Abstract
The authors describe an unusual case of metastatic thyroid follicular adenocarcinoma presenting with sciatica in a 79-year-old woman. The primary thyroid tumour was undiagnosed until this clinical presentation. The patient gave a short history of back pain and right-sided sciatica, which was progressive and nocturnal in nature. Neuroimaging revealed an enhancing intradural mass lesion, which was completely excised through a right L1-L3 hemilaminectomy. Histopathological examination of the excised tissue revealed a follicular thyroid carcinoma. Subsequent metastatic investigation revealed a heterogeneously attenuating mixed solid cystic mass in a retrosternal thyroid gland, with multiple solid pulmonary nodules suggestive of metastatic disease. She opted for palliative radiotherapy for the primary thyroid cancer and made remarkable postoperative improvement. The authors conclude that surgical treatment of solitary metastatic lesion may produce good symptomatic relief irrespective of patient’s age and primary pathology, while emphasising the need for detailed clinical evaluation of patients with ‘red flag’ symptoms.
Background
Thyroid carcinomas are classified by the WHO as papillary (and variants), follicular, poorly-differentiated, undifferentiated (anaplastic) and medullary.1 Intradural metastasis to spine is an unusual presentation for follicular thyroid carcinoma, occurring more typically during the late stages of the disease and resulting from local extension in the neck or haematogenous spread of the primary tumour. In this article, we discuss the aetiopathogenesis and management of this rare condition
Case presentation
A 79-year-old lady presented with a 5-week history of severe back pain with right sciatica. Pain was of gradual onset, sharp in nature and radiating to the right hip and knee. There was associated loss of appetite and weight loss. Her pain was nocturnal, constant, exacerbated by movement and relieved by analgesia. She did not have sphincter disturbance. There were no asociated symptoms suggestive of thyroid dysfunction.
Her medical history included ischemic heart disease, osteoporosis, hypertension, stress incontinence and Meniere’s disease. She had lived independently until onset of symptoms. She did not smoke or consume alcohol. She had no history of trauma, previous infections or instrumentation in the recent process.
On examination, she had normal power in her upper limbs and left lower limb. Right lower limb power was 3+/5. Her straight leg-raising test was 45°. Reflexes were intact. Sensation was normal. There were no cranial nerve deficits. Neck examination did not reveal any abnormalities.
Her medications at presentation included aspirin 75 mg daily, rampril 5 mg daily, clopidogrel 75 mg daily, atorvastatin 20 mg nocte, oxybutynin 3 mg twice daily, imidir 30 mg daily, oromorph 40 mg twice daily and sevredol 10 mg four time daily.
Investigations
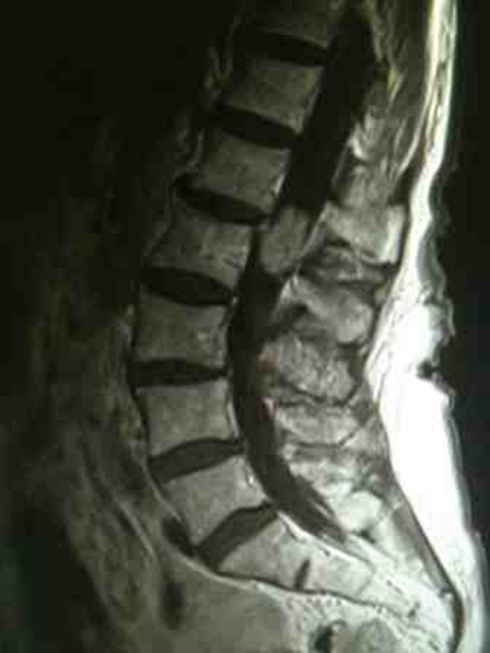
Full blood count and haematocrit were normal. Serum sodium, potassium and calcium were normal including the thyroid function tests (TFTs). Spinal x-ray showed old vertebral fractures in T11 and L1 only. Lumbar spine MRI showed a 2×2 cm extra axial intradural ovoid enhancing mass lesion compressing the cauda equina roots, with focal internal increased T2 and T1 signal in keeping with internal haemorrhage (figures 1 and 2).
Figure 1.
Sagittal post contrast MRI T1-weighted scan of the lumbar spine demonstrating the intradural lesion.
