Abstract
Thoracic aortic aneurysm is a rare clinical entity that is usually asymptomatic. Failure to treat this type of aneurysm can prove fatal. Here, the authors report a case of thoracic aortic aneurysm causing chronically worsening compressive symptoms including dysphagia. This was diagnosed following a suspicious chest radiograph, and confirmed with thoracic CT angiogram. These symptoms remitted over a period of months following thoracic endovascular repair of the aneurysm. Aneurysmal compression and deviation of the oesophagus is noticeably reduced following repair. This is one of few cases in the literature of a remittance of dysphagia following endovascular aneurysm repair, and highlights that rare causes of dysphagia ought not to be disregarded.
Background
Thoracic aortic aneurysm (TAA) is a rare clinical entity, with an incidence of 5–10 per 100 000 in the UK.1 The majority of patients with TAA are asymptomatic. TAAs may be diagnosed as an incidental finding, as a result of compression of adjacent structures, or secondary to complications such as dissection or rupture. Pain is the commonest presenting symptom, usually thoracic or epigastric in nature, radiating to the back. Compressive symptoms have been described including hoarseness, cough, stridor and superior vena caval obstruction. Dysphagia aortica, dysphagia secondary to oesophageal compression by the adjacent aorta, has been described in the literature. However, here we present a case of dysphagia aortica in which symptomatic improvement occurred following thoracic endovascular aneurysm repair (TEVAR).
Case presentation
We present the case of a 55-year-old gentleman with a background of hypertension and smoking, who presented with a 9 month history of shortness of breath and heartburn. Of note, he had a family history of abdominal aortic aneurysm. Following a chest radiograph performed in primary care, which was suggestive of an aortic arch aneurysm, a subsequent thoracic CT angiogram revealed an 8 cm long aneurysm affecting the thoracic aorta. This began distal to the origin of the left subclavian artery (type I). Its maximal diameter was 6.5 cm.
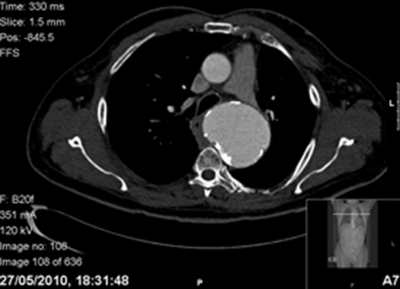
At the time of review by vascular surgery, 6 months after the initial chest radiograph and 15 months after the initial onset of symptoms, the patient was admitted with worsening symptoms and new dysphagia. He was investigated with a new thoracic CT angiogram which showed an increase in size of the aneurysm to 7.2 cm, the aorta’s calcified aneurysmal sac now compressing the adjacent oesophagus to the right (figure 1). There was no evident aneurysmal leak. He was discharged from hospital with a view to elective endovascular aneurysm repair within 1 month.
Figure 1.
Axial section of thoracic CT angiogram demonstrating the large aneurysmal sac of the thoracic aorta compressing the oesophagus to the right.
A TEVAR using a thoracic stent graft was performed around 1 month after this hospital admission, with no intraoperative complication. The patient made an uncomplicated recovery, spending 1 day on the surgical high dependency unit. He was discharged 2 days postoperatively following a thoracic CT angiogram that showed correct stent placement.
He progressed well in the immediate and mid-term postoperative period. However, at review 4 months post-TEVAR, the patient was still complaining of dysphagic symptoms. At this time, thoracic CT angiogram showed the aneurysmal sac to be similar in size to that at the time of repair. The patient was referred for review by an upper gastrointestinal surgeon.
An oesophagogastroduodenoscopy was performed 6 months after the onset of dysphagia. This revealed Barrett’s oesophagus and a benign oesophageal stricture likely secondary to gastro-oesophageal reflux. At review by the upper gastrointestinal surgeon, the patient was tolerating a soft diet. It was suggested that the symptom of dysphagia was likely secondary to compression of the oesophagus by a large residual thrombotic sac. It was recommended that he should start a high dose proton-pump inhibitor as treatment for the Barrett’s oesophagus, and it was suggested that a barium swallow be performed if there was no remittance of the dysphagic symptoms.
Outcome and follow-up
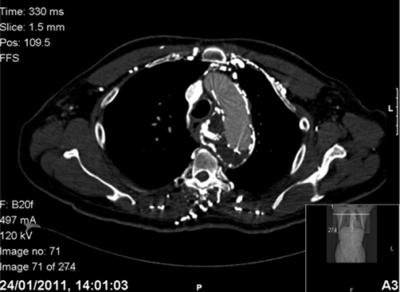
At the most recent review of the gentleman, 9 months after TEVAR and 3 months after initiation of the proton-pump inhibitor, thoracic CT angiogram showed a decrease in aneurysmal size from 7.2 to 5.7cm, with significantly less oesophageal compression compared to the previous CT scan (figure 2). Clinically, his dysphagia has significantly improved. He is now discharged from the care of the upper gastrointestinal surgeon.
Figure 2.
Axial section of thoracic CT angiogram 7 months post thoracic endovascular aneurysm repair demonstrating a smaller aneurysmal sac, with noticeably less compression and deviation of the adjacent oesophagus.
