Abstract
The effect of pretreatment with FK506 on renal ischemia and reperfusion (I/R) injury was investigated using a rat model. Animals were assigned to one of two groups (20 rats each). Group 1 animals (controls) received 0.5 ml saline while group 2 animals received FK506 (0.3 mg/kg), administered intravenously 24 hr prior to the induction of renal ischemia. A 60-min period of ischemia of the right kidney was induced, and upon reperfusion a left nephrectomy was performed. Blood samples for estimation of BUN, creatinine, and tumor necrosis factor were collected on days 0 (preischemia), 1, 2, 3, 5, 7, and 10 (postischemia). Rats were sacrificed after day 10 and renal tissue was examined histologically. All animals survived the ischemic episode. FK506 pretreatment significantly reduced the serum levels of BUN (P<0.02), creatinine (P<0.02), and TNF (P<0.05) as compared with that seen in controls. Histologically, at day 10, the kidneys showed the expected sequelae of prior renal I/R with various degrees of tubular damage. However, no objective differences were evident between the two groups. Based upon these data, it can be concluded that (1) FK506 pretreatment ameliorates the functional renal injury associated with I/R, (2) renal ischemia induces the release of TNF, and (3) FK506 pretreatment results in a significant inhibition of TNF production. These data suggest that the release of TNF may be responsible for the increasing of BUN and creatinine levels seen after renal I/R and that pretreatment of renal donors with FK506 may improve renal function in the immediate post-transplant period.
FK506 has been shown to be an effective and safe immunosuppressive agent capable of inducing long-term allograft acceptance in renal transplantation (1, 2). Nevertheless, the graft injury associated with ischemia and reperfusion (I/R) experienced during organ harvesting and engraftment still poses a major problem in clinical renal transplantation.
In previous experiments, FK506 has been shown, in addition to being a potent immunosuppressant, to be a hepatoprotective against I/R injury occurring in rats subjected to a sixty-minute period of total ischemia of the right hepatic lobe followed by resection of the nonischemic median and left hepatic lobes (3–7). The hepatoprotective effect of FK506 has been related partly to its ability to inhibit the production of several cytokines including tumor necrosis factor, a critical early mediator of organ injury (7). The purpose of the present study was to further extend this issue by examining the effect of FK506 pretreatment on the renal injury associated with I/R in a rat model.
MATERIALS AND METHODS
Animals
Male inbred Lewis rats weighing 200–250 g were used for all experiments (Harlan-Sprague-Dawley, Indianapolis, IN). All animals were acclimatized to the Animal Research Lab for one week prior to being utilized. They were given free access to food and water both before and after the experimental renal ischemia.
Surgical procedure
Anesthesia was induced and maintained using metofane inhalation. A midline abdominal incision was made. The right perirenal fascia was dissected free, leaving only the pedicle of renal vessels and ureter. The right renal vessels were identified and clamped using temporary microvascular clamps, inducing a 60-minute period of total ischemia. Upon removal of the clamps and reperfusion of the right kidney, a left nephrectomy was performed. All animals were allowed to recover spontaneously and survival was observed at 12-hr intervals for a total of 10 days, after which they were sacrificed.
Experimental protocol
Animals were randomly assigned to one of two different experimental groups consisting of 20 rats each. Group 1 animals (controls) were injected intravenously with 0.5 ml saline solution while group two rats received FK506 (0.3 mg/kg in 0.5 ml saline) administered intravenously, 24 hr prior to the induction of the experimental renal ischemia. Blood samples for the estimation of blood urea nitrogen and creatinine and for the bioassays of circulating TNF were collected via the tail vein on days 0 (preischemia), 1, 2, 3, 5, 7, and 10 (postischemia). No more than three samples were withdrawn from each animal, and at each draw the blood withdrawn was immediately replaced by an equal volume of lactated Ringer's solution. The serum was separated immediately and stored at —20°C until being assayed. The animals were weighed at the start and end of the experimental period.
