Abstract
Melanomas display poor response rates to adjuvant therapies because of their intrinsic resistance to proapoptotic stimuli. This study indicates that such resistance can be overcome, at least partly, through the targeting of eEF1A elongation factor with narciclasine, an Amaryllidaceae isocarbostyril controlling plant growth. Narciclasine displays IC50 growth inhibitory values between 30–100 nM in melanoma cell lines, irrespective of their levels of resistance to proapoptotic stimuli. Normal noncancerous cell lines are much less affected. At nontoxic doses, narciclasine also significantly improves (P=0.004) the survival of mice bearing metastatic apoptosis-resistant melanoma xenografts in their brain. The eEF1A targeting with narciclasine (50 nM) leads to 1) marked actin cytoskeleton disorganization, resulting in cytokinesis impairment, and 2) protein synthesis impairment (elongation and initiation steps), whereas apoptosis is induced at higher doses only (≥200 nM). In addition to molecular docking validation and identification of potential binding sites, we biochemically confirmed that narciclasine directly binds to human recombinant and yeast-purified eEF1A in a nanomolar range, but not to actin or elongation factor 2, and that 5 nM narciclasine is sufficient to impair eEF1A-related actin bundling activity. eEF1A is thus a potential target to combat melanomas regardless of their apoptosis-sensitivity, and this finding reconciles the pleiotropic cytostatic of narciclasine.—Van Goietsenoven, G., Hutton, J., Becker, J.-P., Lallemand, B., Robert, F., Lefranc, F., Pirker, C., Vandenbussche, G., Van Antwerpen, P., Evidente, A., Berger, W., Prévost, M., Pelletier, J., Kiss, R., Goss Kinzy, T., Kornienko, A., Mathieu, V. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas.
Keywords: narciclasine, growth inhibition, apoptosis resistance, actin
The incidence of melanoma is steadily increasing among the Caucasian population and affects 1 in 55 cancer patients in the United States (1). One-third of early stage melanoma patients will develop metastases, and metastatic melanomas are associated with dismal prognosis. Such patients have a median overall survival of 6 to 8 months despite significant efforts to develop adjuvant therapies (1). The response rate to the standard FDA-approved treatment dacarbazine, administered as a single agent therapy, is as low as 16%. The response rates to other cytotoxic drugs, such as cisplatin, nitrosurea, vinca alkaloids, or temozolomide, given alone or in combination, are even lower (1, 2). Significantly, combination treatments with IL-2 or IFNα2b improve the response rate without any impact on the overall survival of metastatic melanoma patients (1).
These highly disappointing statistics are likely related to the resistance of melanoma cells to apoptosis. Indeed, while in many other cancer types chemoresistance is acquired from radiation and chemotherapy, melanomas belong to cancer types that are intrinsically resistant to apoptosis (3). This feature has been attributed to numerous molecular changes in proliferation, survival, and death signaling pathways (3–6), including death receptor signaling impairment (5), activation of proliferative and survival pathways Raf/MAPK, PI3K/Akt and NF-κB pathways (3, 4, 6), and modifications to the mitochondrial apoptosis regulating proteins including IAPs and Apaf-1 (3, 4, 6). Actin cytoskeleton and associated proteins have been shown more recently to induce and participate in mitochondrial apoptosis via pathways that are yet to be fully deciphered (7–9). The targeting of actin cytoskeleton is an approach that has not received its due attention (10), despite its significant potential to affect both cell proliferation (cytokinesis) and migration. eEF1A is an abundant evolutionarily conserved protein that binds to and delivers aa-tRNA to the empty A site of elongating ribosomes. However, it has been shown more recently that >60% of eEF1A can be bound to fibrillar actin, promoting bundling and inhibiting both polymerization and depolymerization. Therefore, eEF1A plays critical roles in actin cytoskeleton organization and functions involved in cell migration, cell morphology, protein synthesis, and cell death (11–13). Targeting eEF1A could thus be a promising strategy to combat apoptosis-resistant cancers and melanomas in particular.
For >2000 yr, plants belonging to the Amaryllidaceae family have been used in traditional medicine for various anticancer applications. Over 100 alkaloids and isocarbostyrils, exhibiting diverse biological activities, have been recently isolated from various Amaryllidaceae species (14). Lycorine was the first alkaloid isolated from these plants (15) and found to possess anticancer activities both in vitro and in vivo (16–18). More recently, several isocarbostyril constituents of the Amaryllidaceae, such as narciclasine, pancratistatin, trans-dihydronarciclasine, and their congeners (for structure examples, see Fig. 1), have been isolated or semisynthesized (19, 20). Narciclasine, known for >40 yr, is isolated from narcissus bulbs and serves as an inhibitor of plant growth (21), which was first characterized for its antimitotic effects (22). Pancratistatin, whose chemical structure is similar to that of narciclasine, induces rapid apoptosis in neuroblastoma cells but not in normal cells. The apoptosis induction has been shown to occur via mitochondrial targeting (23), which could further synergize with other anticancer agents, such as tamoxifen (24). Comparable apoptosis induction has been also described for narciclasine in breast cancer cells (25). Although it has been shown that the Amaryllidaceae isocarbostyrils (AIs) inhibit peptide bond formation step and thus protein synthesis (26, 27), recent data obtained in glioblastoma and prostate cancer cells indicate that narciclasine could also target actin cytoskeleton and related proteins (28, 29). The present study demonstrates that eEF1A is a target for the AIs at their growth inhibitory IC50 values, reconciling their effects on protein synthesis and actin cytoskeleton. Thus, using AIs as a chemical tool, we demonstrated that eEF1A targeting represents a useful strategy to bypass apoptosis resistance of melanoma cells. Conceivably, eEF1A targeting also occurs in plants, since the observed effects in plants are similar, and eEF1A is a highly conserved protein in the biological kingdom.
Figure 1.
