The unnatural isotope fluorine–18 (18F) is used as a positron emitter in molecular imaging. Currently, many potentially useful 18F-labeled probe molecules are inaccessible for imaging, because no fluorination chemistry is available to make them. Syntheses must be rapid on account of the 110-minute half-life of 18F and benefit from using [18F]fluoride due to practical access and suitable isotope enrichment. But [18F]fluoride chemistry has been limited to nucleophilic fluorination reactions. Here we report the development of a palladium-based electrophilic fluorination reagent derived from fluoride and its application to the synthesis of aromatic 18F-labeled molecules via late-stage fluorination. Late-stage fluorination enables the synthesis of conventionally unavailable positron emission tomography (PET) tracers for anticipated applications in pharmaceutical development as well as pre-clinical and clinical PET imaging.
Positron emission tomography (PET) is a noninvasive imaging technology used to observe and probe biological processes in vivo (1,2). While several positron-emitting isotopes can be used for PET imaging, fluorine–18 (18F) is the most clinically relevant radioisotope (3,4). For example, the radiotracer [18F]fluorodeoxyglucose ([18F]FDG) has revolutionized clinical diagnosis in oncology. Despite the success of PET and decades of research, there remains a major deficiency in the ability to synthesize complex PET tracers; in fact, no general method is available to radiolabel structurally complex molecules with 18F. In organic molecules, fluorine atoms are typically attached by carbon–fluorine bonds (5), yet carbon–fluorine bond formation is challenging, especially in the presence of the variety of functional groups commonly found in structurally complex molecules (6). For PET applications, chemical challenges are exacerbated by the short half-life of 18F (110 minutes), which dictates that carbon–fluorine bond formation occur at a late-stage in the synthesis to avoid unproductive radioactive decay before injection in vivo.
The unnatural isotope 18F is generated using a cyclotron, either as nucleophilic [18F]fluoride or as electrophilic [18F]fluorine gas ([18F]F2). [18F]Fluoride, formed from proton bombardment of oxygen–18-enriched water, is significantly easier to make and handle than [18F]F2. Moreover, [18F]F2 gas is liberated from the cyclotron with [19F]F2; 19F is the natural, PET-inactive isotope of fluorine. As a result, the 18F/19F ratio, quantified as specific activity, is substantially lower when [18F]F2 is used than when [18F]fluoride is used. High specific activity is often critical to PET imaging. If a biological target of a radiotracer is saturated with the non-positron-emitting 19F-isotopologue of the tracer, a meaningful PET image cannot be obtained. Many biological targets cannot be visualized with PET tracers of low specific activity due to low concentration of the targets. For example, imaging neurotransmitter receptors in the brain typically necessitates tracers of high specific activity (3).
Research toward PET tracer development has focused on the use of [18F]fluoride to make PET tracers in high specific activity. Incorporation of 18F still most commonly relies on simple nucleophilic substitution reactions, a class of reactions originally developed more than 100 years ago (7) and often not suitable to address modern challenges in imaging. Recent advances in nucleophilic fluorination (8–11) include a palladium-catalyzed fluorination reaction of aryl triflates with anhydrous cesium fluoride developed by the Buchwald group, in which carbon–fluorine bond formation proceeds by reductive elimination from palladium(II) aryl fluoride complexes (12,13). Challenges associated with the application of fluorination reactions to PET include the requirement of short reaction times as well as different reaction conditions for 18F-chemistry compared to 19F-chemistry. For example, extensive drying of fluoride is readily achieved for 19F-chemistry, but can be impractical for radiochemistry, which is typically executed on a nanomole scale. The smaller ratio of fluorine to water, when transitioning from 19F-chemistry to 18F-chemistry, can be problematic, because hydrated fluoride has diminished nucleophilicity. As a consequence, even promising modern fluorination reactions developed for 19F-chemistry are often not translated to radiochemistry.
Electrophilic fluorination reactions allow access to a complementary set of molecules when compared to nucleophilic fluorination reactions (6). Yet, all electrophilic 18F-fluorination reactions developed to date use electrophilic fluorination reagents that ultimately originate from [18F]F2. In 1997, Solin developed a method to generate [18F]F2 in higher specific activity than is common for [18F]F2, by minimizing the amount of [19F]F2 used (14). By employing [18F]F2 made via the Solin method, Gouverneur succeeded in synthesizing [18F]F-TEDA, an electrophilic 18F-fluorination reagent more useful and selective than [18F]F2 (15). However, nucleophilic [18F]fluoride is currently the only practical and generally available source of fluorine to prepare high specific activity PET tracers (3). If an electrophilic fluorination reagent were to be made from fluoride (16,17), without the need for F2, electrophilic fluorination could become a general and widely used method to prepare PET tracers that are presently inaccessible via conventional nucleophilic fluorination chemistry.