Biochemical analyses and TNF bioassays
In each rat, serum levels of BUN and creatinine were determined using standard laboratory methods and Sigma diagnostic kits. The measurement of TNF was based upon quantitation of the cytolytic activity of TNF in lysing L-M (murine mouse connective tissue) cells in the presence of actinomycin D, as measured by the uptake of crystal violet dye by residual viable cells (Genentech Inc, San Francisco, CA) (8). Brief1y, L-M cells are cultivated in serum-free medium (M199 containing 0.5% bactopeptone). Conf1uent monolayers are detached using sterile glass beads, resuspended in M199 supplemented with 0.5% bactopeptone, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml insulin, and 0.024 M Hepes buffer. The cells are then seeded at a density of 4×104 cell per well into a 96-well microtiter plate. Following incubation for 24 hr at 37°C in a humidified atmosphere with 5% CO2, the medium in the wells is replaced with fresh medium containing actinomycin D at a final concentration of 1 μg/ml. The plates are then incubated for an additional 18–20 hr at 37°C and stained with 0.5% crystal violet, washed, dried, and read by a plate-reader at 540 nm. Activity levels are reported as units/ml, where 1 U/ml equals approximately 8.8 pg TNF/ml.
Histological studies
At day 10 following renal ischemia and sacrifice, the kidneys were fixed in 10% formalin, dehydrated, embedded in paraffin, sectioned at 5 μ and stained for histological examination with hematoxylin and eosin. Histological variables at day 10 postischemia were subjectively scored and compared using a 0–4+ grading system where 0 = no regenerative tubules; 1 = 0–25% regenerative tubules, 2 = 25–50%, 3 = 50–75%, and 4 = 75–100%), Likewise, tubular dilatation, which also reflects recovery from tubular damage, was assessed using the same scoring system from 0–4+. Other variables such as calcification in the renal tubules, vascular vacuolization, vascular thrombosis, and evidence of infarcts were also estimated and compared.
Statistical analysis
Data are reported as the mean and SEM. BUN and creatinine data were analyzed using the two-way analysis of variance to examine the main treatment effects of FK506 and of time following ischemia and reperfusion. The possible interaction of the two main treatment effects were evaluated. The chi square test was used to test for differences in proportions. A P value of <0.05 was considered to be significant.
RESULTS
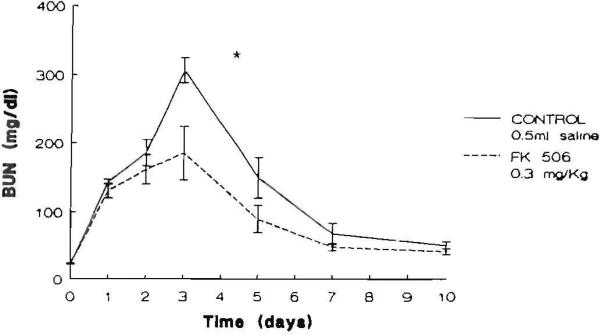
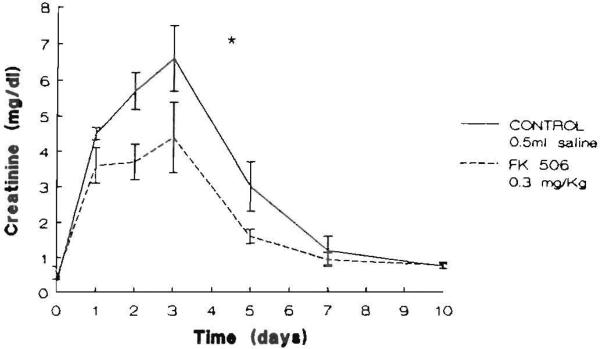
All animals survived the ischemic episode. The animals in both groups lost weight averaging 8% of their body weight during the 10 days of the experiment. No difference between groups for weight was evident before or at the end of the experimental period. Graphic representations of the time courses of the mean serum levels of BUN and creatinine in the saline- and FK506-pretreated animals are shown in Figures 1 and 2, respectively. Serum BUN and creatinine levels in both groups were elevated after I/R peaked at 72 hr and declined thereafter toward their preischemic levels at day 10. There was a significant effect of pretreatment with FK506 with respect to both BUN (P<0.02) and creatinine (P<0.02).