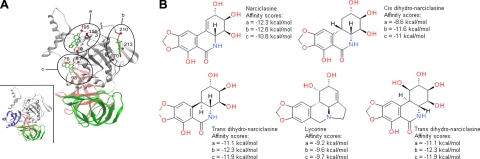
Potential binding sites for the Amaryllidaceae isocarbostyrils on eEF1A protein. A) Three potential binding pockets of narciclasine were found in the 3-D structure of yeast eEF1A in independent docking experiments. They are shown here on the same structure. Pocket a corresponds to the GTP binding site. Pocket c is located in the binding region of the nucleotide exchange factor. The 3 eEF1A domains are colored in gray, red, and green. Positions of residues lining each binding pocket are indicated as red spheres, and their numbers are indicated. Inset: same view of eEF1A structure in association with the nucleotide exchange factor (blue). B) Molecular structures of various AIs and alkaloid lycorine, together with their theoretical affinity scores, calculated for all compounds in each binding site.
MATERIALS AND METHODS
Molecular docking
Docking was performed using the Glide 4.5 software (Schrodinger LLC, Mannheim, Germany) (30), which positions the 3-D structure of flexible ligands in the 3-D structure of rigid or partially flexible receptors. First, docking runs were performed to explore the whole surface of the rigid protein exhaustively. From this exploration step, the locations of the AIs with the best affinity scores were examined to select their potential binding regions on the protein surface. In the second step, induced fit docking (30) experiments were performed in these putative binding regions to account for protein conformational changes induced by the presence of the ligands. For each docking run, the binding zones were defined as cubes with a length of 20 Å. The initial 3-D structures of the ligands were generated with the CORINA program (31).
Narciclasine binding to eEF1A
Human eEF1A isoform 2 recombinant protein was purchased from Novus Biologicals (Littleton, CO, USA), while endogenous yeast eEF1A was prepared as described previously (32). Various concentrations of narciclasine were incubated in phosphate buffer with 100 nM of eEF1A protein overnight at 37°C. The samples were centrifuged through 5-kDa filters (Sartorius, Vilvoorde, Belgium) at 14,000 rpm for 15 min to retain the proteins with bound narciclasine. The concentrations of unbound narciclasine in the ultrafiltrates were measured by fluorescence (excitation: 360 nm; emission: 480 nm) at pH 12 and compared to a standard curve of narciclasine-filtered solutions.
The cell assay was performed as follows: 2 melanoma cell lines, VM-21 and VM-48 (see below), were treated for 16 h with narciclasine at 50 and 100 nM. Cells were collected in lysis buffer (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% Triton X-100; and 1 mM EDTA). The totality of each sample was loaded in a 96-well plate coated with 3 μg/ml of an anti-eEF1A antibody (Millipore, Brussels, Belgium) and incubated for 2 h with agitation at room temperature. After 4 washes with lysis buffer, eEF1A-bound narciclasine was released by methanol and measured using the fluorescent technique described above. Each assay was performed in triplicate.
Cell cultures and compounds
The human SKMEL-28 (ATCC HTB-72) and the mouse B16F10 (ATCC CRL-6475) melanoma cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in our laboratory as detailed previously (33). Primary melanoma cell cultures (VM-21, VM-47, and VM-48) were established at the Institute of Cancer Research, Medical University Vienna, as described previously (33, 34). Their melanocyte origin was confirmed by the presence of melanosomes in electron microscopy, by immunohistochemical S100 and HMB-45 stainings, and by melanin and tyrosinase determination. Temozolomide (TMZ) was purchased from Schering Plough (Brussels, Belgium). Narciclasine was extracted from Narcissus bulbs as described previously (35).
In vitro overall growth determination
Overall cell growth was assessed using the 3-[4,5-dimethylthiazol-2yl]-diphenyltetrazolium bromide (MTT) colorimetric assay (Sigma, Bornem, Belgium), as detailed elsewhere (29, 33). All determinations were performed in sextuplicate.
Determination of apoptosis by flow cytometry
Detection of apoptosis was performed by flow cytometric analysis of double-staining with propidium iodide and annexin-V FITC as detailed elsewhere (25, 33) using the APO AF apoptosis detection kit (Sigma).
Alteration of the outside mitochondrial membrane potential was monitored with the fluorescent dye JC-1 (Calbiochem, Nottingham, UK) as described by Dumont et al. (25). The dye forms red fluorescent aggregates inside the mitochondria, while its monomeric cytoplasmic form fluoresces green (25). Mitochondrial outside membrane permeabilization (MOMP) is therefore characterized by a shift from red to green fluorescence (25). Fluorescence was analyzed immediately after the staining procedures on an Epics XL MCL flow cytometer (Beckman Coulter, Fullerton, CA, USA). All experiments were conducted in triplicate.
Computer-assisted phase-contrast microscopy
Quantitative videomicroscopy was performed as detailed previously (28). Each experiment was conducted in triplicate.
Actin fluorescent staining
Actin cytoskeleton organization was visualized in formol-fixed cells using fluorescent probes with high affinities for either fibrillar actin (Alexa Fluor 488-conjugated phallacidin; Molecular Probes; Invitrogen, Merelbeke, Belgium) or globular actin (Alexa Fluor 594-conjugated DNase I; Molecular Probes), as detailed elsewhere (28).
Actin bundling
Evaluation of eEF1A bundling of F-actin was assayed by light scattering. The effect of narciclasine on the ability of purified rabbit eEF1A to bundle F-actin was analyzed using right angle light scattering as described by Liu et al. (36). Briefly, G-actin purified from S. cerevisiae was allowed to polymerize for 2 h at 25°C. A FluoroMax-3 fluorescence spectrophotometer (Horiba Jobin Yvon, Longjumeau, France) was used with 600-nm emission/excitation wavelengths combined with a slit width of 5 nm. Prior to eEF1A addition baseline, readings of buffer and F-actin alone were obtained. Data were collected and analyzed using FluorEssence (Horiba Jobin Yvon).
Protein synthesis assays
Polysome organization analysis was conducted as described by Pelletier et al. (37). Briefly, cells left untreated (negative control), treated with puromycin (100 μg/ml; positive control) or narciclasine (50 or 100 nM) were collected in PBS and centrifuged. The cell pellets were resuspended in hypotonic lysis buffer. After centrifugation, the supernatants were loaded onto 10–50% sucrose gradients and centrifuged in a SW 60 Ti rotor (Beckman) at 39,000 rpm for 2 h. Gradients were divided into 33 fractions for absorbance measurement at 254 nm.
