Abstract
INTRODUCTION
The risk of ipsilateral breast tumour recurrence (IBTR) following breast conservation surgery (BCS) for invasive breast cancer (IBC) and radiotherapy is dependent on patient-, tumour- and treatment-related variables. In the Cambridge Breast Unit, breast conserving surgery has been performed with a target radial margin of 5 mm for IBC, in combination with 40-Gy hypofractionated (15 fractions) breast radiotherapy, since 1999.
PATIENTS AND METHODS
An audit was performed of cases treated between 1999 and 2004. A total of 563 patients underwent BCS for invasive breast cancer with 90.4% receiving radiotherapy (RT) and 60.4% of patients receiving boost RT (3 fractions of 3-Gy).
RESULTS
After a median follow-up of 58 months, five of the 563 (0.9%) patients developed IBTR. The 5-year actuarial IBTR rate was 1.1%. In terms of distant disease recurrence (DDR), 29 of the 563 (5.2%) had DDR during follow-up, giving a 5-year actuarial DDR rate of 5.4%. The 5-year breast cancer specific survival was 95%, with the poorer NPI groups having worse breast cancer specific survival (Log-rank, P < 0.0001). More importantly, patients with IBTR had a shorter breast cancer-specific survival than those who were IBTR-free (Log-rank, P < 0.0001).
CONCLUSIONS
Our treatment regimen, combining BCS with a 5-mm target margin and hypofractionated 40-Gy RT, results in an extremely low rate of IBTR, and compares favourably with the target IBTR rate of < 5% defined by the Association of Breast Surgeons (ABS) at BASO guidelines.
Keywords: Breast conserving surgery, Radiotherapy, Ipsilateral breast tumour recurrence
Breast conserving surgery (BCS) and radiotherapy (RT) is now the standard of care for the treatment of early-stage invasive breast cancer. With BCS, the issue of ipsilateral breast tumour recurrence (IBTR) is an important one. The risk of IBTR is dependent on patient-, treatment- and tumour-related variables. Patient-related factors that predispose to IBTR include young age, although the cut-off age remains controversial,1–4 and pre-menopausal status.2 Treatment-related factors (such as a positive resection margin) predispose to a higher risk of IBTR,4–6 whilst the use of chemotherapy and hormonal therapy may lower the risk of IBTR.5,7 In terms of surgical therapy, there is still a debate among breast surgeons about what constitutes an adequate surgical margin. Furthermore, there are variations in the radiation regimens used by different breast units and the optimal dose of adjuvant RT remains uncertain. The tumour-related risk factors associated with IBTR include increasing tumour size,1,8,9 high histological grade,1 positive lymph node status,7,8 multicentric disease,10 vascular invasion11 and adverse biological factors such as tumoural p53 mutations.10
In the Cambridge Breast Unit (CBU), BCS with a target radial margin of 5-mm for invasive breast cancer, in combination with hypofractionated 40-Gy whole breast RT regimen, has been used since 1999. The aim of the current audit was to assess the effectiveness of our treatment regimen in terms of local recurrence rate and other outcome measures in 563 patients who underwent BCS and RT for invasive breast cancer. More specifically, we aimed to assess our compliance in terms of local recurrence rate with gold standard being the target rate of 5% as proposed by the British Association of Surgical Oncology (BASO).12
Patients and Methods
Between January 1999 and December 2004, 563 women with unilateral invasive breast carcinoma (T1–3, N0–1, M0 based on the International Union Against Cancer (UICC) classification) were treated with BCS and RT in the Cambridge Breast Unit, Addenbrooke's Hospital. Patients who required mastectomy following initial BCS were not included in this audit. Medical records were reviewed to extract information on clinicopathological variables, including local therapy (surgery, surgical margin, re-excision and radiotherapy regimen), systemic therapy (chemotherapy and hormonal therapy), disease recurrence (local and distant disease recurrences), survival and cause of death for each patient.
Surgical therapy
Prior to surgery, all patients were assessed by a multidisciplinary team (MDT) comprising of two consultant breast surgeons, two clinical oncologists, two medical oncologists and dedicated breast pathologists. In general, patients with extensive disease, multifocal disease or patients in whom RT was contra-indicated were considered ineligible for BCS.