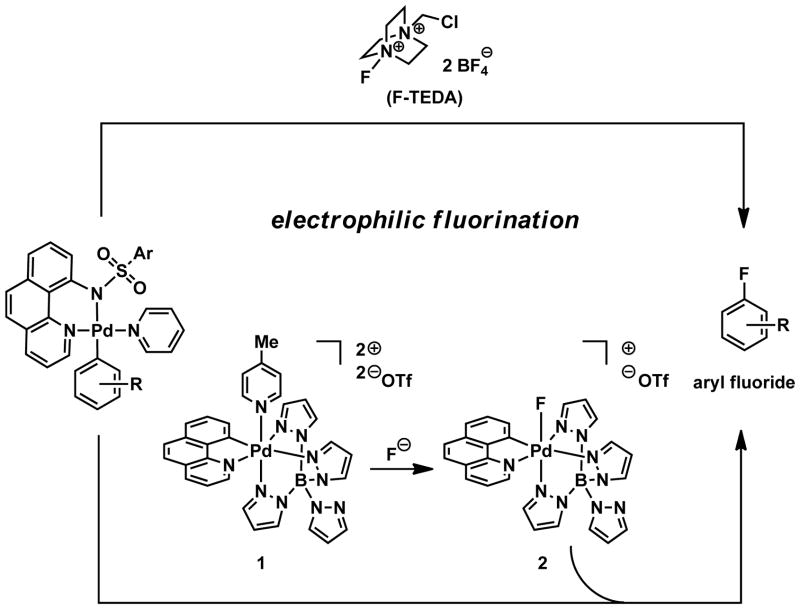
Previously, we reported the fluorination of palladium aryl complexes with the electrophilic fluorination reagent F-TEDA (Fig. 1) (18). F-TEDA can oxidize palladium(II) aryl complexes, which subsequently afford aryl fluorides by carbon–fluorine reductive elimination from palladium(IV) aryl fluoride complexes (19,20). Replacement of F-TEDA by a fluorination reagent that mimics the function of F-TEDA, but is made from fluoride has the potential for use in the synthesis of high specific activity 18F-radiotracers. Here we report the design and synthesis of an organometallic complex made from fluoride (1 → 2, Fig. 1) that behaves as an electrophilic fluorination reagent, and the use of the reagent for the synthesis of 18F-labeled small molecules via late-stage fluorination.
Fig 1.
Electrophilic fluorination of palladium aryl complexes to afford aryl fluorides. Top: With the electrophilic fluorination reagent F-TEDA. Bottom: With the Pd(IV) fluoride complex 2, made from fluoride (F⊖). Ar, aryl; Me, methyl; Tf, trifluoromethanesulfonyl.