Protein elongation assay was conducted using the Click-iT AHA kit following the manufacturer's instructions (Invitrogen). Cells were treated with puromycin (100 μg/ml; positive control) or narciclasine (50 nM or 100 nM) for 5 and 24 h. After 1 h incubation in a methionine-free medium (Invitrogen), the cells were incubated 5 h with methionine-free medium supplemented with 100 μM l-azidohomoalanine. Cells were harvested after 30 min incubation in a lysis buffer and centrifuged 5 min at 14,000 g at 4°C. Azide/alkyne reaction was performed on 200 μg of azide-labeled protein by addition of CuSO4 in the reaction buffer to obtain biotinylated triazole conjugates. Proteins were precipitated and resolubilized in the loading buffer for further electrophoretic analysis.
In vivo orthotopic xenografts
Human melanoma brain metastatic cells (106; VM-48) were stereotactically implanted into the brains of nude mice (6-wk-old female nu/nu mice; 21–23 g; Iffa Credo; Charles River, Arbresle, France) as described previously (33). Each experimental group contained 11 mice. This experiment was performed on the basis of Authorization LA1230509 of the Animal Ethics Committee of the Federal Department of Health, Nutritional Safety and the Environment (Belgium).
RESULTS
Docking of the AIs to the yeast eEF1A
The known effects of altered eEF1A activities closely resemble those induced by AIs. Therefore, we hypothesized that the latter could target this elongation factor. Docking experiments revealed that AIs are indeed potential eEF1A ligands. Three binding pockets were found in independent docking experiments conducted with the crystallographic structure of the yeast eEF1A (PDB code 1G7C) (Fig. 1A). One of the pockets corresponds to the GTP binding site (Fig. 1A, pocket a) and another to the binding region of the nucleotide exchange factor eEF1Bα (Fig. 1A, pocket c). The molecular structures of the docked AIs and alkaloid lycorine are presented in Fig. 1B, together with their binding free energy score in each pocket. Narciclasine features affinity scores ranging from −12.3 to −10.8 kcal/mol. For comparison, the GDPNP, an analog of the natural ligand GTP, has a free energy score of −9.8 kcal/mol (Fig. 1). Lycorine, characterized by higher IC50 growth inhibitory values than narciclasine and pancratistatin, (i.e., ∼5000 vs. ∼50–100 nM; refs. 11, 21; see Fig. 4A), also displays weaker affinity to eEF1A in this theoretical model (Fig. 1).
Figure 4.
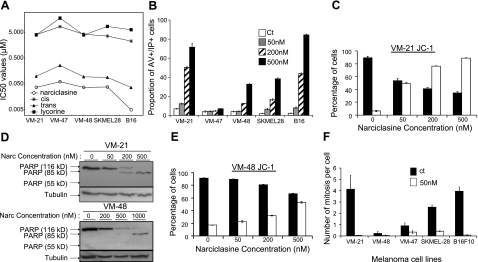
AI-induced effects on melanoma cell death and proliferation. A) Mean IC50 values obtained with 5 cell lines for 4 AIs by means of the colorimetric MTT test. B) Percentage of cells in a late apoptotic status (double-positive cells for propidium and annexin V stainings analyzed by flow cytometry) when treated with various concentrations of narciclasine. C, E) Flow cytometric analysis of JC-1 staining. Open columns represent green fluorescence of the dye; solid columns represent red fluorescence. D) Western blot analysis of PARP cleavage: PARP full protein (116 kDa) is cleaved during apoptosis in fragments of 85 kDa, while necrotic fragments are of 55 kDa. Tubulin blots assess equal loading and protein integrity. F) Mitosis number per cell over a 72-h period was counted in 5 melanoma cell lines by the videomicroscopic device. Solid bars, control; open bars, 50 nM narciclasine. Results are expressed as means ± se.
eEF1A is a target for AIs
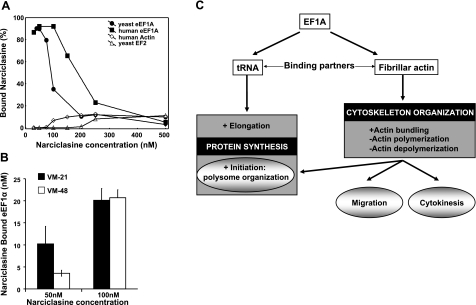
To validate these modeling results, we incubated 100 nM human eEF1A recombinant protein (commercial isoform 2 coupled with GST) and authentic yeast eEF1A with narciclasine at 37°C at various concentrations in vitro. The percentage of bound narciclasine was calculated based on the amount of unbound narciclasine measured by fluorescence. Figure 2A indicates that human eEF1A protein binds nearly all narciclasine molecules until equal molarity between the ligand and its target is reached. At higher narciclasine concentrations, the binding appears to be saturated. The assay was also conducted with other potential binding partners; e.g., human actin (37°C) and yeast elongation factor 2 (EF2; 30°C), but no significant binding could be detected (Fig. 2A). Similarly, narciclasine did not bind to GST alone (internal control, data not shown). The binding affinity for the eEF1A yeast isoform is lower than that for its human counterpart; however, this protein was not coupled with GST, and thus its conformation could be unstable. Moreover, these proteins are only 80% identical, which may account for differences in binding as well. Two melanoma cell lines were treated with narciclasine at 50 and 100 nM for 16 h. Measurement of eEF1A-bound narciclasine after immunoimmobilization of the protein confirmed that narciclasine penetrates melanoma cells and binds to eEF1A intracellularly (Fig. 2B).
Figure 2.
eEF1As is a target for the AIs. A) Narciclasine binding to eEF1A, actin and EF-2 in vitro: various concentrations of narciclasine were incubated overnight with recombinant yeast eEF1A or human eEF1A isoform 2, human actin, and yeast eEF-2. Bound fraction of narciclasine (expressed as percentage) is calculated on the basis of free narciclasine measured by fluorescence. B) Narciclasine binding to eEF1A in mammalian melanoma cells. C) Summary of the various roles of eEF1A in protein synthesis and actin cytoskeleton organization.