Surgery was performed by two consultant breast surgeons. The surgery consisted of wide local excision with a 5-mm target radial margin, which could be wire-guided, ultrasound-marked or without any localisation if clinically palpable. During the early years of the audit period, cavity shavings were taken at the time of primary operation to ensure adequate clearance. This technique had been previously described.13 If the final surgical radial margin was less than 5 mm, re-excision was performed at the discretion of the MDT. Reasons for accepting a margin of less 5 mm included no further breast tissue to take or acceptance by the MDT due to other clinicopathological reasons. Re-excision of the primary tumour site was performed in 10.9% of patients (61 women). At the time of BCS, patients also underwent Level I and II axillary lymph node dissection (ALND), with or without sentinel lymph node biopsy as follows: sentinel lymph node biopsy (19%), level I (3%), level II (74%) and level III (3%) clearance.
Pathological evaluation
All patients underwent specialist breast pathology review including pathological tumour size, tumour grade, lymph node status, lymphovascular invasion as well as margin assessment. Definition of radial margin distance was based on the distance of invasive carcinoma or ductal carcinoma in situ (DCIS) from the edge of the resection sample. If cavity shavings were taken, the radial margin was determined by the sum of disease-free thickness of the shaving together with the disease-free margin from the tumour within the primary resection sample. ER levels were determined by immunohistochemical analyses. During the period of this study, HER2 receptor status was not routinely tested.
Adjuvant systemic therapy
Adjuvant chemotherapy with an anthracycline-based regimen was recommended for patients with an NPI > 4.4 (i.e. who had histological evidence of axillary lymph node involvement or grade 3) and who were felt to be appropriate candidates for chemotherapy by the MDT. Chemotherapy was commenced 4 weeks following surgery and prior to breast RT. Tamoxifen was recommended for all patients with oestrogen receptor (ER) positive tumours. In patients who also received systemic chemotherapy, tamoxifen was started 4 weeks following completion of chemotherapy.
Radiation therapy
All women received adjuvant external-beam RT to the ipsi-lateral breast. RT was delivered using tangential fields and 6–15 MV photon energy and a standard hypofractionated dose of 40-Gy in 15 fractions, delivered over 3 weeks. During initial part of the audit period, 17 patients received 50-Gy in 25 fractions, delivered over 5 weeks, as part of the START trial.14 A boost to the primary tumour bed, consisting of a boost dose of 9-Gy in three fractions, was routinely given except in low-risk patients (T1 tumours, grade 1–2, node negative, ER positive and no lymphovascular invasion). A supraclavicular (SCF) RT field was added for patients with four or more positive axillary nodes. Axillary fields were not normally used unless there was evidence of macroscopic residual disease following axillary clearance.
Follow-up
Follow-up data were obtained up to October 2007. Women were followed up with breast examination, 6-monthly during years 1–2 and annually from years 3–5, together with annual bilateral mammograms. After 5 years, the women were referred to the National Health Service breast screening programme for 3-yearly mammography.
Data and statistical analyses
The NPI was calculated for each case using the formula: NPI = 0.2 × tumour size (cm) + nodal stage (1–3) + grade (1–3). The NPI calculation and its prognostic groups have previously been described.15
Follow-up time was calculated from the date of surgery to the date of last follow-up or death. IBTR was defined as re-appearance of histologically-proven invasive breast carcinoma at the site of previous surgery in the treated breast and was counted only if it occurred alone or before distant disease recurrence. Distant disease recurrence (DDR) was defined as appearance of carcinoma outside the treated breast including ipsilateral axillary lymph node recurrence and metastasis to distant organs. DDR was counted only when it occurred alone, or more than 3 months before IBTR. The development of a contralateral breast carcinoma was not considered as DDR.
