Abstract
A 74-year-old patient presented with constitutional symptoms and was found to have acute kidney injury. He was known to have a prosthetic aortic valve. He was febrile with splenomegaly and vasculitic lesions on both hands. Nephritic screen revealed strongly positive cytoplasmic-antineutrophil cytoplasmic antibodies (c-ANCA). Differential diagnosis thus included a small vessel vasculitis or infective endocarditis. Transoesophageal echocardiography demonstrated no vegetations and serial blood cultures were negative. Immunosuppression for presumed granulomatosis with polyangiitis (Wegeners granulomatosis) was therefore instituted. The patient deteriorated, requiring multi-organ support. Renal biopsy showed a proliferative glomerulopathy and complements were low. Atypical screen for culture negative endocarditis revealed a strongly positive IgG-antibody titre against Bartonella henselae. Immunosuppression was discontinued and treatment for chronic Bartonellosis commenced. The patient made a remarkable recovery. His renal function quickly returned to normal, and ANCA titres and complements normalised. He was discharged home after completing a 6 week course of antibiotic therapy.
Background
This case highlights the difficulties in differentiating between infective endocarditis, particularly culture negative, and antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis in light of their similar spectrum of presentation and overlapping symptomatology. The importance of taking a clear history and performing thorough and tailored investigations is reinforced. Interpretation of results, in this case particularly the low complements, is key and without identification of a culture negative organism, the correct diagnosis may never have been made and the outcome may have differed dramatically.
Case presentation
A 74-year-old man presented with 10 weeks of general malaise, lethargy, anorexia and a ten kilogram weight loss (13% body weight). He denied any other symptoms. History from his wife revealed two brief episodes of confusion.
His medical history comprised a minimally-invasive bovine aortic valve replacement in 2005 for severe aortic regurgitation, paroxysmal atrial tachyarrhythmias, hypertension, hypercholesterolaemia and two transurethral prostate operations for benign prostatic hyperplasia. His premorbid condition was good and he regularly swam and cycled. He was taking aspirin 75 mg and simvastatin 40 mg only. Travel history included a trip to Cape Verde in 2008 and to Texas in 2006. He had two cats at home.
Prior to hospital presentation, he had attended his general practitioner with general malaise when it was noted that his serum creatinine was 264 micromol/l, having been 84 micromol/l the previous year. An urgent nephrology outpatient appointment was made.
In the meantime, he presented as an emergency with worsening lethargy and dyspnoea and it was found that his renal function had further deteriorated (creatinine of 486 µmol/l, with active urinary sediment and protein: creatinine ratio (PCR) of 300 mg/mmol). Clinically he had splenomegaly and a soft pansystolic murmur at the apex. He was febrile with a temperature of 38.5°Celsius. Blood tests revealed pancytopenia (white cell count 2.7×109/l, haemoglobin 9.5 g/dl, platelets <42×109/l). Three sets of blood cultures were negative. A full nephritic screen showed a strongly positive c-ANCA (PR 3 ELISA >100 AU/ml, antimyeloperoxidase antibodies <5 AU/ml), low complement C3 and C4 levels, negative ANA, negative rheumatoid factor and cryoglobulins and negative antiglomerular basement membrane antibodies. Serum electrophoresis revealed a diffuse increase in α-globulins.
In view of his prosthetic valve, he was investigated for infective endocarditis by both transthoracic (TTE) and transoesophageal (TOE) echocardiography, neither of which revealed any valvular lesion. He also had a bone marrow aspirate to investigate the pancytopenia which showed a reactive picture. Abdominal ultrasound demonstrated normal sized kidneys and confirmed 15 centimetre splenomegaly. He was then referred to our hospital for further assessment.