No evidence indicated that narciclasine competes with the GDP binding in pocket a, at least up to concentrations of 250 nM of narciclasine (GDP displays a calculated Kd value of 300 nM; data not shown). This finding suggests that the GDP binding site is not where narciclasine binds eEF1A. Further ongoing tests involving mutational studies and/or crystallography are required to determine more precisely the binding site of narciclasine to eEF1A.
AIs affect actin cytoskeleton organization and impair protein synthesis at both initiation and elongation steps
The main eEF1A activities are summarized in Fig. 2C. Ample data have shown that eEF1A modulates actin cytoskeleton by stimulating actin bundling as well as altering polymerization (12, 13). Narciclasine markedly impairs eEF1A-mediated actin bundling at doses as low as 5 nM (Fig. 3A). Actin bundling impairment has been confirmed further via actin cosedimentation assays (data not shown). Narciclasine has been shown to interfere with actin cytoskeleton in glioblastoma and pancreatic cancer cells (28, 29). We therefore analyzed the effects of narciclasine on actin cytoskeleton in apoptosis-sensitive VM-21 (Fig. 4B) and apoptosis-resistant VM-48 (Fig. 4B) human melanoma cells by fluorescent staining. After incubation with 50 nM narciclasine for 15 min, VM-21 cells displayed increased fibrillar actin, whereas these effects occurred only after 3 h incubation in VM-48 cells (Fig. 3B).
Figure 3.
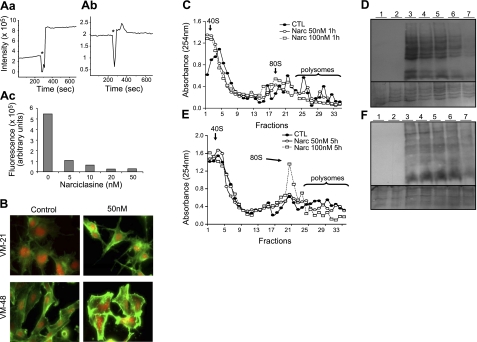
Narciclasine-induced effects on actin cytoskeleton and protein synthesis. A) Right-angle light scattering was measured for 1.5 μM of preassembled F-actin. Then 0.5 μM rabbit eEF1A was added in the presence of 0–50 nM narciclasine, and scattering was measured for another 400 s. a, b) Representative graphs at 0 nM (a) and 20 nM narciclasine (b). Asterisk indicates emission shutter closed, eEF1A ± narciclasine added. c) Increased fluorescence intensity on addition of eEF1A to F-actin is reduced in the presence of narciclasine. B) Narciclasine-induced effects on the actin cytoskeleton of VM-21 and VM-48 human melanoma cells treated with 50 nM (IC50 value) narciclasine for 15 min and 3 h, respectively, highlighted by fluorescence. Fibrillar actin is represented in green and globular actin in red. C, E) Spectrometry analysis of polyribosome status in VM-21 (C) and VM-48 (E) human melanoma cells treated with 0 nM (solid circles), 50 nM (open circles), and 100 nM narciclasine (open squares) for 1 h (C) and 5 h (E), of cell extracts centrifuged in sucrose gradients and separated into 33 fractions. D, F) Protein synthesis in VM-21 (D) and VM-48 (F) human melanoma cells left untreated or treated with 50 or 100 nM of narciclasine for 5 and 24 h. After treatment, cells were incubated with a methionine analog that was incorporated in nascent proteins and further biotinylated. Lane 1: cells incubated with the methionine analog alone (negative control). Lanes 2–7: cells incubated for 5 h with the methionine analog. Lane 2: cells treated with 100 μg/ml puromycin for 2 h (positive control). Lane 3: untreated cells. Lanes 4 and 5: cells treated for 5 h with 50 nM and 100 nM narciclasine, respectively. Lanes 6 and 7: cells treated for 24 h with 50 nM and 100 nM narciclasine, respectively. Bottom panels correspond to the Coomassie blue staining of the membranes assessing equal total (labeled and nonlabeled) protein loading and integrity.
eEF1A is essential for maintaining the translational status of the cell, while specific mutants of eEF1A separate this canonical activity from the role of the protein in actin organization (38). Furthermore, the intact cytoskeleton has been shown to be essential for appropriate polysome organization in an eEF1A-dependent manner (38). Figure 3C, E clearly shows that narciclasine induces dose-dependent modifications of polysome organization: When cells were treated with narciclasine at 50 or 100 nM, polysomes were reduced in favor of 80S ribosome complexes (VM-48 cells, 5h of treatment; Fig. 3E) and 40S ribosomes (VM-21 cells, 1 h of treatment; Fig. 3C). Narciclasine-induced actin cytoskeleton disorganization, demonstrated in the present study, occurred earlier and could therefore lead to impairment of cytokinesis as well as polysome disorganization, which could in turn impair protein synthesis at the initiation step, as previously reported (38).
Effects of narciclasine on the elongation step of protein synthesis were evaluated by the incorporation of l-azidohomoalanine, a methionine analog, in nascent proteins of VM-21 and VM-48 melanoma models. Figure 3D, F shows that narciclasine at 50 and 100 nM induces a dose-dependent decrease in newly synthesized protein in VM-21 cells and VM-48 cells, occurring several hours after the disorganization of polysomal structures. However, the effects were more marked (50 vs. 100 nM) and occurred earlier in VM-21 cells than in VM-48 cells (5 vs. 24 h, respectively). The mechanisms underlying these effects can be associated with eEF1A-dependent actin organization and altered initiation (38), as well as a direct inhibition of peptide bond formation, as described by Carrasco et al. (26). These results are consistent with our hypothesis of eEF1A as a target for AIs because eEF1A is an elongation factor that delivers aa-tRNA to the ribosomes.