Actuarial curves of IBTR and DDR were calculated using the Kaplan–Meier method. Recurrence-free survival time was calculated from the date of surgery to the date of diagnosis of recurrence or the last follow-up or death. Women were censored from the calculation of IBTR at the time of last local recurrence-free follow up or when death occurred. Women were censored from calculation of DDR rate at the time of last distant disease-free follow-up or when death occurred. Breast cancer-specific survival was defined as the interval between primary BCS and last follow-up or death. Patients who were alive or died of a cause other than breast cancer were censored for analysis of breast cancer-specific survival. For overall survival (i.e. all-cause mortality), the survival time was the interval between primary BCS and last follow-up or death but patients were censored only if they were alive. The survival curves were compared using the log-rank test. P < 0.05 was considered significant. SPSS v10.1 software (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
Patient characteristics
Between 1999 and 2004, 563 patients who underwent BCS, as their final surgical procedure, followed by RT for invasive breast cancer were identified. The median age at diagnosis was 58 years (range, 29–92 years; Table 1). Most of the patients in this series presented with a symptomatic tum our (62.7%) whilst 34.3% of patients had a screen-detected tumour. The overall median follow-up was 58 months (4.8 years). The median follow-up for women who were not lost to follow-up was 60 months (range, 13–99 months). Twenty-seven women (4.8%) were lost to follow-up. The overall follow-up is relatively short as this is accounted by the follow-up protocol of 5 years following the diagnosis of breast cancer at the unit, before discharging the women back to the national screening programme for further screening mammography.
Table 1.
Patient and tumour characteristics
| Characteristics (n = 563) | |
|---|---|
| Age | |
| Median (years) | 58 |
| Range (years) | 29–92 |
| Presentation | |
| Screen-detected | 193 (34.3%) |
| Symptomatic | 353 (62.7%) |
| Unknown | 17 (3.0%) |
| Tumor size, pathologic | |
| Median (mm) | 14 |
| Range (mm) | 8–54 |
| Histological type | |
| Invasive ductal | 405(71.9%) |
| Invasive lobular | 49 (8.7%) |
| Tubular | 47 (8.3%) |
| Mixed | 42 (7.5%) |
| Mucinous | 9 (1.6%) |
| Medullary | 2 (0.4%) |
| Other | 9 (1.6%) |
| Histopathological grade | |
| I | 129 (22.9%) |
| II | 254(45.1%) |
| III | 172 (30.6%) |
| Lymph node status | |
| Negative | 381 (67.7%) |
| Positive | 176(31.3%) |
| Unknown | 6(1.1%) 481 (85.4%) |
| Oestrogen receptor status | |
| Positive | |
| Negative | 77 (13.7%) |
| Unknown | 5 (0.9%) |
| Nottingham Prognostic Index | (NPI) groups |
| Group 1 (< 2.4) | 94 (16.7%) |
| Group 2 (2.42–3.4) | 175(31.1%) |
| Group 3 (3.42–4.4) | 147 (26.1%) |
| Group 4 (4.42–5.4) | 87 (15.5%) |
| Group 5 (5.42–6.4) | 28 (5.0%) |
| Group 6 (> 6.4) | 15(2.7%) |
Tumour and treatment characteristics
The pathological features of the tumour, including Nottingham prognostic index (NPI), are summarised in Table 1. The target surgical radial margin of > 5 mm was achieved in 81.9% of patients. To achieve this, 10.9% of patients underwent re-excision operation(s) to achieve adequate margins. All patients received fractionated 40-Gy breast radiotherapy following surgery. Three hundred and forty (60.4%) patients received tumour bed boost radiotherapy. Twenty-four women (4.3%) received breast and ipsilateral supraclavicular irradiation. For adjuvant therapy, 20.2% of patients underwent chemotherapy whilst 78.5% of patients received hormonal therapy. The treatment characteristics are summarised in Table 2.
Table 2.