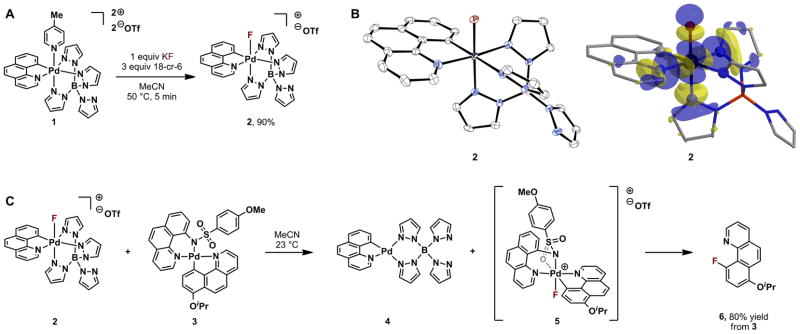
Palladium complex 2 was envisioned to accomplish oxidative fluorine transfer by serving as an electrophile in SN2 reactions with nucleophilic attack occurring at the fluorine substituent. The complexes 1 and 2 were designed based on the following five considerations (Fig. 2): i) The palladium center in 1 carries three formal positive charges (counter charges to two triflate anions and one negatively charged borate ligand) and should therefore capture negatively charged fluoride from solution. High fluorophilicity of the palladium complex 1 is required for radiochemistry applications due to the low effective concentration of fluoride in solution (roughly 10−4 M). ii) The palladium centers in 1 and 2 are in the oxidation state +IV (Pd(IV)), a high oxidation state for palladium. Late transition metals such as palladium, when in a high oxidation state, can function as an oxidant and transfer a ligand to a nucleophile, while being reduced in the process to a lower oxidation state (21). The palladium in 2 can function as an electron acceptor, which rationalizes the reactivity of 2 as an oxidant. iii) The supporting benzo[h]quinolyl and tetrapyrazole borate (Tp) ligands are multidentate ligands that were selected to impart stability on both 1 and 2 toward undesired reductive processes such as carbon–fluorine reductive elimination. Reductive elimination from 2 would likely require dissociation of one ligand to form a pentacoordinate palladium complex (19,20), and multidentate ligands such as Tp are less likely to dissociate and hence reduce the rate of potential reductive elimination. iv) An octahedral Pd(IV) complex was chosen to avoid undesired nucleophilic attack at the transition metal. The palladium–fluorine bond is polarized toward fluorine, with partial negative charge on fluorine and positive charge on palladium. Solely based on coulombic interactions, nucleophilic attack is expected at palladium rather than fluorine. However, the orbitals available for nucleophilic attack on octahedral (t2g)6 (eg)0 Pd(IV) complexes, the dx2-y2 and dz2 orbitals, are high in energy, and nucleophilic attack on high energy orbitals is disfavored (22). v) The multidentate supporting ligands in 2 feature aromatic substituents to prevent nucleophilic attack on the carbon and nitrogen atoms coordinated to palladium. Complex 2 was devised to act as an electrophilic fluorination reagent through nucleophilic attack at the antibonding palladium–fluorine-based orbital (σ*Pd–F) at fluorine in an SN2 reaction. In such a putative SN2 reaction, palladium would function as a leaving group, with concomitant reduction to the oxidation state +II (Pd(II)). Nucleophilic attack would occur at the lowest unoccupied molecular orbital (LUMO) of 2. The calculated LUMO of 2 (Fig. 2B), shows that only the lobe on fluorine points into unoccupied space; no other LUMO lobe is available for nucleophilic attack because the aromatic ligands block the trajectories.
Fig. 2.
Reactivity and structure of 1 and 2. (A) Nucleophilic fluoride capture by Pd(IV) complex 1 to form electrophilic fluorination reagent 2. (B) X-ray structure of 2 (ORTEP drawing at 50% probability; hydrogen atoms and counteranion omitted for clarity), and calculated lowest unoccupied molecular orbital (LUMO) of 2. (C) Fluorination of Pd(II) aryl complex 3 by reagent 2 to form Pd(IV) aryl fluoride complex 5, followed by C–F reductive elimination from 5. Reductive elimination from reactive intermediate 5 to 6 proceeds at 23 °C; the highest yield of 6 (80%) was obtained upon heating 2 and 3 at 85 °C. 18-cr-6, 18-crown-6; iPr, isopropyl.
Treatment of the palladium complex 1 with nucleophilic fluoride (KF) afforded the palladium fluoride complex 2 within five minutes in 90% yield (Fig. 2A). The ability of 2 to function as an electrophilic fluorination reagent was confirmed by fluorination of Pd(II) aryl complex 3 (Fig. 2C). Fluorine transfer from 2 to 3 results in oxidation of 3 to form Pd(IV) aryl fluoride intermediate 5, assigned by 1H and 19F nuclear magnetic resonance (NMR). Carbon–fluorine reductive elimination from reactive intermediate 5 occurs at a rate comparable to oxidation of 3 with 2. Oxidation of 3 with the electrophilic fluorination reagent F-TEDA afforded 5 at a faster rate than oxidation with 2, which allowed independent synthesis and identification of 5 (see Supporting Online Material, Fig. S2). Reduction of palladium from Pd(IV) in oxidant 2 to Pd(II) was established by isolation of Pd(II) complex 4.