Antitumor activities of AIs are mainly related to their cytostatic rather than proapoptotic effects
Three AIs, narciclasine and cis- and trans-dihydronarciclasine, as well as alkaloid lycorine, were evaluated for their in vitro growth inhibitory activities by means of the colorimetric MTT test. Figure 4A illustrates the IC50 values obtained on 5 melanoma cell lines, whose sensitivities to the compounds were found to be similar. Narciclasine exerted the most potent anticancer activities, with IC50 ∼ 40 nM, which is comparable to the NCI data (NCI database).
Induction of apoptosis was studied by means of double-annexin V and propidium iodide staining. Figure 4B shows that narciclasine induces typical late apoptotic changes after 72 h of treatment in a dose-dependent manner in 4 of the 5 melanoma models investigated. These results were confirmed further by a Western blot analysis of PARP (poly-ADP-ribose polymerase) cleavage in VM-21 cells (displaying apoptotic features at 200 and 500 nM) and VM-48 cells (resistant to apoptotic induction in Fig. 4B at doses <500 nM; Fig. 4D). Intact PARP expression (116 kDa) decreased while the apoptotic fragments (83–89 kDa) increased in a dose-dependent manner from 200 to 500 nM in VM-21 cells; nevertheless this effect was observed only at higher doses (from 500 to 1000 nM) in VM-48 cells (Fig. 4D). These events have been related further to the changes in mitochondrial outside membrane permeabilization, analyzed by flow cytometry with JC-1 dye: Fig. 4C, E shows a dose-dependent increase in green fluorescence paralleled by a dose-dependent decrease in red fluorescence in VM-21; these features were much less pronounced for VM-48 treated cells. However, VM-21 and VM-48 display similar IC50 values (∼40 nM), not high enough to induce apoptosis even in the apoptosis-sensitive VM-21 model (Fig. 4B). We then investigated by means of quantitative videomicroscopy whether narciclasine induces cytotoxic vs. cytostatic effects when exerting growth inhibition of human melanoma cells. This experimental approach enabled us to quantify the number of mitoses during the 72 h of the experiment: It was found that narciclasine clearly impairs proliferation at 50 nM (Fig. 4F), without inducing death of melanoma cells during this 72 h-period of observation (data not shown).
In vivo therapeutic benefits of eEF1A targeting by narciclasine in an apoptosis-resistant brain metastatic melanoma orthotopic model in immunocompromized mice
Pharmacokinetic studies performed previously in mice revealed that high doses of narciclasine that can induce cancer cell death (200–500 nM) could not be reached in plasma due to toxicity effects (29). However, narciclasine is bioavailable either via intravenous or oral routes, and safe repeated administrations of 1 mg/kg of narciclasine lead to plasmatic concentrations close to the in vitro IC50 value (∼40 nM; ref. 29). These concentrations were sufficient to provide significant therapeutic benefits in the case of primary and secondary brain tumors (28, 29).
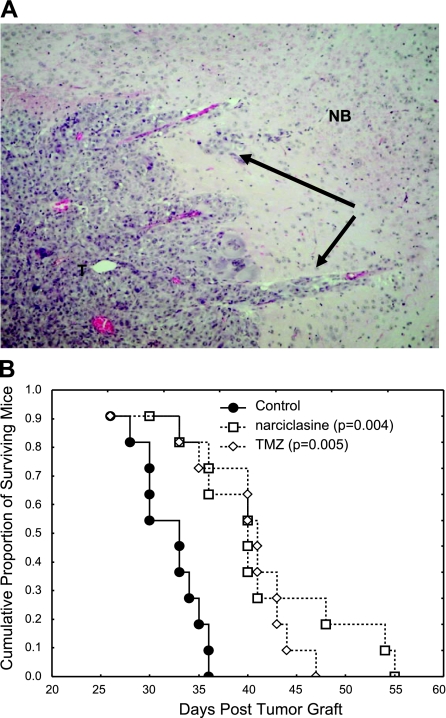
We grafted human brain metastatic and apoptosis-resistant melanoma cells (VM-48) directly into the brains of immunocompromized mice to mimic melanoma brain metastasis (Fig. 5A). The mice were treated with narciclasine or the drug temozolomide, chosen as a reference. Kaplan-Meier survival analyses revealed that narciclasine provided significant therapeutic benefits in this aggressive melanoma model and that its effects were similar to temozolomide (Fig. 5B).
Figure 5.
Narciclasine improves the survival of mice bearing brain melanoma metastatic xenografts. A) Typical hematoxylin eosin staining of a tumor that developed in the brain of a nude mouse 1 mo after the stereotactical graft of human melanoma brain metastatic cells (×50). T, tumor; NB, normal brain tissue; black arrows indicate invasive islets). B) Kaplan-Meier survival analysis of the tumor-bearing mice left untreated, treated with narciclasine (1 mg/kg p.o.; 2 administrations/wk for 3 wk) or with temozolomide (TMZ, 40 mg/kg p.o.; 3 administrations/wk for 3 wk).