Figure 1.
Serum BUN levels (mg/dl) in the animals studied. The points represent the mean level and the brackets indicate the SEM ([*] P<0.02).
Figure 2.
Serum creatinine levels (mg/dl) in the animals studied. The points represent the mean level and the brackets indicate the SEM ([*] P<0.02).
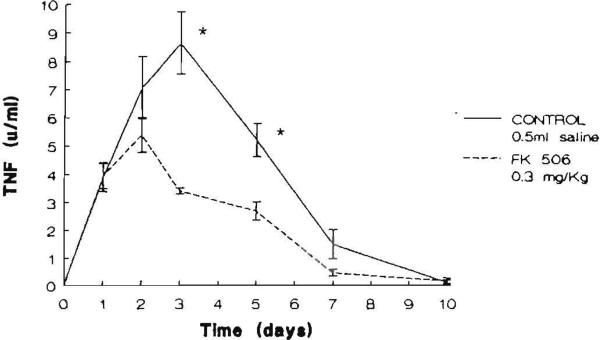
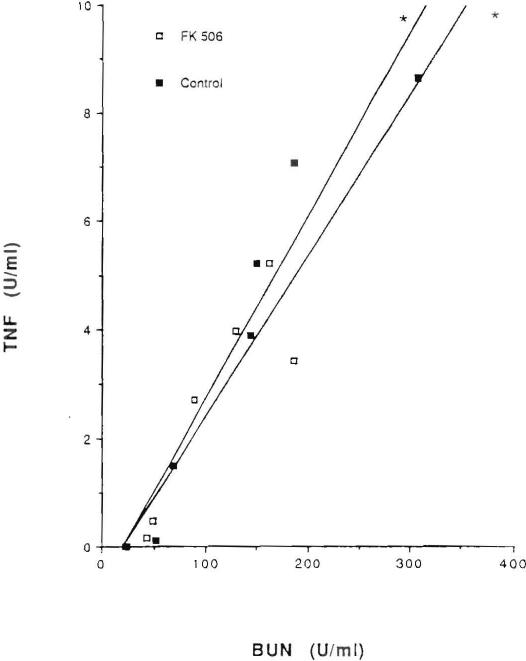
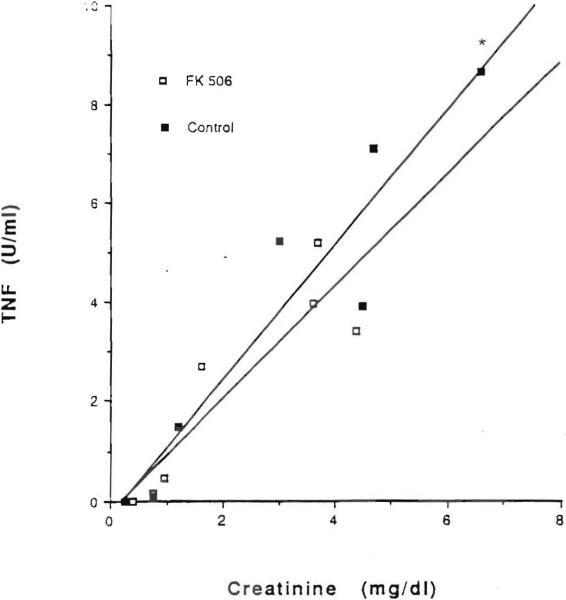
TNF was measurable in the serum of all experimental animals after renal ischemia and reperfusion. In contrast, TNF was undetectable in the preischemic samples obtained from the animals in both groups. The mean peak TNF level in saline-pretreated controls was 8.66+1.8 U/ml with a range of 5.83–11.98 U/ml and it occurred at 72 hr postischemia. The mean peak TNF level was 5.21+0.7 U/ml with a range of 3.1–7.74 U/ml in FK506-pretreated rats and it occurred at 48 hr after the ischemic episode (Fig. 3). At days 3 and 5 following ischemia, the mean value of TNF was significantly lower in the rats receiving FK506 than it was in the controls (P<0.05). A significant positive correlation was found between serum TNF levels and serum BUN levels (P<0.05) and serum TNF levels and creatinine (P<0.05) levels (Figs. 4 and 5, respectively).