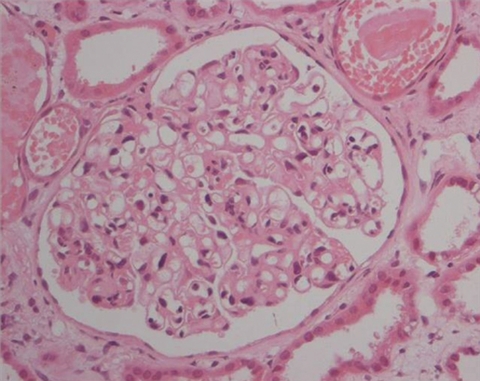
The summary to date was that of a patient with acute kidney injury, a bovine aortic valve, a strongly positive c-ANCA (PR3> 100 AU/ml), consumed complements, splenomegaly and thrombocytopenia. Because of thrombocytopenia he was deemed high risk for a renal biopsy and was initially treated with both three pulses of methylprednisolone for vasculitis, and covered for infective endocarditis with vancomycin and flucloxacillin, upon microbiology advice. Within 3 days, his platelet count improved sufficiently to perform a renal biopsy. This showed acute tubular injury and a mildly proliferative glomerulonephritis with neutrophils (figure 1). The glomerular changes suggested an infection- related process rather than small vessel vasculitis, although no immune complexes were seen on immunohistochemistry.
Figure 1.
Renal biopsy H&E.
In view of this histology and the lack of evidence of a focal and segmental necrotising crescentic glomerulonephritis, which would be expected with a vasculitis, no further immunosuppression was administered. Antibiotic therapy was continued. Several sets of blood cultures remained negative. A further TTE and TOE (done 7 and 10 days later respectively) again showed no vegetations, although the aortic valve leaflets were thickened and there was now moderate aortic regurgitation.
The clinical suspicion of endocarditis remained, and in the face of negative cultures and fairly recent foreign travel, atypical serology for culture negative organisms was requested. The patient, however, then deteriorated rapidly and was admitted to the intensive care unit (ICU), requiring mechanical ventilation and renal replacement therapy. Purpuric vasculitic skin lesions on his face and arms were noted, and repeat abdominal ultrasound scan of the abdomen showed multiple splenic lesions suggestive of granulomata. High-resolution CT of his chest revealed no evidence of pulmonary granulomata or haemorrhage. CT of chest, abdomen and pelvis revealed no evidence of malignancy. A repeat TOE showed that his left ventricular cavity was now dilated with globally severely impaired systolic function. MRI of the brain was also reported as showing vasculitic changes.
Now 1 month post admission, the diagnosis remained unclear. There had been no response to antibiotic therapy for endocarditis (by now including vancomycin, piperacillin, imipenem and flucloxacillin upon microbiology advice), and he had a persistently raised PR3 of >100 AU/ml. It was felt that there was little option but to treat as an ANCA-associated small vessel vasculitis. He was given three pulses of 0.5 grams of methylprednisolone and initially improved with a sharp decrease in his inotropic and ventilatory requirements. He was also commenced on cyclophosphamide 100 mg once daily and prednisolone 60 mg once daily and discharged from the ICU. Within a few days, however, he again deteriorated and required readmission to ICU.
At this point, the results of the atypical organism screen were obtained, showing a strongly positive titre for Bartonella Henselae IgG (1: 512) and a weakly positive titre for Coxiella spp (1:80). Hepatitis A, B and C, Treponema pallidum, Leishmaniasis, Schistosomiasis, cytomegalovirus, Chlamydia serology, Ebstein–Barr virus and the human immunodeficiency virus were negative.
On the basis of this serology and the lack of response to immunosuppression and conventional antibiotics, cyclophosphamide was discontinued and the steroid dose reduced. Treatment with clarithromycin 500 mg twice daily with gentamicin 80 mg once daily was commenced for presumed Bartonellosis.