Treatment characteristics and clinical outcomes of patients who had breast conservation surgery
| Variables (n = 563) | |
|---|---|
| Treatment characteristics | |
| Re-excision (no. of operations) | |
| 0 | 502 (89.2%) |
| 1 | 60 (10.7%) |
| 2 | 1 (0.2%) |
| Margin achieved | |
| < 1 mm | 8(1.4%) |
| 1–2 mm | 22 (3.9%) |
| 3–4 mm | 67(11.9%) |
| ≥ 5 mm | 461 (81.9%) |
| Unknown | 5 (0.9%) |
| Radiation boost | |
| Yes | 340 (60.4%) |
| No | 223 (39.6%) |
| Chemotherapy | |
| Yes | 114(20.2%) |
| No | 442 (78.5%) |
| Unknown | 7(1.2%) |
| Hormonal therapy | |
| Yes | 442 (78.5%) |
| No | 85 (15.1%) |
| Unknown | 36 (6.4%) |
| Clinical outcomes | |
| Follow-up: median (range) | 4.8 (0.08–8.3) yrs |
| 5-year actuarial IBTR rate | 1.1% |
| 5-year crude IBTR rate | 0.9% (5 of 563) |
| 5-year actuarial DDR rate | 5.4% |
| 5-year crude DDR rate | 5.2% (29 of 563) |
| 5-year breast cancer specific survival | 95.0% |
| 5-year breast cancer specific survival by NPI groups | |
| Group 1 (excellent prognostic group) | 100% |
| Group 2 (good) | 99.2% |
| Group 3 (moderate I) | 96.1% |
| Group 4 (moderate II) | 86.6% |
| Group 5 (poor) | 88.6% |
| Group 6 (very poor) | 75.2% |
| Overall 5-year survival | 90.3% |
IBTR, ipsilateral breast tumour recurrence; DDR, distant disease recurrence; NPI, Nottingham Prognostic Index.
Treatment outcomes: IBTR and DDR rates
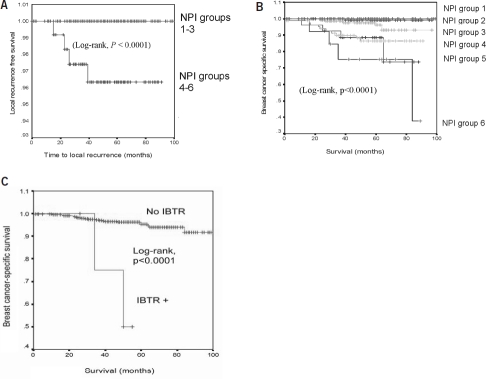
After a median follow-up of 58 months, five of the 563 (0.9%) patients developed IBTR. The 5-year actuarial IBTR rate was 1.1%. Among the five patients with IBTR, the median time to IBTR was 26 months. One out of 192 (0.52%) patients with an initial screen-detected tumour developed IBTR following BCS, whilst four out of 353 (1.13%) patients with an initial symptomatic tumour developed IBTR. The 5-year actuarial IBTR rate is 0.7% for the screen-detected group and 1.4% for the symptomatic patients. The clinico-pathological features for each IBTR case are given in Table 3. All patients with IBTR were treated by salvage mastectomy. When dichotomising the cohort into good and adverse NPI groups (i.e. NPI groups 1–3 vs NPI groups 4–6), the adverse NPI group had significantly higher IBTR rate (log rank test, P < 0.0001; Fig. 1A). Comparing the IBTR-free survival curves of the group who achieved the target margin (radial margin ≥ 5 mm) and the group who did not (< 5 mm), women with achieved surgical margin of < 5 mm were not predisposed to IBTR (log-rank test, P = 0.29; data not shown). There were only 17.3% of patients who had less than target margin as most patients had undergone re-excision for inadequate margin.
Table 3.
Local recurrences: clinical characteristics
| Age (yrs) | Presentation | Tumour type | Size (mm) | Tumour grade | No. of (+) nodes | ER status | NPI Score | Interval (mts) | Radial margin (mmm) | Re-excision | RT | RT bose | Hormone therapy | Chemotherapy | Systemic recurrence | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | Symptomatic | IDC | 15 | 2 | N/A | + | N/A | 49 | 15 | Yes | No | No | No | No | No | See below* |
| 50 | Screening | IDC | 14 | 3 | 1 | − | 5.28 | 39 | 21 | No | Yes | Yes | No | Yes | No | |
| 55 | Symptomatic | IDC | 18 | 3 | 1 | + | 5.36 | 23 | 10 | No | Yes | Yes | Yes | Yes | Yes | |
| 44 | Symptomatic | IDC | 16 | 3 | 2 | + | 5.32 | 15 | 20 | No | Yes | Yes | Yes | Yes | Yes | |
| 53 | Symptomatic | IDC | 24 | 3 | 2 | + | 5.48 | 26 | 13 | No | Yes | Yes | Yes | Yes | No |
Patient refused axillary surgery, hence NPI not applicable
IDC, infiltrating ducta carcinoma; ER, oestrogen receptor; (+) positive; (−) negative; N/A not applicable; RT, radiotherapy.