Figure 3.
Serum TNF levels (U/ml) in the animals studied. The points represent the mean level and the brackets indicate the SEM ([*] P <0.05).
Figure 4.
Correlation between mean serum levels of TNF and BUN in saline-pretreated animals (r=0.929, [*] P<0.05) and in FK506-pretreated animals (2=0.824, [*]P<0.05).
Figure 5.
Correlation between mean serum levels of TNF and creatinine in saline-pretreated animals (r=0.90, [*] P<0.05) and in FK506-pretreated animals (r=0.82, [*] P<O.05) and in FK506-pretreated animals (r=0.82, [*]P<0.05).
Histologically, kidneys at day 10 showed sequelae of tubular damage in both the control and the experimental groups. This was manifest by occasional necrotic tubular epithelial cells, areas of tubular dilatation, and areas of basophilic regenerative epithelium with somewhat predominant nucleoli and occasional mitoses. Focal areas of calcification and interstitial widening with focal mononuclear infiltrates were seen also in both groups. There was no evidence of infarcts or of vascular thrombosis in the sections examined. The extent of changes was variable within each group, and no objective difference could be distinguished between the two groups. Although a trend toward less damage was observed in the FK506-pretreated group as a whole, it was not possible to tell into which group a single case fell on the basis of the histology alone.
DISCUSSION
The large increase in the number of organ transplantations in recent years has resulted in growing interest in the patho-physiological events that are associated with ischemia and reperfusion injury.
In previous experiments we have shown the ability of FK506 pretreatment to ameliorate the hepatic injury associated with I/R, as evidenced by improved animal survival (3–7). Improvement in survival was reflected by a restoration of hepatic ATP content; a reduction in serum levels of ALT and LDH (5, 6), as well as TNF and interleukin 6 (7); an amelioration of hepatic necrosis and neutrophilic infiltration; and an increase in the mitotic activity of the liver (6). A hepatoprotective effect of pretreatment with cyclosporine has also been shown by several investigators (9, 10). However, a well-known side effect of CsA administration is nephrotoxicity (11), which may become more pronounced in the presence of a renal ischemic insult (12–16). Both experimental (17) and clinical (18) results reported from Pittsburgh have shown that FK506 does not have the severe pattern of nephrotoxicity found with CsA that leads to renal failure and the need for dialysis in some patients (19).
Currently, it is believed that ischemic renal damage occurs in two stages—one that is induced during blood flow interruption and one that occurs in the immediate reperfusion period (20). This concept is supported by the presence of minimal histologic damage before the reperfusion process and the protection conferred by therapeutic interventions imposed during early ret10w (21, 22).
Blood flow may be reduced by mechanical obstruction to the vascular lumen produced by endothelial swelling (23) or by vascular congestion with erythrocytes (trapping) (24, 25) resulting from neutrophil “capillary plugging” (26–28) and/or capillary wall injury (29) with consequent “capillary leakage,” extravasation of plasma, and local hemoconcentration (30, 31). This is supported by the fineting that renal function improved considerably when medullary congestion was reduced by a lowering of the hematocrit (32). Alternatively renal vasoconstriction due to humoral or neural stimuli may be responsible for the increase in renal vascular resistance in the first few hours following ischemic injury (33). It has also been suggested that the depletion of ATP during ischemia, leads to an irreversible renal cell damage (5).