Investigations
Figure 1 – Renal biopsy H&E
Differential diagnosis
In general, a positive ANCA is associated with the small vessel vasculitides (SVV). Other causes of ANCA positivity are reported and include both infectious and non-infectious diseases, including Bartonella endocarditis.1–3 The categories of associations are listed in figure 2. In this case particularly infective endocarditis, SVV and malignancy were considered. The differences between infective endocarditis presenting with a positive ANCA, and a SVV presenting with endocardial involvement have been previously documented.4 These are noted in table 1 for comparison and many of these were pertinent to our case. Hypocomplementaemia is only associated with certain renal lesions, the most common of which are postinfectious glomerulonephritis, infective endocarditis and lupus nephritis, and would not routinely be expected in a case of vasculitis. The low C3 and C4 in this case thus favour the diagnosis of endocarditis. Causes of a positive ANCA are illustrated in figure 2.
Figure 2.
Causes of a positive antineutrophil cytoplasmic antibodies.
Table 1.
Differentiation between vasculitis and endocarditis
| Features common to both vasculitis and infective endocarditis | Features seen predominantly in infective endocarditis |
| Presentation with constitutional symptoms | Thrombocytopenia |
| Fever | Hypocomplementaemia |
| Active urinary sediment | Immune complexes |
| Skin involvement | Other positive autoantibodies |
| Renal impairment | Low titre antineutrophil cytoplasmic antibodies/ELISA negative |
| Raised inflammatory markers | Splenomegaly |
Outcome and follow-up
Within 3 days, the patient’s clinical condition dramatically improved. He became alert and orientated, was weaned off ventilatory support and renal replacement and underwent rehabilitation. A repeat TOE demonstrated improvement in his left ventricular systolic function. After 3 weeks, his PR3 titres had sharply fallen to 15 AU/ml and his complement levels had normalised. The patient was discharged home after completing the recommended 6 weeks of antibiotics. He was mentally alert and was able to walk with a stick.
Discussion
There are several cases reported in the literature of infective endocarditis presenting as and masquerading as an ANCA-associated vasculitis.5 6 Here we report a rare case of culture negative endocarditis presenting as a cytoplasmic ANCA-associated vasculitis (c-ANCA) with acute kidney injury, and highlight the diagnostic and therapeutic challenges such a case poses.
ANCA and endocarditis
Increasing evidence exists that ANCA is pathogenic in the development of small vessel vasculitis rather than being simply an epiphenomenon.7 8 It has been shown that there can be transient induction of, particularly PR3, antibodies during infection.1 9 Studies indicating the close link between granulomatosis with polyangiitis (Wegeners granulomatosis) and chronic nasal Staphylococcus aureus carriage were key in linking the development of ANCA with infection.10–12 Further work done on molecular mimicry,13 the role complementary PR314 15 and a new subset of ANCA, lysosomal-associated membrane protein 2,8 16 17 further serve to reinforce this link.
Both infectious and non-infectious diseases have been reported as causing ANCA positivity by immunofluorescence, but these are often at low titre or ELISA negative.2 Bacterial endocarditis presenting with both c-, and less commonly p-ANCA positivity, have been documented.5 6
Non-infectious endocardial involvement is also known as a feature of ANCA-associated vasculitides (in particular c-ANCA).8 18 19 Clinically, infective endocarditis and SVV have a very similar spectrum of presentation and clearly the differentiation between the two is of paramount importance in terms of initiating treatment. Comparison has been made between infective endocarditis occurring in association with ANCA versus endocardial involvement with idiopathic ANCA.4 This concluded subtle differences in clinical presentation, presence of splenomegaly (rare in SVV), presence of other autoimmune markers (also rare in SVV), levels of complement (should be normal in SVV) and echocardiographic findings and clinical and valvular course, which are pertinent to our case.
Diagnostic challenge
The diagnosis was confounded by several factors. Initially, the patient’s presentation with renal failure on the background of a bovine aortic valve replacement and systemic symptoms favoured a diagnosis of infective endocarditis, which could have explained the splenomegaly and consumed complements. The persistently negative blood cultures and cardiac investigations, however, coupled with a lack of clinical response to antibiotic therapy refuted this. It is known that ANCA is 99% specific for vasculitis in the correct clinical context20 and the consistently high PR3 titres, pauci-immune appearance of the biopsy and possibility of both coronary and cerebral vasculitis steered the diagnosis in favour of a small vessel vasculitis. We therefore decided to initiate immunosuppressive therapy, which was administered for 2 weeks but did not lead to any sustained clinical improvement. This treatment was discontinued upon receiving a positive result for B henselae, prompting treatment with clarithromycin and gentamicin. The testing for and identification of Bartonella was key in the progression of the management of the case.
