Abstract
A 23-year-old female who presented with advanced renal failure was subsequently diagnosed with renal vein thrombosis and antiglomerular basement membrane (GBM) antibody disease. A previous case of renal vein thrombosis has been reported in association with anti-GBM disease, but to our knowledge, this is the first reported case in which the presentation of anti-GBM disease and renal vein thrombosis was concurrent. Further study is essential to understand if the association of anti-GBM disease and renal vein thrombosis as seen in our case was pure coincidence or is in fact occurs more frequently. It may be that the dual diagnosis is not made as establishing one sufficient diagnosis for renal failure may halt further investigations for additional diagnoses.
Background
Antiglomerular basement membrane (anti-GBM) disease is a rare disorder characterised by crescentic glomerulonephritis which generally manifests as rapidly progressive renal failure, and in the majority of patients, concurrent pulmonary haemorrhage.1 Disease incidence is noted to peak in the third and sixth decades. The term ‘Goodpasture’s disease’ is used by different authors with alternative meanings, but is most commonly reserved for those individuals with anti-GBM antibodies, glomerulonephritis and pulmonary haemorrhage.1 The disease has been noted to be associated with various renal insults, including lithotripsy2 and other forms of glomerulonephritis.3 To our knowledge, this is the second reported case of renal vein thrombosis occurring in association with anti-GBM disease.
Case presentation
A 23-year-old Caucasian female presented with an 11 day history of vomiting and oliguria. She had no significant medical history, was a non-smoker and was taking the combined contraceptive pill, Microgynon. Examination was unremarkable.
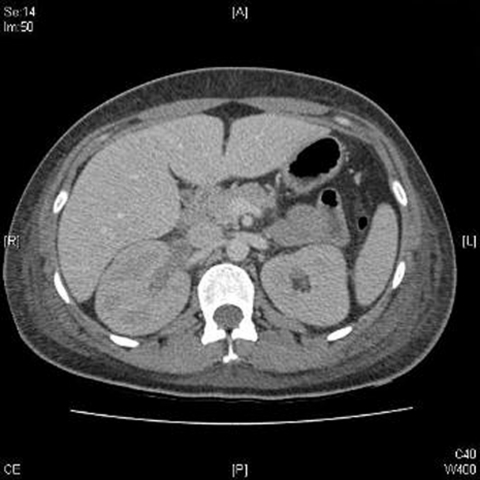
Investigations revealed a serum urea 33.3 mmol/l and serum creatinine 957 µmol/l, corresponding with an eGFR 4 mls/min/1.73m2. C-reactive protein was 600 and her serum albumin was 32 g/l. Full blood count revealed Hb 11.2 g/dl, white cell count 17.5×109/l and platelets 814×109/l. Urinalysis revealed heavy frank haematuria and 2+ protein. CT of chest/abdomen/pelvis ruled out pulmonary infiltration and revealed right renal vein thrombosis (figure 1). Lung function tests revealed a normal corrected carbon monoxide transfer factor (KCO) suggesting an absence of alveolar basement membrane involvement. Anti-GBM antibodies were strongly positive (>200 U/ml) and all other serological tests were negative. A renal biopsy revealed 100% active crescentic glomerulonephritis with ruptured basement membrane and linear IgG staining along the GBM. The diagnoses of anti-GBM antibody mediated glomerulonephritis and renal vein thrombosis were made.
Figure 1.
CT abdomen and pelvis with contrast revealing right renal vein thrombosis.
By day 3, the patient had become anuric and had been commenced on renal replacement therapy. Immunosuppression was not felt to be indicated due to the unlikelihood of renal recovery in a patient with 100% crescents on renal biopsy, the need for immediate dialysis at presentation and the absence of non-renal involvement. The patient was discharged on warfarin therapy and haemodialysis with outpatient follow-up for transplantation investigation. A thrombophilia screen performed after the acute event was negative.
Discussion
To our knowledge, this is the first reported case of the simultaneous presentation of renal vein thrombosis and anti-GBM (renal limited) disease, and in the absence of nephrotic syndrome.
A review of the literature reveals only one previous reported case of renal vein thrombosis in association with anti-GBM disease: this occurred in the context of nephrotic syndrome, a known significant risk factor for thrombosis.4 This report describes renal vein thrombosis diagnosed in a patient 2 weeks prior to the diagnosis of anti-GBM disease. At the time of initial presentation, the patient had nephrotic syndrome and renal failure, but after the diagnosis of renal vein thrombosis was made no further renal investigations, including a renal biopsy or testing for anti-GBM antibodies, were undertaken. It was only after the patient was discharged, her clinical condition deteriorated and she re-presented with pulmonary haemorrhage that the diagnosis of anti-GBM disease was made. The authors of this case propose that the renal vein thrombosis in their patient resulted from anti-GBM-related nephrosis that preceded the more typical features of Goodpasture’s disease.