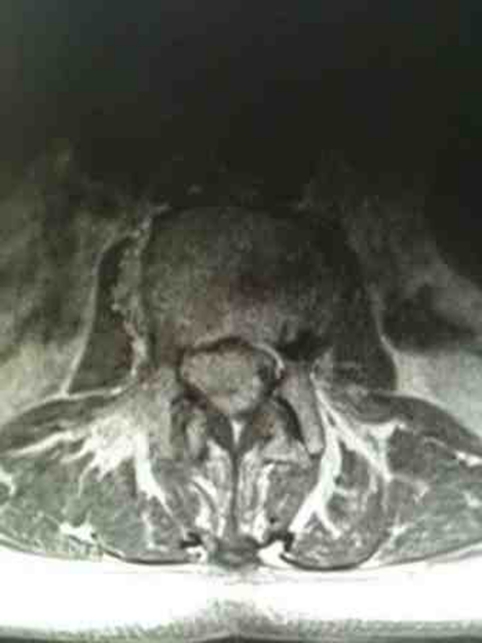
Figure 2.
MRI T1- weighted axial imaging of the lumbar spine at the corresponding level of the lesion demonstrating the enhancing intradural mass occupying the whole of spinal canal.
Differential diagnosis
Meningioma, schwannoma, neurofibroma, metastatic lesion, ependymoma or paraganglioma.
Treatment
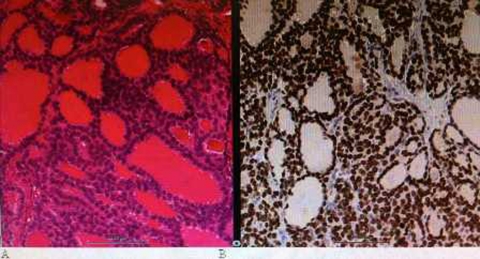
The patient was offered two options, symptomatic management in the form of pain relief and surgical excision. The patient opted for surgical treatment. Following this she underwent a right L1-L3 hemilaminectomy and complete excision of the intradural lesion. At surgery, the tumour was round, firm and vascular, anterior to the cauda equina/conus. Pathological examination of the excised tissue revealed multiple small colloid-filled glands typical of follicular thyroid carcinoma. Immunocytochemistry was strongly positive for thyroid transcription factor and epithelial markers AE1/3 (figure 3).
Figure 3.
(A) H&E stained section of tumour showing follicular cells with colloid, which demonstrated immunoreactivity for thyroid transcription factor (B).
Postoperative imaging
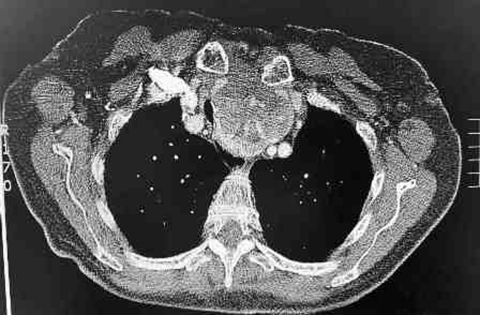
Postoperative CT thorax, abdomen and pelvis revealed multiple solid pulmonary nodules within the lung parenchyma, suspicious of metastatic deposits. There was a mixed solid-cystic mass in the thyroid gland with heterogeneous signal intensity. It contained areas of calcification, measured 6×5 cm and extended retrosternally (figure 4).
Figure 4.
CT thorax demonstrating the thyroid carcinoma with retrosternal extension.
Outcome and follow-up
The patient made a good symptomatic recovery and her sciatica completely resolved postoperatively. A repeat neck examination did not reveal any neck swelling. MRI of the whole spine did not show evidence of any other spinal metastases. She has received palliative radiotherapy to the neck for the thyroid cancer and currently remains well 6 months postoperatively.
Discussion
Overall, only 5% of patients with thyroid cancer have metastases beyond the cervical or mediastinal area on initial presentation and a spinal metastasis as the presenting feature of thyroid cancer is unusual.2–7 In the Memorial Sloan-Kettering thyroid series of 1038 patients,8 follicular cancer was found to be the most common (10.5%) to present with distant metastasizes consistent with Ruegemer et al’s9 report of 19% follicular cancer. This was found to be inconsistent with Pomoski and Bartos’s study,10 which demonstrated only 1.9% follicular, compared with 4% papillary cancer. The overall incidence of a differentiated thyroid cancer presenting with a distant metastasis was only 4%. When metastases do occur in this category, they preferentially tend to affect the lung and bone.9
Tumour metastasising to the spinal column is a common clinical occurrence but asymptomatic thyroid malignancy presenting, as sciatica in an older patient is uncommon. Compression of the spinal cord and nerve roots is the second most frequent complication of cancer, after brain metastasis.11 Spinal cord compression is the initial feature of malignancy in approximately 10% of patients with any form of malignancy.11–15
Routine TFTs are unlikely to be suggestive of thyroid cancer. Ragni et al reported an incidence of thyroid cancer16 as low as 0.3% in patients with raised TFTs. Similarly, thyroid cancers have been detected in less than 6% of hyperthyroid patients with goitres large enough to cause tracheal or oesophageal compression.17 In our case, our patient’s thyroid function was normal.