Figure 1.
(A) Kaplan–Meier analysis for local recurrence-free survival in patient cohort dichotomized into Nottingham Prognostic Index (NPI) groups using two categories: good category, NPI groups 1–3 and adverse category, NPI groups 3–6. The good NPI category had significantly lower ipsilateral breast tumour recurrence compared to the adverse NPI category as assessed using log-rank test (P = 0.0003). (B) Kaplan–Meier curves for breast cancer specific survival for individual NPI groups. The poor prognostic NPI groups were significantly associated with poorer breast cancer specific survival (log rank test, P < 0.0001). (C) Kaplan–Meier curves for breast cancer-specific survival comparing patients who had ipsilateral breast tumour recurrence (IBTR) with those who were IBTR-free. Patients who experienced IBTR had a shorter median breast cancer-specific survival time than those who were IBTR-free (50 months vs 57 months; log rank test, P < 0.0001).
Twenty-nine of the 563 (5.2%) patients had DDR (axillary and systemic), giving a 5-year actuarial DDR rate of 5.4%. Treatment outcomes are summarised in Table 2.
Treatment outcomes: breast cancer-specific and overall survival
During follow-up, 25 out of 563 women died from breast cancer. The 5-year breast cancer specific survival was 95%. Of note, patients with IBTR had a shorter breast cancer-specific survival than those without IBTR (median survival, 50 vs 57 months, log-rank P < 0.0001; Fig. 1C). Overall 5-year survival (all-cause) was 90.3%.
When separating the cohort into the six NPI groups,15 the breast-cancer specific survival curves delineated into a pattern whereby progressively worse NPI groups had lower 5-year breast cancer-specific survival rates (log-rank test, P < 0.0001; Table 2 and Fig. 1B).
Discussion
The current audit established the local recurrence rate of the Cambridge Breast Unit for women with invasive breast cancer (T1–3, N0–1, M0) treated with BCS and RT. Our findings suggest that our treatment regimen, combining a surgical radial margin of 5-mm with hypofractionated 40-Gy breast RT, is associated with a low IBTR rate. This compared favourably with the target local recurrence rate of 5% at 5 years of follow-up as proposed by BASO.12 To achieve the surgical margin, we have an acceptable re-excision rate of 10.9%.
It is commonly accepted that early IBTR after BCS is due to residual tumour cells in the breast tissue. Although surgical margin is a marker of residual disease following primary excision, the optimal margin has not been identified. Multiple studies have reviewed the impact of differing surgical margins on local recurrence rates. Studies that used target margins ranging from 1 – 5 mm reported comparable IBTR rates, varying from 0–10%.16 Our 5-year IBTR rate (1.1%), using a surgical margin of 5 mm, compares favourably with studies that used a similar target margin. Horiguchi et al.17 reported a 5-year actuarial IBTR rate of 3.4% using a 5-mm margin, whilst Schmidt-Ullrich et al.18 using the same target margin reported no IBTR in a patient cohort after a median follow-up of 60 months.
In the current audit, there was no difference in IBTR rates between patients who achieved the target margin (i.e. 5 mm) and those who did not. We could account for this by several reasons. First, the relatively small group with inadequate margins may have obscured margin status as a risk factor for IBTR. Furthermore, there were often good clinical grounds behind the decision not to re-excise in those patients who had less than target margin. One common reason is that there was no residual breast tissue at the margin of tumour cavity to re-excise, especially in tumours at the breast periphery. In such cases, the lack of an adequate margin may not affect the IBTR rate. Lastly, the surgical margin in this study is relatively generous. Hence, in patients with less than the target margin, the achieved margin appears to be adequate to prevent IBTR, particularly, when studies have shown that target margin of 1 mm may be adequate for local control.5,19 Alternatively, there was no IBTR in our patient cohort with close or involved margins suggesting that our RT regimen did eliminate any residual tumour cells.