Several mechanisms have been proposed for reperfusion injury. The first is the production of xanthine oxidase–mediated free radical production (34, 35). In support of this hypothesis is the improvement in renal function by treatment with allopurinol (36) or superoxide dismutase (4, 37). The second is increased neutrophil adherence to damaged endothelium (38) that may intensify injury (39, 40), possibly by exacerbating the “no-reflow” phenomenon or by contributing to oxidant damage (41). Third, the delivery of calcium during reperfusion to an energy-depleted organ may produce cellular calcium overload that can exacerbate tissue damage (42). Fourth, the incomplete adenine nucleotide recovery in the postischemic period leaves the cells relatively energy-deficient (43).
TNF is produced by-activated macrophages, and receptors for TNF are present in all somatic cells, with the exception of erythrocytes (44). TNF causes augmented neutrophil aggregation and adherence to endothelial cells (45), increased superoxide anion regeneration (46), enhancement of neutrophilic phagocytic (47) and cytotoxic activities (48), induction of endothelial production of IL-1 and procoagulant factors, augmentation of lymphocyte production of and response to IL-2, and induction of a general shock-like state (44). Thus the vascular injury that follows renal I/R may occur as a consequence of the action of TNF on endothelial cells and neutrophils. This may also explain the presence of TNF during renal allograft rejection (49).
In the present study, the data show that FK506 pretreatment ameliorates the renal injury associated with I/R, as evidenced by a significant reduction in the subsequent BUN and creatinine levels (Figs. 1 and 2, respectively). The results also show that, similar to hepatic I/R, TNF production is triggered by renal I/R injury and is significantly inhibited by a single dose intravenous dose of FK506 (0.3 mg/kg) administered 24 hr prior to the induction of renal ischemia (Fig. 3). While the precise mode of inhibition of TNF activity by FK506 remains unknown, one explanation may be that FK506 has a direct effect on macrophages (independent of lymphocytes) or on the communication between macrophages and lymphocytes. It is conceivable that during I/R either oxidants or oxidant-stimulated proinflammatory factors such as platelet-activating factor stimulate lymphocytes to release lymphokines that consequently initiate neutrophil infiltration (50). Since both esA (51) and FK506 (52) have been shown to inhibit the release of lymphokines (from activated lymphocytes) with chemotactic activity for neutrophils it is possible that FK506 modulates neutrophil infiltration through its effect on lymphocytes. Whether a similar effect upon renal function would be obtainable were animals treated with cyclosporine rather than FK506 prior to I/R is unknown. The results of such an experiment would be of considerable interest. Such a group was not included in the present study because the investigators were convinced that cyclosporine would be overtly nephrotoxic and as such would result in needless animal deaths.
The reversibility of ischemic acute renal failure depends on the ability of renal tubular cells to regenerate and replace damaged areas of epithelium along the nephrons (53). It is conceivable that ischemic renal injury may activate a cytotoxic immune reaction against renal tubular cells and other cells present in the kidney, as a consequence of an induced structural alteration caused by ischemia, and thereby impair the ability of the renal tubular cells to regenerate. Such an action might be unmasked by the powerful T cell–specific immunosuppression exhibited by FK506. A further possible mechanism for the protective effect of FK506 herein observed is through restoration of cellular ATP levels following renal I/R—an action similar to its effect in the case of hepatic I/R (6) that needs further investigation.
No discernible differences were observed histologically with light microscopy at day 10 postischemia in saline- and FK506-pretreated animals, which is consistent with the decline toward the baseline of BUN, creatinine, and TNF levels at day 10 in both groups. Had a more precise measure of renal function such as insulin clearance peen performed as part of the present study, evidence fora long-lasting effect of FK506 as compared with saline-treated controls might have been seen.