Conversely, serum thyroglobulin has been found to be useful in detecting thyroid cancers. Thyroglobulin is expressed by both normal thyroid tissue and 95% of differentiated thyroid carcinomas.18–22 Serum levels of thyroglobulin in patients with metastatic thyroid cancer have been reported to be greater than 500 ng/ml but only 13 ng/ml in normal subjects. Levels in metastatic disease were however indistinguishable from levels in patients with non-toxic nodular goitre.23 Comprehensive preoperative imaging investigations are imperative as they may reveal asymptomatic sites in the spine as well as other tissues. A combination of CT head, neck, thorax, abdomen and pelvis as well as MRI of the whole spine is required in the management.
Our patient had surgical excision of the spinal metastatic lesion, despite her age, and she made significant clinical improvement. Whole spine MRI did not reveal any other metastatic deficit. Saillant et al6 suggest the use of the most radical therapy for spinal metastasis of thyroid origin, while Sumimura et al5 describe using external radiation of 52 Gy, total thyroidectomy, 131I therapy and embolisation of lumbar arteries for the successful treatment of a thyroid cancer metastasis to the lumbar spine.
Table 1 summarises the literature review of metastatic follicular thyroid carcinoma over the past 12 years. There seems to be equivocal results with surgical and non-surgical treatment modality. The primary follicular adenocarcinoma in our case was managed non-operatively as the patient was asymptomatic and opted for symptomatic treatment.
Table 1.
Literature review of metastatic follicular thyroid carcinoma over the past 12 years
| S/N | Authors and year | Journal | Patient’s age (year) and gender | Site(s) of metastasis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | Scarrow A M et al (1999)7 | Clinical Neurology and Neurosurgery | 50, Male | C6 | Surgery and Radiotherapy | Alive |
| 2 | Izzard M et al (2006)1 | Australia and New Zealand Journal of surgery | 57, Female | L5/S1 | Radiotherapy | Alive |
| 61, Male | Multiple cervical, thoracic and sacral levels | Radiotherapy | Dead | |||
| 71, Male | T2 | Radiotherapy and decompressive surgery | Alive | |||
| 3 | Fone-Ching Hsiao et al (2008)24 | Journal of Medical Science | 55, Female | L5–S5 | Surgery | Dead |
Sciatica and low back pain is a very common clinical problem in the society and majority of the patients in geriatric age when they present with similar symptoms there is a small possibility of them being overlooked. One should be extremely cautious in eliciting the clinical history in this age group and any ‘red flag’ symptoms (new onset nocturnal back pain, sphincter disturbance and neurological deficits in the lower limbs in the form of foot drop associated with back pain and associated weight loss) should be promptly investigated. We would also like to emphasise the fact that surgical option may be beneficial providing a good quality of life in this category of patient
Learning points.
-
▶
Red flag symptoms such as nocturnal pain needs prompt neuroimaging in the form of MRI scan.
-
▶
Solitary spinal metastasis can occur with a silent primary thyroid neoplasm.