Our RT regimen, using hypofractionated 40-Gy (15 fractions over 3 weeks), has been in use since 1999. The UK Standardisation of Breast Radiotherapy Trial (START) has shown, in a randomised trial of over 2000 patients after a median follow-up of 6 years, the rate of IBTR at 5 years was 2.0% (95% CI 1.1–2.8%) in the 40-Gy group and 3.3% (95% CI 2.2–4.4%) in the 50-Gy group.20 Furthermore, there were lower rates of adverse cosmetic effects after 40-Gy RT and this trial confirmed the efficacy of the hypofractionated RT regimen.14,20 Of note, our 5-year IBTR rate was 1.1% which compares favourably with the 40-Gy arm of the START trial and we speculate this may be in part due to our strict adherence to the surgical margin.
Interestingly, all the IBTR cases in this series had an adequate margin (i.e. > 5 mm). In these patients, despite apparent removal of all tumour cells and use of breast RT, local recurrence occurred, implying that the aggressive biology of tumour cells was probably accountable. This corroborates with findings of our review of cases of local recurrence in our audit series, whereby there is a trend that patients with local recurrence have higher NPI scores. NPI can be viewed as a surrogate marker for biological aggressiveness of the cancer.21 Taking these together, one could speculate that, in a patient cohort in whom the local therapy is adequate (i.e. adequate margin and radiotherapy), the biology of the tumour probably becomes the dominant factor in predicting IBTR. Surrogate markers of tumour aggressiveness can be obtained from conventional pathological analyses of the specimen. For instance, tumour grade, size and lymphovascular invasion have been shown to be predictive of IBTR,22 DDR23,24 and overall patient survival.25 Consistent with this, genomic studies of breast cancer specimens have identified gene expression signatures that predict biological aggressiveness and hence, local recurrence.26 Furthermore, in our cohort, NPI did clearly stratify the patients into groups with differing breast cancer-specific survivals confirming NPI as a reproducible and reliable prognosticator in breast cancer patients.15
Conclusions
This current audit suggests that our treatment regimen, combining BCS with a 5-mm target margin and hypofractionated 40-Gy RT, results in an extremely low rate of IBTR, and compares favourably with the target IBTR rate of < 5% defined by the Association of Breast Surgeons (ABS) at BASO guidelines.
Acknowledgments
S-S Liau is in receipt of the Cancer Research UK Core Skills Bursary. Prof. Gordon C Wishart receives research funding from the Cambridge National Institute of Health Research Biomedical Research Centre. The authors gratefully acknowledge the statistical assistance of Dr Chris Palmer, Centre for Applied Medical Statistics, University of Cambridge.
References
- 1.Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992;84:683–9. doi: 10.1093/jnci/84.9.683. [DOI] [PubMed] [Google Scholar]
- 2.Calle R, Vilcoq JR, Zafrani B, Vielh P, Fourquet A. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys. 1986;12:873–8. doi: 10.1016/0360-3016(86)90379-2. [DOI] [PubMed] [Google Scholar]
- 3.Fredriksson I, Liljegren G, Palm-Sjovall M, Arnesson LG, Emdin SO, et al. Risk factors for local recurrence after breast-conserving surgery. Br J Surg. 2003;90:1093–102. doi: 10.1002/bjs.4206. [DOI] [PubMed] [Google Scholar]
- 4.Leong C, Boyages J, Jayasinghe UW, Bilous M, Ung O, Chua B, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004;100:1823–32. doi: 10.1002/cncr.20153. [DOI] [PubMed] [Google Scholar]
- 5.Cabioglu N, Hunt KK, Buchholz TA, Mirza N, Singletary SE, et al. Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer. 2005;104:20–9. doi: 10.1002/cncr.21121. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, et al. Risk factors for breast recurrence in premenopausal and postmenopausal patients with ductal cancers treated by conservation therapy. Cancer. 1990;65:1867–78. doi: 10.1002/1097-0142(19900415)65:8<1867::aid-cncr2820650833>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Mirza NQ, Vlastos G, Meric F, Buchholz TA, Esnaola N, et al. Predictors of locoregional recurrence among patients with early-stage breast cancer treated with breast-conserving therapy. Ann Surg Oncol. 2002;9:256–65. doi: 10.1007/BF02573063. [DOI] [PubMed] [Google Scholar]