Nonetheless, from the data presented, it can be concluded that FK506 pretreatment ameliorates the acute renal injury associated with I/R, as demonstrated by the decrease in BUN and creatinine levels over a 10-day period; renal I/R induces the release of TNF; there is a positive correlation between TNF levels and BUN and TNF levels and creatinine; and FK506 pretreatment results in a significant inhibition of TNF production. Based upon these data, it can be speculated that the release of TNF may be responsible, at least in part, for the increase in BUN and creatinine levels seen after I/R and that pretreatment of renal donors with FK506 may be associated with improvement in immediate posttransplant function. Such a speculation merits further evaluation.
Footnotes
This work was supported in part by Grants NIDDK AM32556 and NIDDK AM39789.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, Demetris J, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK-506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 3.Sakr M, Hassanein T, Zetti G, Van Thiel D. FK 506 ameliorates the hepatic injury associated with ischemia. Life Sci. 1990;47:687. doi: 10.1016/0024-3205(90)90623-y. [DOI] [PubMed] [Google Scholar]
- 4.Sakr M, Zetti G, Isaaccson Y, Van Thiel D. The hepatotrophic effect of FK 506 may reduce ischemic injury and improve donor organ performance. In: Montorsi M, Zennaro F, editors. Nutrition, infections, and intensive care transplantation. Monduzzi Editore SPA; Bologna, Italy: 1990. p. 281. [Google Scholar]
- 5.Sakr M, Zetti G, Farghali H, et al. Protective effect of FK 506 against hepatic ischemia in rats. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 6.Sakr M, Zetti G, Hassanein T, et al. FK 506 ameliorates the hepatic injury associated with ischemia and reperfusion in rats. Hepatology. in press. [PMC free article] [PubMed] [Google Scholar]
- 7.Sakr M, McClain C, Gavaler J, Zetti G, Starzl TE, Van Thiel D. FK 506 inhibits tumor necrosis factor and interleukin 6 production associated with hepatic ischemia/reperfusion. Hepatology. doi: 10.1016/s0168-8278(05)80209-0. in press. [DOI] [PubMed] [Google Scholar]
- 8.Kramer SM, Carver ME. Serum-free in vitro bioassay for the detection of tumor necrosis factor. J Immunol Methods. 1986;93:201. doi: 10.1016/0022-1759(86)90189-4. [DOI] [PubMed] [Google Scholar]
- 9.Francavilla A, Barone M, Todo S, et al. Augmentation of rat liver regeneration by FK 506 compared with cyclosporin. Lancet. 1989;2:1248. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Scotti-Foglieni CL, Porter KA, et al. Studies of the hepatotrophic qualities of FK 506 and CyA. Transplant Proc. 1990;22:93. [PMC free article] [PubMed] [Google Scholar]
- 11.Kahan BD. Cyclosporine. N Engl J Med. 1989;312:1725. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 12.Chow S, Thorner P, Baumal R, Wilson D. Cyclosporine and experimental renal ischemic injury. Transplantation. 1986;41:152. doi: 10.1097/00007890-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jabolonski P, Harrison C, Howden B, et al. Cyclosporine and the ischemic rat kidney. Transplantation. 1986;41:147. doi: 10.1097/00007890-198602000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gianello P, Ramboux A, Poelart D, et al. Prevention of acute cyclosporine nephrotoxicity by atrial natriuretic factor after ischemia in the rat. Transplantation. 1989;47:512. [PubMed] [Google Scholar]
- 15.Kanazi G, Stowe N, Steinmuller D, Ho-Hsieh H, Novick A. Effect of cyclosporine upon the function of ischemically damaged kidneys in the rat. Transplantation. 1986;41:782. doi: 10.1097/00007890-198606000-00023. [DOI] [PubMed] [Google Scholar]
