distinct clinical entity? Sleep Medicine Reviews. 2003;7:203–214. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- Geyer JD, Lichstein KL, Carney PR, Dillard SC. Paradoxical insomnia. In: Winkelman JW, Henderson JH, Kotagal S, Lee-Chiong TL, Lichstein KL, Murray BJ, Schenck CH, editors. Case book of sleep medicine—A learning companion to the International Classification of Sleep Disorders, diagnostic and coding manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2008. pp. 25–28. [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:626–636. [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological assessment and management. New York: Guilford; 1993. [Google Scholar]