Oxidative fluorine transfer from 2 to 3 could proceed by SN2 reaction as designed, with nucleophilic attack of the dz2-based orbital on palladium of 3 (Fig. S17) on the σ*Pd–F-based orbital of the LUMO of 2, or via electron transfer (23,24) from 3 to 2 with interposed or subsequent fluorine transfer. Current data cannot distinguish between these mechanisms. In addition, other pathways that intercept intermediates such as 5 could potentially be used for carbon–fluorine bond formation. Palladium complex 2 is both an oxidant and a fluoride donor. The oxidation equivalents are inherent in the transition metal oxidation state, and the fluorine substituent is negatively polarized, because fluorine is the most electronegative element. But oxidation of 3 with an external oxidant followed by reaction with an independent fluoride source could also provide 5. In such a stepwise approach, potential side reactions of putative intermediates, such as undesired reductive elimination reactions, must be prevented. Conceptually, several combinations of an oxidant and a fluoride source are conceivable for successful oxidative fluorination.
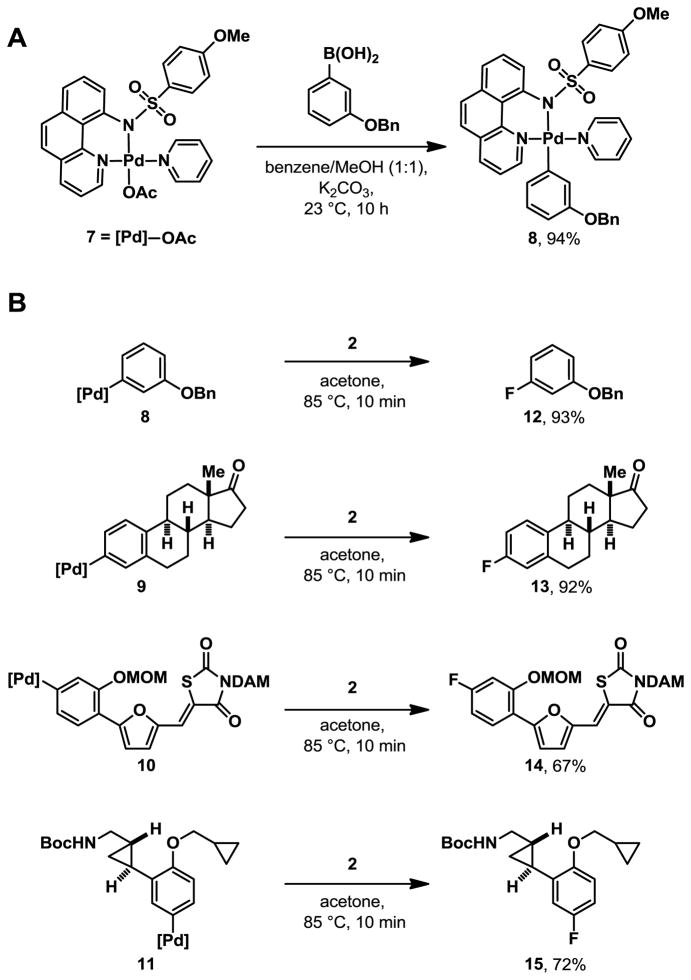
The fluorination reagent 2 was subsequently evaluated for late-stage fluorination. Fluorination of the Pd(II) aryl complexes 8–11 with 2 afforded aryl fluorides 12–15 in 67–93% yield (Fig. 3). We propose that Pd(IV) fluoride complex 2 oxidizes the Pd(II) aryl complexes by fluorine transfer to form high-valent Pd(IV) aryl fluoride complexes, analogous to 5, from which carbon–fluorine reductive elimination can occur to form aryl fluoride products 12–15. The aryl fluoride molecules shown in Figure 3 were selected based on their structure and exhibition of a variety of functional groups, akin to potential small-molecule PET tracers (25,26). The molecules are electron-rich arenes, which would be challenging to prepare by conventional fluorination reactions with [18F]fluoride.
Fig. 3.
Synthesis and fluorination of palladium aryl complexes. (A) Representative synthesis of a palladium aryl complex from palladium acetate complex 7. (B) Fluorination of palladium aryl complexes 8–11 with electrophilic fluorination reagent 2. Ac, acetate; Bn, benzyl; Boc, tert-butoxycarbonyl; MOM, methyoxymethyl; DAM, di-(p-anisyl)methyl.