Bartonella infection
Bartonella species (spp), first isolated in 1988,21 are intracellular, fastidious gram-negative proteobacteria and are associated with diseases such as cat scratch disease (B henselae, as in our patient), trench fever (Bartonella quintana, more common in the homeless and alcohol dependent population) and oroya fever or verruga peruvana (Bartonella bacilliformis, most prevalent in South America). Across various studies, the seroprevalence of antibodies to B henselae in healthy persons ranges from 3.6% to 6% and in many patients, the disease is self-limiting. Initially, a primary inoculation skin lesion can be observed at the site of the scratch, and regional lymphadenopathy is a common finding. Bacteraemia can lead to systemic infection and multi-organ involvement. Systemic disease is more commonly found in immunosuppressed patients, but can also occur in healthy people.
Symptoms include general malaise, myalgia, arthralgia, anorexia, weight loss and intermittent fever. It is recognised that kidney injury, splenomegaly, granuloma formation and encephalopathy may develop,22 all of which our patient displayed.
Confirmation of the diagnosis can be challenging. Bartonella spp can be cultured on selective media but sensitivity of this method is at best 20 per cent. Direct smear with Warthin-Starry Silver stain of the tissues, including heart valves, could help in establishing the diagnosis. Our patient underwent biopsy of the skin lesions but histopathological examination with this staining did not reveal any positive findings. A high titre of IgG antibody against Bartonella spp is usually suggestive of chronic infection as was in our patient with a titre of >1:512 against B henselae. An IgG titre of greater than 1:256 is considered diagnostic of current or past Bartonella infection. PCR can also be performed on various samples, for example EDTA blood sample and skin lesions. The PCR results of EDTA blood sample and skin biopsies of our patient were both negative. It is described that any other samples apart from heart valves may not yield high sensitivity.23
Bartonella spp have been associated with culture negative endocarditis and account for up to 3% of infective endocarditis cases.24 A retrospective study performed among 348 patients in France suggested that Bartonella spp was responsible for 28% cases of culture negative endocarditis.25 B quintana was three times more prevalent than B henselae as the cause of culture negative endocarditis.25 It has been described that more than half of the patients with Bartonella spp infective endocarditis have had known valvular disease. Fever may not be a common feature.26 Infection with Bartonella spp mimicking small vessel vasculitis has also been described.3 27 28 Myocarditis is a known but rare feature of Bartonella infection.29 30
Learning points.
-
▶
Differentiating between infective endocarditis and SVV is challenging and it is important to be aware of their similar presentation and overlapping symptomatology.
-
▶
The importance of clear history taking and performing tailored investigations is reinforced.
-
▶
Although c-ANCA is highly specific for vasculitis, it is vital to be aware of causes of false-positivity as the treatment options vary significantly – beware of culture negative endocarditis.
-
▶
Here, without the correct diagnosis, the outcome may have differed dramatically and the patient might not have survived his illness.