-
▶
Surgical excision is an acceptable option in the management of metastatic solitary spinal lesion and may be preferred for pain relief and preservation of function irrespective of the patient’s age.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Izzard M, McIvor N, Chaplin J, et al. Neurological deficit as a presentation of occult metastatic thyroid carcinoma. ANZ J Surg 2006;76:953–6 [DOI] [PubMed] [Google Scholar]
- 2.Vicente P, Rovirosa A, Gallego O, et al. [Spinal cord compression as a primary manifestation of occult thyroid carcinoma]. An Med Interna 1992;9:334–6 [PubMed] [Google Scholar]
- 3.Patchefsky AS, Keller IB, Mansfield CM. Solitary vertebral column metastasis from occult sclerosing carcinoma of the thyroid gland: report of a case. Am J Clin Pathol 1970;53:596–601 [DOI] [PubMed] [Google Scholar]
- 4.Goldstein SI, Kaufman D, Abati AD. Metastatic thyroid carcinoma presenting as distal spinal cord compression. Ann Otol Rhinol Laryngol 1988;97(4 Pt 1):393–6 [DOI] [PubMed] [Google Scholar]
- 5.Sumimura J, Nakagawa K, Kawamura J, et al. [Thyroid cancer metastasis to the lumbar spine successfully treated by embolization and radioiodine. A case report]. Nihon Geka Gakkai Zasshi 1990;91:910–3 [PubMed] [Google Scholar]
- 6.Saillant G, Enkaoua EA, Aimard T, et al. [Spinal metastases of thyroid origin. Apropos of a series of 37 cases]. Rev Chir Orthop Reparatrice Appar Mot 1995;81:672–81 [PubMed] [Google Scholar]
- 7.Scarrow AM, Colina JL, Levy EI, et al. Thyroid carcinoma with isolated spinal metastasis: case history and review of the literature. Clin Neurol Neurosurg 1999;101:245–8 [DOI] [PubMed] [Google Scholar]
- 8.Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg 1997;174:474–6 [DOI] [PubMed] [Google Scholar]
- 9.Ruegember J, Hay J, Bergstralh E, et al. Distant metastasis in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab 1988;67:501–8 [DOI] [PubMed] [Google Scholar]
- 10.Pomorski L, Bartos M. Metastasis as the first sign of thyroid cancer. Neoplasma 1999;46:309–12 [PubMed] [Google Scholar]
- 11.Boogerd W, van der Sande JJ. Diagnosis and treatment of spinal cord compression in malignant disease. Cancer Treat Rev 1993;19:129–50 [DOI] [PubMed] [Google Scholar]
- 12.Rodichok LD, Harper GR, Ruckdeschel JC, et al. Early diagnosis of spinal epidural metastases. Am J Med 1981;70:1181–8 [DOI] [PubMed] [Google Scholar]
- 13.Posner JB. Management of central nervous system metastases. Semin Oncol 1977;4:81–91 [PubMed] [Google Scholar]
- 14.Siegal T, Siegal T. Current considerations in the management of neoplastic spinal cord compression. Spine 1989;14:223–8 [DOI] [PubMed] [Google Scholar]
- 15.Stark RJ, Henson RA, Evans SJ. Spinal metastases. A retrospective survey from a general hospital. Brain 1982;105(Pt 1):189–213 [DOI] [PubMed] [Google Scholar]
- 16.Ragni F, Pinelli D, Facchini M, et al. [Thyroid carcinoma in hyperthyroid syndromes]. G Chir 1996;17:158–65 [PubMed] [Google Scholar]
- 17.Zanella E, Rulli F, Muzi M, et al. Prevalence of thyroid cancer in hyperthyroid patients treated by surgery. World J Surg 1998;22:473–7; discussion 477–8 [DOI] [PubMed] [Google Scholar]
- 18.Lo Gerfo P, Li Volsi V, Colacchio D, et al. Thyroglobulin production by thyroid cancers. J Surg Res 1978;24:1–6 [DOI] [PubMed] [Google Scholar]
- 19.Valenta LJ. Thyroid peroxidase, thyroglobulin, cAMP and DNA in human thyroid. J Clin Endocrinol Metab 1976;43:466–9 [DOI] [PubMed] [Google Scholar]
- 20.Valenta LJ, Kynel F, Niederle B. Soluble proteins in thyroid neoplasia. J Clin Endocrinol Metab 1968;22:442–50 [DOI] [PubMed] [Google Scholar]
- 21.Valenta L, Lemarchand-Béraud T. Thyroglobulin and thyroid acid protease activity in thyroid disease. J Clin Endocrinol Metab 1970;31:422–7 [DOI] [PubMed] [Google Scholar]
- 22.van den Hove-Vandenbroucke MF, De Visscher M, Couvreur-Eppe M. Secretory activity of isolated thyroid adenomas. J Clin Endocrinol Metab 1976;43:178–81 [DOI] [PubMed] [Google Scholar]
- 23.Gerfo PL, Colacchio T, Colacchio D, et al. Thyroglobulin in benign and malignant thyroid disease. JAMA 1979;241:923–4 [PubMed] [Google Scholar]
- 24.Hsiao FC, Chen CL, Lin TY, et al. Metastatic spinal cord compression as an initaial presentation of occult follicular thyroid carcinoma. J Med Sci 2008;28:089–94 [Google Scholar]