The transition from 19F-chemistry to 18F-chemistry is challenging because the concentration of the limiting reagent, fluoride, changes from mM to μM, and syntheses must be performed in the presence of ionizing radiation and under a time constraint due to the 110-minute half-life of 18F. Therefore, reaction chemistry applicable to 18F-chemistry must be robust and as simple as possible for broad applications in medical imaging. Organometallic synthesis in a hospital setting would be impractical. However, the palladium complexes discussed here can be prepared conveniently on scale, stored, and subsequently transported to imaging sites when needed. The palladium(II) aryl complexes such as 8–11 can be purified by chromatography or recrystallization and can be stored and transported in air at ambient temperature. Palladium complex 1 is stable at room temperature and can be manipulated briefly in air. Palladium complex 2 is stable toward heat (no observed decomposition for 24 hours at 100 oC), and water (no observed decomposition in 10% aqueous acetonitrile solution after 3 hours at 23 oC). Thermal stability and tolerance toward water are beneficial for practical PET applications, because reaction mixtures are often heated to increase reaction rates and [18F]fluoride is made from oxygen–18-enriched water.
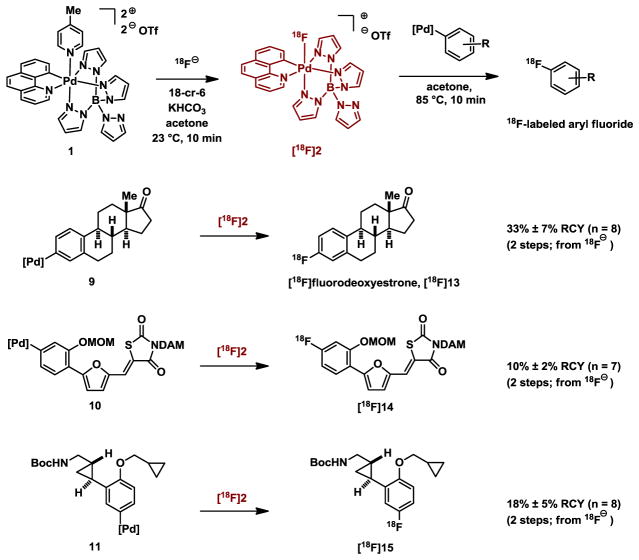
The synthesis of 18F-radiolabeled molecules using the presented fluoride-derived reagent is operationally simple. Pd(IV) complex 1 reacts with conventionally prepared solutions of [18F]fluoride in acetone and forms the 18F-reagent [18F]2 within ten minutes (Fig 4). Subsequent filtration over a polymer-supported resin and addition of the Pd(II) aryl complexes 9–11 afforded, upon heating for ten minutes, the 18F-labeled aryl fluorides [18F]13–15, respectively. Azeotropic drying of aqueous [18F]fluoride, the two-step reaction sequence (fluoride capture (1 → [18F]2) followed by fluorine transfer (e.g. 9 → [18F]13), and subsequent purification of the 18F-labeled molecules by high-performance liquid chromatography (HPLC) results in an overall synthesis time of less than 60 minutes. Automated syntheses with up to 1 Ci of radioactivity have been accomplished. The efficiency of radiochemical synthesis is given in radiochemical yield, which is based on decay-corrected radioactivity rather than on mass of isolated material (4). Radiochemical yields (RCYs) as high as possible are desired, but RCYs as low as 5% can provide meaningful PET imaging (27). The 18F-fluorination method described here has afforded RCYs higher than 30%.
Fig. 4.
Late-stage fluorination to form 18F-labeled aryl fluorides. [18F]Fluoride capture by Pd(IV) complex 1 to form electrophilic fluorination reagent [18F]2 and subsequent fluorination of palladium aryl complexes 9–11. Reported radiochemical yields (RCYs) are averaged over n experiments at a 500 μCi scale.
Stoichiometric amounts of precious transition metals such as palladium are typically avoided in syntheses of health care products due to toxicity and cost considerations. However, such considerations are different for the synthesis of PET tracers due to the small amount that is required (28). High-specific activity PET tracers are administered at a dose at least two orders of magnitude smaller than is common for pharmaceuticals because pharmacological effects are not sought (29), and purification is often straightforward due to the small amount of tracer to be purified. For example, inductively coupled plasma mass spectrometry (ICP-MS) analysis of [18F]13 (Fig. 4), purified by conventional HPLC technique (20 minutes), afforded material with 5 parts per billion (ppb) palladium residue, commensurate with international recommendations on the palladium impurity profile for samples injected into humans, which demand less than 1000 ppb palladium (30). Similarly, the synthesis cost for the palladium complexes is small compared to the cost of 18F-isotope infrastructure and the cost associated with clinical imaging (31).