Acknowledgments
Dr Ravindra Rajakariar, Dr Michael Millar, Dr Michael Sheaff.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Csernok E, Lamprecht P, Gross WL. Clinical and immunological features of drug-induced and infection-induced proteinase 3-antineutrophil cytoplasmic antibodies and myeloperoxidase-antineutrophil cytoplasmic antibodies and vasculitis. Curr Opin Rheumatol 2010;22:43–8 [DOI] [PubMed] [Google Scholar]
- 2.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol 2010;29:893–904 [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama H, Sahara M, Imai Y, et al. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Cardiology 2009;114:208–11 [DOI] [PubMed] [Google Scholar]
- 4.Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol 2007;26:590–5 [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Lamprecht P, Niles JL, et al. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheum 2000;43:226–31 [DOI] [PubMed] [Google Scholar]
- 6.de Corla-Souza A, Cunha BA. Streptococcal viridans subacute bacterial endocarditis associated with antineutrophil cytoplasmic autoantibodies (ANCA). Heart Lung 2003;32:140–3 [DOI] [PubMed] [Google Scholar]
- 7.Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 2006;17:1235–42 [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Kallenberg CG. New advances in the pathogenesis of ANCA-associated vasculitides. Clin Exp Rheumatol 2009;27(1 Suppl 52):S108–14 [PubMed] [Google Scholar]
- 9.Tiliakos AM, Tiliakos NA. Dual ANCA positivity in subacute bacterial endocarditis. J Clin Rheumatol 2008;14:38–40 [DOI] [PubMed] [Google Scholar]
- 10.Charlier C, Henegar C, Launay O, et al. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis 2009;68:658–63 [DOI] [PubMed] [Google Scholar]
- 11.Popa ER, Stegeman CA, Kallenberg CG, et al. Staphylococcus aureus and Wegener’s granulomatosis. Arthritis Res 2002;4:77–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegeman CA, Tervaert JW, de Jong PE, et al. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med 1996;335:16–20 [DOI] [PubMed] [Google Scholar]
- 13.Kallenberg CG, Brouwer E, Mulder AH, et al. ANCA-pathophysiology revisited. Human Immunology 2004;65:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Woude FJ, Daha MR, van Es LA. The current status of neutrophil cytoplasmic antibodies. Curr Opin Rheumatol 2007;19:17–2417143091 [Google Scholar]
- 15.Pendergraft WF, 3rd, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med 2004;10:72–9 [DOI] [PubMed] [Google Scholar]
- 16.Kain R, Exner M, Brandes R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 2008;14:1088–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salama AD, Pusey CD. Shining a LAMP on pauci-immune focal segmental glomerulonephritis. Kidney Int 2009;76:15–7 [DOI] [PubMed] [Google Scholar]
- 18.Grant SC, Levy RD, Venning MC, et al. Wegener’s granulomatosis and the heart. Br Heart J 1995;73:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horino T, Takao T, Taniguchi Y, et al. Non-infectious endocarditis in a patient with cANCA-associated small vessel vasculitis. Rheumatology (Oxford) 2009;48:592–4 [DOI] [PubMed] [Google Scholar]
- 20.Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998;53:743–53 [DOI] [PubMed] [Google Scholar]
- 21.English CK, Wear DJ, Margileth AM, et al. Cat-scratch disease. Isolation and culture of the bacterial agent. JAMA 1988;259:1347–52 [DOI] [PubMed] [Google Scholar]
- 22.Centre for disease and control and prevention. Encephalitis associated with cat scratch disease, Broward and palm beach Counties, Florida, 1994, MMWR. Morbidity and Mortality Weekly Reports 1994;43:915–16. [PubMed]
- 23.Goldenberger D, Künzli A, Vogt P, et al. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol 1997;35:2733–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med 1996;125:646–52 [DOI] [PubMed] [Google Scholar]
- 25.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–73 [DOI] [PubMed] [Google Scholar]
- 26.Raoult D, Fournier PE, Vandenesch F, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med 2003;163:226–30 [DOI] [PubMed] [Google Scholar]
- 27.Vikram HR, Bacani AK, DeValeria PA, et al. Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol 2007;45:4081–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JW, Pien BC, Ardoin SA, et al. A man with chest pain and glomerulonephritis. Lancet 2005;365:2062. [DOI] [PubMed] [Google Scholar]
- 29.Meininger GR, Nadasdy T, Hruban RH, et al. Chronic active myocarditis following acute Bartonella henselae infection (cat scratch disease). Am J Surg Pathol 2001;25:1211–4 [DOI] [PubMed] [Google Scholar]
- 30.Shah SS, McGowan PP. Rickettsial, Ehrlichial and Bartonella infections of the myocardium and pericardium. Front Biosci 2003;8:197–201 [DOI] [PubMed] [Google Scholar]