- 16.The Canadian Multicenter Transplant Study Group A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med. 1983;309:809. doi: 10.1056/NEJM198310063091401. [DOI] [PubMed] [Google Scholar]
- 17.Nalesnik M, Lai H, Murase N, Todo S, Starzl TE. The effect of FK 506 and CyA on the Lewis rat renal ischemia model. Transplant Proc. 1990;22:87. [PMC free article] [PubMed] [Google Scholar]
- 18.McCauley J, Fung J, Jain A, Todo S, Starzl TE. The effects of FK 506 on renal function after renal transplantation. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers BD. Cyclosporine nephrotoxicity. Kidney Int. 1986;30:964. doi: 10.1038/ki.1986.280. [DOI] [PubMed] [Google Scholar]
- 20.Thornton MA, Zager RA. Brief intermittent reperfusion during renal ischemia: effects on adenine nucleotides, oxidant stress, and the severity of renal failure. J Lab Clin Med. 1990;115:564. [PubMed] [Google Scholar]
- 21.Zager RA, Mahan J, Merola AJ. Effects of mannitol on the post-ischemic kidney: biochemical, functional, and morphological assessments. Lab Invest. 1985;53:433. [PubMed] [Google Scholar]
- 22.Zager RA, Gmur DJ, Bredl CR, Eng MJ. Degree and time sequence of hypothermic protection against experimental ischemic acute renal failure. Circ Res. 1989;65:1263. doi: 10.1161/01.res.65.5.1263. [DOI] [PubMed] [Google Scholar]
- 23.Flores J, Dibona DR, Beck CH, Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972;51:118. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason J, Welsch J, Torhorst J. The contribution of vascular obstruction to the functional defect that follows renal ischemia. Kidney Int. 1987;31:65. doi: 10.1038/ki.1987.10. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsson J, Odlind B, Tufveson G, Wahlberg J. Effects of cold ischemia and reperfusion on trapping of erythrocytes in the rat kidney. Transplant Int. 1988;1:75. doi: 10.1007/BF00353823. [DOI] [PubMed] [Google Scholar]
- 26.Barosso-Aranda J, Schmid-Schonbein GW, Zweifach BW, Engler RL. Granulocytes and no-flow phenomenon in irreversible hemorrhagic shock. Circ Res. 1988;63:436. doi: 10.1161/01.res.63.2.437. [DOI] [PubMed] [Google Scholar]
- 27.Engler R, Dahlgren S, Morris DD, et al. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986;251:H314. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- 28.Engler R, Schmid-Shonbein GW, Pavelee S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983;111:98. [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez LA, Grisham M, Twohig B, et al. Role of neutrophils in ischemia-reperfusion induced microvascular injury. Am J Physiol. 1987;253:H699. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 30.Hellberg POA, Bayat A, Kallskog O, Wolgast M. Red cell trapping after ischemia and long-term kidney damage: influence of hematocrit. Kidney Int. doi: 10.1038/ki.1990.107. in press. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Holm-Rutili L, Perry MA, et al. Role of neutrophils in hemorrhagic shock–induced gastric mucosal injury in the rat. Gastroenterology. 1987;466 doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- 32.Hellberg O, Kallskog O. Influence of preischemic hematocrit on long-term kidney damage in the rat [Abstract].. Proceedings of the Xth International Congress of Nephrology; London. 1987.p. 463. [Google Scholar]
- 33.Levenson DJ, Simmons CE, Brenner BM. Arachidonic acid metabolism, prostaglandins, and the kidney. Am J Med. 1982;72:354. doi: 10.1016/0002-9343(82)90826-9. [DOI] [PubMed] [Google Scholar]
- 34.McCoy RN, Hill KE, Ayon MA, Stein JH, Burk RF. Oxidant stress following renal ischemia: changes in the glutathione redox ratio. Kidney Int. 1988;33:812. doi: 10.1038/ki.1988.72. [DOI] [PubMed] [Google Scholar]