We have shown that the presented late-stage fluorination reaction can access 18F-labeled functionalized molecules, which would be particularly difficult to prepare with conventional fluorination reactions. The availability of a high specific activity fluorination reagent that functions as an electrophile may find applications in 18F-fluorination of pharmaceutical candidates for evaluation of their biodistribution to accelerate drug development, as well as in the development of previously unavailable 18F-PET tracers for clinical care.
Supplementary Material
Acknowledgments
Funding was provided by NIH-NIGMS (GM088237), NIH-NIBIB (EB013042), NIH–NCRR (1S10RR017208-01A1), NIH-NIDA (5P30DA028800-02), the Richard and Susan Smith Family Foundation, the Massachusetts Life Science Center, the Harvard Catalyst, NSF-GRFP (DGE0644491), and the Harvard Accelerator Fund. We thank S.-L. Zheng for X-ray crystallographic analysis. DFT computation was performed using the computer facilities at the Odyssey cluster at Harvard University. Metrical parameters for the crystal structures of compounds 1, 2, 3, 8, and S10 are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 829427, 829428, 840744, 829429, and 839058, respectively. A patent application has been filed through Harvard on methods and reagents presented in this manuscript. TR is a Sloan fellow, a Lilly Grantee, an Amgen Young Investigator, a Camille Dreyfus Teacher-Scholar, and an AstraZeneca Awardee.
Footnotes
Materials and Methods
Experimental Data
Spectroscopic Data
References
References
- 1.Phelps ME. Proc Natl Acad Sci USA. 2000;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler JS, Wolf AP. Acc Chem Res. 1997;30:181. [Google Scholar]
- 3.Ametamey SM, Honer M, Schubiger PA. Chem Rev. 2008;108:1501. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 4.Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem Int Ed. 2008;47:8998. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 5.O’Hagan D. Chem Soc Rev. 2008;37:308. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- 6.Furuya T, Kamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young S. J Chem Soc, Trans. 1881;39:489. [Google Scholar]
- 8.Pike VW, Aigbirhio FI. J Chem Soc, Chem Commun. 1995:2215. [Google Scholar]
- 9.Sun H, DiMagno SG. Angew Chem Int Ed. 2006;45:2720. doi: 10.1002/anie.200504555. [DOI] [PubMed] [Google Scholar]
- 10.Katcher MH, Doyle AG. J Am Chem Soc. 2010;132:17402. doi: 10.1021/ja109120n. [DOI] [PubMed] [Google Scholar]
- 11.Hollingworth C, et al. Angew Chem Int Ed. 2011;50:2613. doi: 10.1002/anie.201007307. [DOI] [PubMed] [Google Scholar]
- 12.Watson DA, et al. Science. 2009;325:1661. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noël T, Maimone TJ, Buchwald SL. Angew Chem Int Ed Early View. doi: 10.1002/anie.201104652. [DOI] [Google Scholar]
- 14.Bergman R, Solin O. Nucl Med Biol. 1997;24:677. doi: 10.1016/s0969-8051(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 15.Teare H, et al. Angew Chem Int Ed. 2010;49:6821. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- 16.Brauner B. Z Anorg Chem. 1894;7:1. [Google Scholar]
- 17.Christe KO. Inorg Chem. 1986;25:3721. [Google Scholar]
- 18.Furuya T, Kaiser HM, Ritter T. Angew Chem Int Ed. 2008;47:5993. doi: 10.1002/anie.200802164. [DOI] [PubMed] [Google Scholar]
- 19.Furuya T, Ritter T. J Am Chem Soc. 2008;130:10060. doi: 10.1021/ja803187x. [DOI] [PubMed] [Google Scholar]
- 20.Furuya T, et al. J Am Chem Soc. 2010;132:3793. doi: 10.1021/ja909371t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl SS, Labinger JA, Bercaw JE. Angew Chem Int Ed. 1998;37:2180. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Cotton FA. Chemical Applications of Group Theory. 3. Chap 9 Wiley; New York: 1990. [Google Scholar]
- 23.Taube H, Myers H. J Am Chem Soc. 1954;76:2103. [Google Scholar]
- 24.Haim A. Prog Inorg Chem. 1983;30:273. [Google Scholar]
- 25.Pomel V, et al. J Med Chem. 2006;49:3857. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 26.Kozikowski AP, et al. ChemMedChem. 2010;5:1221. doi: 10.1002/cmdc.201000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin FT, et al. Mol Imaging Biol. 2008;10:82. doi: 10.1007/s11307-007-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyohara J, et al. Ann Nucl Med. 2009;23:301. doi: 10.1007/s12149-009-0240-x. [DOI] [PubMed] [Google Scholar]
- 29.U. S. Department of Health and Human Services, Food and Drug Administration. [accessed September 5, 2011.]; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078933.pdf.