DISCUSSION
The connection between actin cytoskeleton and protein synthesis has become more and more evident in recent years: While microtubules link to mRNA in large cell types, such as oocytes, neurons, or oligodendrocytes, for transport to specific locations, actin cytoskeleton plays this role in smaller, and most somatic, cell types (39, 40). Association of mRNA and/or polysomes with actin cytoskeleton has been evidenced by fractionation experiments; elongation factors, such as EF-2, colocalize with polysomes and actin fibers (39). Mutagenic studies of elongation factors, in particular eEF1A, which binds actin or aa-tRNA, have been recently conducted in yeast (38). The results clearly indicate that improper actin cytoskeleton organization leads to translation impairment, while elongation inhibition is not sufficient to affect cytoskeleton organization (38). Actin disruption, through modifications of actin-binding proteins, actin itself, or elongation factor interactions, does not affect protein translation at the elongation step, but rather does so at the initiation step, as it induces changes in the ratio of 80S free ribosomes and polysome translation complexes (38). The present study validates this hypothesis in mammalian cells as evidenced by the effects of narciclasine on actin cytoskeleton (Fig. 3), polysome organization, and protein synthesis (Fig. 3). These effects could occur, at least partly, due to the interaction of narciclasine (and likely other AIs) with eEF1A (Fig. 2C). Targeting this protein seems, therefore, to be a promising strategy to bypass the intrinsic resistance of melanoma cells to apoptosis (3). Indeed, the AIs used in the current study displayed potent cytostatic effects toward melanoma cells when used at their respective IC50 growth inhibitory values, with similar efficacy between apoptosis-resistant and sensitive melanoma cell lines. Narciclasine provided significant in vivo therapeutic benefits in an orthotopic brain model related to apoptotis-resistant melanoma brain metastasis (Fig. 5). Narciclasine induced apoptotic features in melanoma cells, but at nonpharmacological doses; i.e., doses that ranged between 5× and 10× the IC50 growth inhibitory values, leading to mitochondria-dependent apoptosis (Fig. 4). At the intracellular level, these events could be related partly to their prominent effects on actin cytoskeleton. Indeed, the actin-severing protein cofilin, shown previously to be inactivated by narciclasine resulting in increased fibrillar actin (27), has been demonstrated to translocate to mitochondria under apoptotic stimuli and to play crucial roles in the induction of apoptosis (8). CAP-1, a cofilin binding protein, also translocates to mitochondria and appears to assist cofilin in its proapoptotic functions (41). For both proteins, their actin-binding domains are required to exert their proapoptotic functions: actin could be the effector, since actin fragments are involved in spontaneous mitochondrial apoptosis induction (9). While eEF1A is involved profoundly in actin cytoskeleton organization (13) and eEF1A expression level has been linked to modulation of apoptosis-sensitivity (42), the proapoptotic effects of narciclasine cannot be directly and exclusively related to eEF1A targeting because the saturation of this protein with this compound occurs at doses of 100 nM (Fig. 2A), which are in turn not high enough to induce apoptosis (Fig. 4). Therefore, when used at cytotoxic doses, narciclasine (and perhaps AIs in general), probably interacts with other lower affinity targets, which are not necessarily the same for all these natural products. Indeed, correlations of the differential cellular sensitivities to these compounds in the NCI 60-cell line screen indicate that the AIs exert a uniform mode of action at cytostatic concentrations; i.e., at their mean GI50 values characteristic of growth inhibition (e.g., the correlation coefficient of >0.7 for 15 nM trans-dihydronarciclasine and 41 nM pancratistatin, Fig. 6). However, the uniformity of the mechanism among these compounds disappears if the correlation is performed at their mean LC50 values, characteristic of cell death (e.g., the correlation coefficient of 0.12 for 5.9 μM trans-dihydronarciclasine and 23.3μM pancratistatin). In addition, differential cellular sensitivity profiles associated with these compounds at their GI50 do not match with any other compounds in the NCI database, emphasizing the unique and new mode of action for AIs in general, and narciclasine in particular. The present study demonstrates that the new mode of action is related to the targeting of eEF1A, and this finding might constitute an important new strategy to combat apoptosis resistant melanomas. In addition, the selective growth inhibitory activities of AIs toward cancer cells as compared to their normal counterparts (23, 25, 29) can be explained by the overexpression of eEF1A in cancer cells, which has been demonstrated in the case of ovarian (43), breast (44), lung (45), and liver cancers (46) with respect to its isoform eEF1A-2. This marks eEF1A as a putative oncogene. In addition, selectivity can stem from the pH difference between cancer and normal cells (47). Indeed, binding of eEF1A to aa-tRNA or to actin could be tuned by the local pH level (36). The affinity of the AIs for eEF1A could thus also be dependent on the pH.
Figure 6.
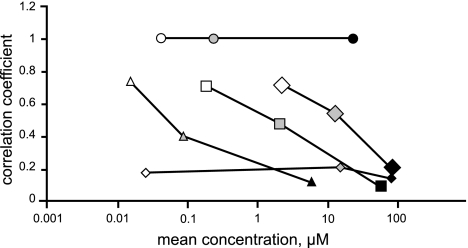
Correlations of the differential cellular sensitivities in the NCI 60 cell line screen using the COMPARE algorithm: compare correlation coefficients (CCCs) were generated by a computerized pattern-recognition algorithm and serve as an indication of similarities in differential cellular sensitivities or characteristic fingerprints for each compound. Pancratistatin (circles), trans-dihydronarciclasine (triangles), 7-deoxynarciclasine (squares), cis-dihydronarciclasine (large diamonds), and paclitaxel (small diamonds) were each used as a seed to find significant correlations with the anticancer agents in the NCI Standard Compound Database, containing pancratistatin as a representative of the AIs, at the GI50, TGI, and LC50 levels (for definitions of these parameters, see DTP human tumor cell line screen; http://dtp.nci.nih.gov/branches/btb/ivclsp.html). At the GI50 level, all correlations, with the exception of paclitaxel identified pancratistatin, ranked first among all the compounds in the database with CCC > 7 (open markers). Correlations with pancratistatin at the TGI (shaded markers) and LC50 levels (solid markers) were significantly inferior and virtually nonexistent, respectively (CCC 0.4–0.5 and 0.1–0.2). Correlations of paclitaxel with pancratistatin were poor and similar to the rest of the seed compounds at the LC50 level.
eEF1A is a large protein composed of 3 domains, and it displays considerable flexibility (48). While the nucleotide (either GTP or GDP) binding pocket is well characterized in both bacterial EF-Tu and eukaryotic eEF1A, the structure of the aa-tRNA-bound form is known only in prokaryotes. The binding sites for fibrillar actin seem to be even more complex (32, 36). These two binding sites, as well as for eEF1Bα, overlap so that the binding of these ligands could be mutually exclusive (32, 36). The competition assay with GDP, one of its natural ligands, leads us to postulate that the nucleotide binding site could not be the one for the AIs. Mutational studies of eEF1A and investigation of effects of pH are currently ongoing to determine and characterize the binding site of the AIs on eEF1A, which we plan to validate by crystallography.
Acknowledgments
The authors thank Jean-François Gaussin and Sébastien Sauvage for the in vivo experiment.
The present work has been supported partly by grants awarded by the Fonds Yvonne Boël (Brussels, Belgium) and by the Fonds National de la Recherche Scientifique (FNRS; Brussels, Belgium). F.L. is a clinical research fellow, V.M. is a senior research assistant, and R.K. is a director of research with the FNRS.