- 8.Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817–27. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 9.Fisher BJ, Perera FE, Cooke AL, Opeitum A, Venkatesan V, et al. Long-term follow-up of axillary node-positive breast cancer patients receiving adjuvant systemic therapy alone: patterns of recurrence. Int J Radiat Oncol Biol Phys. 1997;38:541–50. doi: 10.1016/s0360-3016(97)00001-1. [DOI] [PubMed] [Google Scholar]
- 10.Elkhuizen PH, van Slooten HJ, Clahsen PC, Hermans J, van de Velde CJ, van den Broek LC, et al. High local recurrence risk after breast-conserving therapy in node-negative premenopausal breast cancer patients is greatly reduced by one course of perioperative chemotherapy: a European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group Study. J Clin Oncol. 2000;18:1075–83. doi: 10.1200/JCO.2000.18.5.1075. [DOI] [PubMed] [Google Scholar]
- 11.Borger J, Kemperman H, Hart A, Peterse H, van Dongen J, Bartelink H. Risk factors in breast-conservation therapy. J Clin Oncol. 1994;12:653–60. doi: 10.1200/JCO.1994.12.4.653. [DOI] [PubMed] [Google Scholar]
- 12.Association of Breast Surgery. Guidelines for the management of symptomatic breast disease. Eur J Surg Oncol. 2005;31(Suppl 1):1–21. doi: 10.1016/j.ejso.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Macmillan RD, Purushotham AD, Mallon E, Love JG, George WD. Tumour bed positivity predicts outcome after breast-conserving surgery. Br J Surg. 1997;84:1559–62. [PubMed] [Google Scholar]
- 14.Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiother apy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blamey RW, Ellis IO, Pinder SE, Lee AH, Macmillan RD, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. Eur J Cancer. 2007;43:1548–55. doi: 10.1016/j.ejca.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–93. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 17.Horiguchi J, Iino Y, Takei H, Maemura M, Yokoe T, et al. Surgical margin and breast recurrence after breast-conserving therapy. Oncol Rep. 1999;6:135–8. [PubMed] [Google Scholar]
- 18.Schmidt-Ullrich R, Wazer DE, Tercilla O, Safaii H, Marchant DJ, et al. Tumor margin assessment as a guide to optimal conservation surgery and irradiation in early stage breast carcinoma. Int J Radiat Oncol Biol Phys. 1989;17:733–8. doi: 10.1016/0360-3016(89)90059-x. [DOI] [PubMed] [Google Scholar]
- 19.Schnitt SJ, Abner A, Gelman R, Connolly JL, Recht A, Duda RB, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–51. doi: 10.1002/1097-0142(19940915)74:6<1746::aid-cncr2820740617>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Dewar JA, Haviland JS, Agrawal RK, Bliss JM, Hopwood P, et al. on behalf of the START Trials centres Hypofractionation for early breast cancer: First results of the UK standardisation of breast radiotherapy (START) trials. 2007 ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007;25(Suppl) LBA518. [Google Scholar]
- 21.Miller DV, Leontovich AA, Lingle WL, Suman VJ, Mertens ML, et al. Utilizing Nottingham Prognostic Index in microarray gene expression profiling of breast carcinomas. Mod Pathol. 2004;17:756–64. doi: 10.1038/modpathol.3800114. [DOI] [PubMed] [Google Scholar]
- 22.Hanna WM, Kahn HJ, Chapman JA, Fish EB, Lickley HL, McCready DR. Pathologic characteristics of breast cancer that predict for local recurrence after lumpectomy alone. Breast J. 1999;5:105–11. doi: 10.1046/j.1524-4741.1999.00133.x. [DOI] [PubMed] [Google Scholar]
- 23.Locker AP, Ellis IO, Morgan DA, Elston CW, Mitchell A, Blamey RW. Factors influencing local recurrence after excision and radiotherapy for primary breast cancer. Br J Surg. 1989;76:890–4. doi: 10.1002/bjs.1800760906. [DOI] [PubMed] [Google Scholar]
- 24.Mansell J, Monypenny IJ, Skene AI, Abram P, Carpenter R, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2009;117:91–8. doi: 10.1007/s10549-008-0291-z. [DOI] [PubMed] [Google Scholar]
- 25.Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183–91. doi: 10.1038/modpathol.3800728. [DOI] [PubMed] [Google Scholar]
- 26.Nuyten DS, Kreike B, Hart AA, Chi JT, Sneddon JB, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8 doi: 10.1186/bcr1614. R62. [DOI] [PMC free article] [PubMed] [Google Scholar]