Discussion
Dysphagia is a common presenting symptom of oesophageal disease, and can be caused by a lesion obstructing the oesophageal lumen, within the oesophageal wall, or an externally compressing lesion. Common first-line investigations for dysphagia include a barium swallow or oesophagogastroduodenoscopy.
Here, we present the case of a 55-year-old gentleman with an indolent history of dysphagia, indigestion and shortness of breath, due to an externally compressing aneurysmal sac of the thoracic aorta. This was diagnosed by initial chest radiography followed by a thoracic CT angiogram. Decrease in the size of the aneurysmal sac postrepair by endovascular thoracic aortic stenting led to a remittance in symptoms.
There are few reports in the literature of dysphagia caused by a compressing aorta, referred to by some as dysphagia aortica, a term coined by Pape in 1932.2
One of these cases died of aortic aneurysmal rupture in the community while awaiting urgent tertiary review.3 This 67-year-old lady presented with indolent dysphagia and weight loss over a 3 month period, and was seen by numerous specialties. This aneurysm was evident on both chest radiography and CT. As this patient was stable, she was discharged pending urgent tertiary referral, and died of aneurysmal rupture soon afterwards.
Another case documents an 86-year-old lady who also presented with a chronic presentation of progressive dysphagia that, following investigation with chest radiograph and thoracic CT angiogram, was found to be secondary to thoracic aortic aneurysm. This patient declined invasive intervention and was therefore discharged from hospital.4
The most similar case of this kind to be recently documented was that of an older lady who presented with dysphagia and retrosternal pressure sensation. This was found to be due to a giant penetrating ulcer of the descending aorta, and her symptoms remitted following endovascular aortic stenting.5
In our case, however, dysphagia was due to a large aneurysmal sac with no associated penetrating ulcer, and symptomatic improvement in dysphagia occurred following TEVAR and reduction in aneurysmal sac size. This is the first documented case of endovascular stenting of a simple true atherosclerotic aneurysm causing a subjective remittance in symptoms of dysphagia. In addition, this case involves a 55-year-old gentleman, in contrast to the previous cases of dysphagia due to a compressive thoracic aortic aneurysm that have been documented to have occurred in older women with short stature and kyphosis.
This case, and the previously described cases highlight that in patients with atypical dysphagia, rarer causes such as TAA should be excluded. Although chest radiographs and thoracic CTs are not first-line in the investigation of dysphagia, this case suggests that they ought to be considered to exclude TAA, following an inconclusive oesophagogastroduodenoscopy or barium swallow. Delay in diagnosis of this condition can be fatal, and the prognosis of the untreated TAA is poor.6
In this particular case, the salient points are twofold. First that the aneurysmal sac decreased in size over a period of months, not acutely, and that consequently, the symptom of dysphagia also took months to resolve. Second, that the oesophagogastroduodenoscopy was delayed in the investigation of this man’s dysphagia, despite the fact that it is a first-line test for this symptom. As well as proving that extrinsic compression existed, the endoscopy also revealed Barrett’s oesophagus in this case, an asymptomatic premalignant condition, requiring long-term treatment with a proton-pump inhibitor and endoscopic surveillance. Had this not been performed, this condition, although unrelated to his primary pathology, would have gone undiagnosed.
In conclusion, this case demonstrates that TAA can present with dysphagia, and therefore should be included in the differential diagnosis of the dysphagic patient. This suggests that chest radiographs and CT scans should be considered in chronically dysphagic patients in whom primary investigations have not yielded a source, in order to prevent a delay in diagnosis. It also emphasises that an oesophagogastroduodenoscopy, a first-line investigation, ought not be forgotten, even if an obvious cause for dysphagia is evident through alternative imaging.
Learning points.
-
▶
Thoracic aortic aneurysm rarely causes compressive symptoms such as dysphagia.
-
▶
Here we present a case of chronic dysphagia secondary to a true thoracic aortic aneurysm.
-
▶
This case demonstrates remittance of symptoms following endovascular aneurysmal stenting.
-
▶
Thoracic CT scans may reveal rare causes of dysphagia where first-line investigations such as dysphagia and upper gastrointestinal endoscopy have been inconclusive.
-
▶
First-line investigations for dysphagia ought not to be delayed or omitted even if a likely cause for the symptom is known.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Ramanath VS, Oh JK, Sundt TM, 3rd, et al. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc 2009;84:465–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pape R. Uber einen abnormen verlauf (‘tiefe Rechtslage’) der mesa aotitischen aorta descendens. Fortschr Roetgenstr 1932;46:257–69 [Google Scholar]
- 3.Hiller HG, Lagattolla NR. Thoracic aortic aneurysm presenting with dysphagia: a fatal delay in diagnosis. Thorac Surg Sci 2007;4:Doc01. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Jang SW, Kim DB, et al. A patient With dysphagia due to an aortic aneurysm. Korean Circ J 2009;39:258–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kische S, Werner D, Ince H. A neglected symptom of contained aortic laceration - dysphagia aortica successfully treated by endovascular stentgrafting. Catheter Cardiovasc Interv. 2011 (In Press) doi: 10.1002/ccd.23265. [DOI] [PubMed] [Google Scholar]
- 6.Abraha I, Romagnoli C, Montedori A, et al. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst Rev 2009;1:CD006796. [DOI] [PubMed] [Google Scholar]