REFERENCES
- 1. Hamm C., Verma S., Petrella T., Bak K., Charette M. (2008) Melanoma Disease Site Group of Cancer Care Ontario's Program in evidence-based care. Biochemotherapy for the treatment of metastatic malignant melanoma: a systematic review. Cancer Treat. Rev. 34, 145– 146 [DOI] [PubMed] [Google Scholar]
- 2. Atallah E., Flaherty L. (2005) Treatment of metastatic malignant melanoma. Curr. Treat. Options Oncol. 6, 185– 193 [DOI] [PubMed] [Google Scholar]
- 3. Soengas M. S., Lowe S. W. (2003) Apoptosis and melanoma chemoresistance. Oncogene 22, 3138– 3151 [DOI] [PubMed] [Google Scholar]
- 4. Eberle J., Kurbanov B. M., Hossini A. M., Trefzer U., Fecker L. F. (2007) Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist. Updat. 10, 218– 234 [DOI] [PubMed] [Google Scholar]
- 5. Ivanov V. N., Bhoumik A., Ronai Z. (2003) Death receptors and melanoma resistance to apoptosis. Oncogene 22, 3152– 3161 [DOI] [PubMed] [Google Scholar]
- 6. La Porta C. A. (2007) Drug resistance in melanoma: new perspectives. Curr. Med. Chem. 14, 387– 391 [DOI] [PubMed] [Google Scholar]
- 7. Boldogh I. R., Pon L. A. (2006) Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophys. Acta 1763, 450– 462 [DOI] [PubMed] [Google Scholar]
- 8. Chua B. T., Volbracht C., Tan K. O., Li R., Yu V. C., Li P. (2003) Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat. Cell Biol. 5, 1083– 1089 [DOI] [PubMed] [Google Scholar]
- 9. Franklin-Tong V. E., Gourlay C. W. (2008) A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem. J. 413, 389– 404 [DOI] [PubMed] [Google Scholar]
- 10. Hayot C., Debeir O., Van Ham P., Van Damme M., Kiss R., Decaestecker C. (2006) Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol. Appl. Pharmacol. 211, 30– 40 [DOI] [PubMed] [Google Scholar]
- 11. Yang F., Demma M., Warren V., Dharmawardhane S., Condeelis J. (1990) Identification of an actin-binding protein from Dictyostelium as elongation factor 1a. Nature 347, 494– 496 [DOI] [PubMed] [Google Scholar]
- 12. Murray J. W., Edmonds B. T., Liu G., Condeelis J. (1996) Bundling of actin filaments by elongation factor 1 alpha inhibits polymerization at filament ends. J. Cell Biol. 135, 1309– 1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross S. R., Kinzy T. G. (2005) Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology. Nat. Struct. Mol. Biol. 12, 772– 778 [DOI] [PubMed] [Google Scholar]
- 14. Kornienko A., Evidente A. (2008) Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 108, 1982– 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gheorghiu A., Ionescu-Matiue. (1962) Presence of lycorine and galanthamine in Leucojum aestivum L. Anatomical study of the aerial parts of the plant and the corresponding powders. Ann. Pharm. Fr. 20, 531– 538 [PubMed] [Google Scholar]
- 16. Liu J., Li Y., Tang L. J., Zhang G. P., Hu W. X. (2007) Treatment of lycorine on SCID mice model with human APL cells. Biomed. Pharmacother. 61, 229– 234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X. S., Jiang J., Jiao X. Y., Wu Y. E., Lin J. H., Cai Y. M. (2009) Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells. Cancer Lett. 274, 16– 24 [DOI] [PubMed] [Google Scholar]
- 18. Lamoral-Theys D., Andolfi A., Van Goietsenoven G., Cimmino A., Le Calvé B., Wauthoz N., Mégalizzi V., Gras T., Bruyère C., Dubois J., Mathieu V., Kornienko A., Kiss R., Evidente A. (2009) Lycorine, the main phenanthridine amaryllidaceae alkaloid, exhibits significant anti-tumor activity in cancer cells that are resistant to proapoptotic stimuli: an investigation of SAR and mechanistic insight. J. Med. Chem. 52, 6244– 6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pettit G. R., Gaddamidi V., Herald D. L., Singh S. B., Cragg G. M., Schmidt J. M., Boettner F. E., Williams M., Sagawa Y. (1986) Antineoplastic agents, 120. Pancratium littorale. J. Nat. Prod. 49, 995– 1002 [DOI] [PubMed] [Google Scholar]
- 20. Pettit G. R., Ducki S., Eastham S. A., Melody N. (2009) Antineoplastic agents. 454. Synthesis of the strong cancer cell growth inhibitors trans-dihydronarciclasine and 7-deoxy-trans-dihydronarciclasine (1a). J. Nat. Prod. 72, 1279– 1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bi Y., Guo J., Zhang L., Wong Y. (2003) Changes in some enzymes of microbodies and plastid development in excised radish cotyledons: effect of narciclasine. J. Plant. Physiol. 160, 1041– 1049 [DOI] [PubMed] [Google Scholar]
- 22. Ceriotti G. (1967) Narciclasine: an antimitotic substance from Narcissus bulbs. Nature 213, 595– 596 [DOI] [PubMed] [Google Scholar]
- 23. McLachlan A., Kekre N., McNulty J., Pandey S. (2005) Pancratistatin: a natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis 10, 619– 630 [DOI] [PubMed] [Google Scholar]
- 24. Siedlakowski P., McLachlan-Burgess A., Griffin C., Tirumalai S. S., McNulty J., Pandey S. (2008) Synergy of Pancratistatin and Tamoxifen on breast cancer cells in inducing apoptosis by targeting mitochondria. Cancer Biol. Ther. 7, 376– 384 [DOI] [PubMed] [Google Scholar]
- 25. Dumont P., Ingrassia L., Rouzeau S., Ribaucour F., Thomas S., Roland I., Darro F., Lefranc F., Kiss R. (2007) The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia 9, 766– 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carrasco L., Fresno M., Vazquez D. (1975) Narciclasine: an antitumour alkaloid which blocks peptide bond formation by eukaryotic ribosomes. FEBS Lett. 52, 236– 239 [DOI] [PubMed] [Google Scholar]