- 35.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojteg G, Bayati A, Kallskog O, Wolgast M. Renal capillary permeability and intravascular red cell aggregation after ischemia: effects of xanthine oxidase activity. Acta Physiol Scand. 1987;129:295. doi: 10.1111/j.1748-1716.1987.tb08072.x. [DOI] [PubMed] [Google Scholar]
- 37.Bayati A, Hellberg O, Odlind B, Wolgast M. Prevention of ischemia acute renal failure with superoxidase dismutase and sucrose. Acta Physiol Scand. 1987;130:367. doi: 10.1111/j.1748-1716.1987.tb08150.x. [DOI] [PubMed] [Google Scholar]
- 38.Haral JM. Neutrophil-mediated vascular injury. Acta Med Scand [Suppl] 1987;715:123. doi: 10.1111/j.0954-6820.1987.tb09912.x. [DOI] [PubMed] [Google Scholar]
- 39.Klausner JM, Paterson IS, Goldman G, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 256:F794. doi: 10.1152/ajprenal.1989.256.5.F794. 19889. [DOI] [PubMed] [Google Scholar]
- 40.Hellberg POA, KaJlskog O, Wolgast M, Ojteg G. Effect of neutrophil granulocytes on the inulin barrier of renal tubular epithelium after ischemic damage. Acta Physiol Scand. 1988;134:313. doi: 10.1111/j.1748-1716.1988.tb08496.x. [DOI] [PubMed] [Google Scholar]
- 41.Linas SL, Shanley RP, Whittenberg D, Berger S, Repine JE. Neutrophils accentuate ischemic-reperfusion injury in isolated perfused rat kidneys. Am J Physiol. 1988;255:F728. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- 42.Schrier M, Arnold PE, Van Putten VJ, Burke TJ. Cellular calcium in ischemic acute renal failure: role of calcium channel blockers. Kidney Int. 1987;32:313. doi: 10.1038/ki.1987.211. [DOI] [PubMed] [Google Scholar]
- 43.Van Waarde AV, Stromski ME, Thulin G, et al. Protection of the kidney against ischemic injury by inhibition of 5′-nucleotidase. Am J Physiol. 1989;256:F298. doi: 10.1152/ajprenal.1989.256.2.F298. [DOI] [PubMed] [Google Scholar]
- 44.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 45.Bevilacqua MP, Wheeler ME, Pober JS, et al. Endothelial-dependent mechanisms of leukocyte adhesion: regulation by interleukin-1 and tumor necrosis factor. In: Movat HZ, editor. Leukocyte emigration and its sequelae. Karger; New York: 1979. p. 179. [Google Scholar]
- 46.Tsujimoto M, Yokota S, Vileck J, et al. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986;137:1094. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- 47.Shalby MR, Aggarwal BB, Rinderknecht E, et al. Activation of human polymorphonuclear neutropnil function by interferon-gamma and tumor necrosis factor. J Immunol. 1985;135:2069. [PubMed] [Google Scholar]
- 48.Meyer JD, Yurt RW, Duhaney R, et al. Tumor necrosis factor-enhanced leukotriene B, generation and chemotaxis in human neutrophils. Arch Surg. 1988;123:1454. doi: 10.1001/archsurg.1988.01400360024002. [DOI] [PubMed] [Google Scholar]
- 49.Metzger J, Lauterburg BH. Postischemic ATP levels predict hepatic function 24 hours following ischemia in the rat. Experimentia. 1988;44:455. doi: 10.1007/BF01940546. [DOI] [PubMed] [Google Scholar]
- 50.Braquet P, Rola-Pleszczynski M. Platelet-activating factor and cellular immune responses. Immunol Today. 1987;8:345. doi: 10.1016/0167-5699(87)90010-7. [DOI] [PubMed] [Google Scholar]
- 51.Bunjes D, Hardt C, Rollinghoff M, Wagner H. Cyclosporine A mediates immunosuppression of primary toxic T cell responses by impairing the release of interleukin 1 and interleukin 2. J Immunol. 1981;11:657. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- 52.Kino T, Hatanaka H, Miyata S, et al. FK 506, a novel immunosuppressant isolated from a streptomyces: II. Immunosuppressive effect of FK 506 in vitro. J Antibiot. 1987;40:1256. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 53.Humed HD, Cieslinski DA, Coimbra TM, et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]