- 30.European Medicines Agency. [accessed September 5, 2011.]; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003587.pdf.
- 31.Buck AK, et al. J Nuc Chem. 2010;51:401. [Google Scholar]
- 32.Still WC, Kahn M, Mitra A. J Org Chem. 1978;43:2925. [Google Scholar]
- 33.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518. [Google Scholar]
- 34.Matthews WS, et al. J Am Chem Soc. 1975;97:7006. [Google Scholar]
- 35.Fulmer GR, et al. Organometallics. 2010;29:2176. [Google Scholar]
- 36.Dick AR, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 37.Niedenzu K, Niedenzu PM. Inorg Chem. 1984;23:3713. [Google Scholar]
- 38.Onishi M, Ohama Y, Sugimura K, Hiraki K. Chem Lett. 1976;5:955. [Google Scholar]
- 39.Weiss R, Seubert J. Angew Chem, Int Ed. 1994;33:891. [Google Scholar]
- 40.McDaniel DH, Brown HC. J Org Chem. 1958;23:420. [Google Scholar]
- 41.Muller P, Baud C, Jacquier Y. Can J Chem. 1998;76:738. [Google Scholar]
- 42.Taylor S, et al. J Chem Soc, Perkin Trans 2. 2001;1714 [Google Scholar]
- 43.Hartwell GE, Lawrence RW, Smas MJ. J Chem Soc D: Chem Commun. 1970;912 [Google Scholar]
- 44.Dick AR, Remy MS, Kampf JW, Sanford MS. Organometallics. 2007;26:1365. [Google Scholar]
- 45.Ahmed V, Liu Y, Silvestro C, Taylor SD. Bioorg Med Chem. 2006;14:8564. doi: 10.1016/j.bmc.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Furuya T, Strom AE, Ritter T. J Am Chem Soc. 2009;131:1662. doi: 10.1021/ja8086664. [DOI] [PubMed] [Google Scholar]
- 47.Billingsley KL, Barder TE, Buchwald SL. Angew Chem, Int Ed. 2007;46:5359. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]
- 48.Charette AB, Juteau H, Lebel H, Molinaro C. J Am Chem Soc. 1998;120:11943. [Google Scholar]
- 49.Tang P, Furuya T, Ritter T. J Am Chem Soc. 2010;132:12150. doi: 10.1021/ja105834t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting R, Adam MJ, Ruth TJ, Perrin DM. J Am Chem Soc. 2005;127:13094. doi: 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]
- 51.Frisch MJ, et al. Gaussian 09, Revision A.02. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- 52.Becke AD. J Chem Phys. 1993;98:5648. [Google Scholar]
- 53.Perdew JP, Wang Y. Phys Rev B. 1992;45:13244. doi: 10.1103/physrevb.45.13244. [DOI] [PubMed] [Google Scholar]
- 54.Andrae D, et al. Theor Chim Acta. 1990;77:123. [Google Scholar]
- 55.Andrae D, et al. Theor Chim Acta. 1991;78:247. [Google Scholar]
- 56.Bergner A, et al. Mol Phys. 1993;30:1431. [Google Scholar]
- 57.Ehlers AW, et al. Chem Phys Lett. 1993;208:111. [Google Scholar]
- 58.Höllwarth A, et al. Chem Phys Lett. 1993;208:237. [Google Scholar]
- 59.Hariharan PC, Pople JA. Theor Chim Acta. 1973;28:213. [Google Scholar]
- 60.APEX II. Bruker AXS Inc; Madison, WI: 2009. [Google Scholar]
- 61.Sheldrick GM. Acta Cryst. 2008;A64:112. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.