Anti-GBM disease is characterised by circulating antibodies directed against the non-collagenous domain 1 (NC1) of the α3 chain of type IV collagen.5 The production of circulating autoantibodies appears to be short-lived, in response to an unknown inciting stimulus.6 Possible stimuli noted to be associated with anti-GBM pulmonary disease include cigarette smoking,7 exposure to hydrocarbons.7
The commonly accepted explanation that links these associated and/or possible provoking factors with anti-GBM pulmonary disease is that they may result in damage to the alveolar basement membrane which exposes cryptic immunological epitopes which in turn stimulate a novel autoimmune response. It has been postulated that dissociation of the NC1 hexamers and exposure of the pathogenic epitopes on the α3 chain may be the inciting event that leads to the production of the Goodpasture’s disease autoantibodies.8 It is possible that environmental factors could bring about such a conformational change in the quaternary structure of the NC1 hexamers. The question of whether exposure to a pathogenic stimulus directly elicits the autoimmune response, or uncovers hidden epitopes and triggers autoantibody synthesis, will need further study.9
Disease associations that may trigger similar exposure of hidden glomerular antigens are less clearly described. Direct renal insults for example, lithotripsy2 and other forms of glomerulonephritis including membranous nephropathy3 have been postulated as possible associations.
The concurrent diagnoses of renal vein thrombosis and anti-GBM disease in our patient may be due to random chance. Alternatively, the simultaneous diagnosis of renal vein thrombosis and the anti-GBM disease in our case raises the question of significant association. It is possible that the renal vein thrombosis was, as with lithotripsy and other forms of glomerulonephritis, the primary insult which may have occurred due to known risk factors such as her use of the combined contraceptive pill, Microgynon. The renal insult of renal vein thrombosis may have resulted in damage to the GBM and thus exposed the non-collagenous α-3 type IV collagen antigen to antibodies, pre-existing or newly formed. This could explain the presentation 2 weeks following the acute illness, with anti-GBM disease. Postulating such a disease association is speculative but it fits with the commonly held but yet unproven hypothesis of a primary insult requiring exposure of hidden antigens followed by antibody mediated tissue damage.10
Learning points.
-
▶
This case raises the possibility of a hitherto unrecognised disease association.
-
▶
Further study is needed to establish if such an association between these two conditions is more prevalent and if so whether there is a causative element to this association.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Salama AD, Levy JB, Lightstone L, et al. Goodpasture’s disease. Lancet 2001;358:917–20 [DOI] [PubMed] [Google Scholar]
- 2.Xenocostas A, Jothy S, Collins B, et al. Anti-glomerular basement membrane glomerulonephritis after extracorporeal shock wave lithotripsy. Am J Kidney Dis 1999;33:128–32 [DOI] [PubMed] [Google Scholar]
- 3.Nayak SG, Satish R. Concurrent anti-glomerular basement membrane disease and membranous glomerulonephritis: a case report and literature review. Clin Nephrol 2006;66:120–7 [DOI] [PubMed] [Google Scholar]
- 4.Gottehrer A, Reynolds SD, Libys JJ, et al. Renal vein thrombosis. Initial manifestation of Goodpasture’s syndrome. Chest 1991;99:239–40 [DOI] [PubMed] [Google Scholar]
- 5.Saus J, Wieslander J, Langeveld JP, et al. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem 1988;263:13374–80 [PubMed] [Google Scholar]
- 6.Hudson BG, Tryggvason K, Sundaramoorthy M, et al. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 2003;348:2543–56 [DOI] [PubMed] [Google Scholar]
- 7.Hérody M, Duvic C, Noël LH, et al. Cigarette smoking and other inhaled toxins in anti-GBM disease. Contrib Nephrol 2000;130:94–102 [DOI] [PubMed] [Google Scholar]
- 8.Pedchenko V, Bondar O, Fogo AB, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 2010;363:343–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salant DJ. Goodpasture’s disease–new secrets revealed. N Engl J Med 2010;363:388–91 [DOI] [PubMed] [Google Scholar]
- 10.Serratrice J, Chiche L, Dussol B, et al. Sequential development of perinuclear ANCA-associated vasculitis and anti-glomerular basement membrane glomerulonephritis . Am J Kidney Dis 2004;43:e26–30 [DOI] [PubMed] [Google Scholar]