- 27. Jimenez A., Santos A., Alonso G., Vazquez D. (1976) Inhibitors of protein synthesis in eukarytic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim. Biophys. Acta 425, 342– 348 [DOI] [PubMed] [Google Scholar]
- 28. Lefranc F., Sauvage S., Van Goietsenoven G., Mégalizzi V., Lamoral-Theys D., Debeir O., Spiegl-Kreinecker S., Berger W., Mathieu V., Decaestecker C., Kiss R. (2009) Narciclasine, a plant growth modulator, activates Rho and stress fibers in glioblastoma cells. Mol. Cancer Ther. 8, 1739– 1750 [DOI] [PubMed] [Google Scholar]
- 29. Ingrassia L., Lefranc F., Dewelle J., Pottier L., Mathieu V., Spiegl-Kreinecker S., Sauvage S., El Yazidi M., Dehoux M., Berger W., Van Quaquebeke E., Kiss R. (2009) Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agents. J. Med. Chem. 52, 1100– 1114 [DOI] [PubMed] [Google Scholar]
- 30. Friesner R. A., Murphy R. B., Repasky M. P., Frye L. L., Greenwood J. R., Halgren T. A., Sanschagrin P. C., Mainz D. T. (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177– 6196 [DOI] [PubMed] [Google Scholar]
- 31. Sadowski J., Gasteiger J. (1993) From atoms and bonds to 3-dimensional atomic coordinates – automatic model builders. Chemical Rev. 93, 2567– 2581 [Google Scholar]
- 32. Pittman Y. R., Kandl K., Lewis M., Valente L., Kinzy T. G. (2009) Coordination of eukaryotic translation elongation factor 1A (eEF1A) function in actin organization and translation elongation by the guanine nucleotide exchange factor eEF1Balpha. J. Biol. Chem. 284, 4739– 4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathieu V., Pirker C., de Lassalle E. M., Vernier M., Mijatovic T., DeNeve N., Gaussin J-F., Dehoux M., Lefranc F., Berger W., Kiss R. (2009) The sodium pump alpha1 subunit: a disease progression-related target for metastatic melanoma treatment. J. Cell. Mol. Med. 13, 3960– 3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berger W., Hauptmann E., Elbling L., Vetterlein M., Kokoschka E. M., Micksche M. (1997) Possible role of the multidrug resistance-associated protein (MRP) in chemoresistance of human melanoma cells. Int. J. Cancer 71, 108– 115 [DOI] [PubMed] [Google Scholar]
- 35. Evidente A. (1991) Narciclasine: 1H- and 13C-NMR Data and a new improved method of preparation. Planta. Med. 57, 293– 295 [DOI] [PubMed] [Google Scholar]
- 36. Liu G., Tang J., Edmonds B. T., Murray J., Levin S., Condeelis J. (1996) F-actin sequesters elongation factor 1alpha from interaction with aminoacyl-tRNA in a pH-dependent reaction. J. Cell Biol. 135, 953– 963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pelletier C. L., Maggi L.B., Jr., Brady S. N., Scheidenhelm K., Gutmann D. H., Weber J. D. (2007) TSC1 sets the rate of ribosome export and protein synthesis through nucleophosmin translation. Cancer Res. 67, 1609– 1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gross S. R., Kinzy T. G. (2007) Improper organization of the actin cytoskeleton affects protein synthesis at initiation. Mol. Cell. Biol. 27, 1974– 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jansen R. P. (1999) RNA-cytoskeletal associations. FASEB J. 13, 455– 466 [PubMed] [Google Scholar]
- 40. López de Heredia M., Jansen R. P. (2004) mRNA localization and the cytoskeleton. Curr. Opin. Cell Biol. 16, 80– 85 [DOI] [PubMed] [Google Scholar]
- 41. Wang C., Zhou G. L., Vedantam S., Li P., Field J. (2008) Mitochondrial shuttling of CAP1 promotes actin- and cofilin-dependent apoptosis. J. Cell Sci. 121, 2913– 2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duttaroy A., Bourbeau D., Wang X. L., Wang E. (1998) Apoptosis rate can be accelerated or decelerated by overexpression or reduction of the level of elongation factor-1 alpha. Exp. Cell. Res. 238, 168– 176 [DOI] [PubMed] [Google Scholar]
- 43. Pinke D. E., Kalloger S. E., Francetic T., Huntsman D. G., Lee J. M. (2008) The prognostic significance of elongation factor eEF1A2 in ovarian cancer. Gynecol. Oncol. 108, 561– 568 [DOI] [PubMed] [Google Scholar]
- 44. Edmonds B. T., Wyckoff J., Yeung Y. G., Wang Y., Stanley E. R., Jones J., Segall J., Condeelis J. (1996) Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J. Cell Sci. 109, 2705– 2714 [DOI] [PubMed] [Google Scholar]
- 45. Li R., Wang H., Bekele B. N., Yin Z., Caraway N. P., Katz R. L., Stass S. A., Jiang F. (2006) Identification of putative oncogenes in lung adenocarcinoma by a comprehensive functional genomic approach. Oncogene 25, 2628– 2635 [DOI] [PubMed] [Google Scholar]
- 46. Grassi G., Scaggiante B., Farra R., Dapas B., Agostini F., Baiz D., Rosso N., Tiribelli C. (2007) The expression levels of the translational factors eEF1A 1/2 correlate with cell growth but not apoptosis in hepatocellular carcinoma cell lines with different differentiation grade. Biochimie (Paris) 89, 1544– 1552 [DOI] [PubMed] [Google Scholar]
- 47. Gerweck L. E., Seetharaman K. (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 56, 1194– 1198 [PubMed] [Google Scholar]
- 48. Noble C. G., Song H. (2008) Structural studies of elongation and release factors. Cell. Mol. Life. Sci. 65, 1335– 1346 [DOI] [PMC free article] [PubMed] [Google Scholar]