Abstract
Development of rational therapeutic treatments of Alzheimer disease (AD) requires the elucidation of the etiopathogenic mechanisms of neurofibrillary degeneration and β-amyloidosis, the two hallmarks of this disease. Here we show, employing an adeno-associated virus serotype 1 (AAV1)-induced expression of the C-terminal fragment (I2CTF) of I2PP2A, also called SET, in rat brain, decrease in protein phosphatase 2A (PP2A) activity, abnormal hyperphosphorylation of tau, and neurodegeneration; littermates treated identically but with vector only, i.e., AAV1-enhanced green fluorescent protein (GFP), served as a control. Furthermore, there was an increase in the level of activated glycogen synthase kinase-3β and enhanced expression of intraneuronal Aβ in AAV1-I2CTF animals. Morris water maze behavioral test revealed that infection with AAV1-I2CTF induced spatial reference memory and memory consolidation deficits and a decrease in the brain level of pSer133-CREB. These findings suggest a novel etiopathogenic mechanism of AD, which is initiated by the cleavage of I2PP2A, producing I2CTF, and describe a novel disease-relevant nontransgenic animal model of AD.—Wang, X., Blanchard, J., Kohlbrenner, E., Clement, N., Linden, R. M., Radu, A., Grundke-Iqbal, I., Iqbal, K. The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment.
Keywords: Aβ, adeno-associated virus-induced expression, glycogen synthase kinase-3β, hyperphosphorylation of tau, neurofibrillary degeneration, nontransgenic rat model of Alzheimer disease, SET
Alzheimer's disease (AD) is multifactorial, and different etiopathogenic mechanisms involved are probably responsible for different subgroups of this disease (1). Independent of the etiology, AD is histopathologically characterized by neurofibrillary degeneration of abnormally hyperphosphorylated tau in the presence of β-amyloidosis in the brain. The histopathological changes, the intracellular neurofibrillary tangles (NFTs), and extracellular senile plaques, are particularly severe in the fundus of the forebrain, hippocampus, and cerebral cortex since these brain areas are the first affected. Although the triggering mechanisms of AD pathogenesis are still unclear, the degree and severity of clinical dementia is positively and strongly correlated with the NFT load and spatial brain distribution in AD patients (2).
NFTs are composed of bundles of paired helical filaments, the major protein subunit of which is abnormally hyperphosphorylated tau (3, 4). The activity of protein phosphatase-2A (PP2A), which is the major regulator of tau phosphorylation (5–8) and of several protein kinases that phosphorylate tau (7, 9–12) and modulate the amyloidogenic processing of amyloid precursor protein (13–15), is compromised in the AD brain (16). The mRNA and protein expressions of I1PP2A and I2PP2A, also called SET, which regulate the activity of PP2A (17) are up-regulated in AD brain (18). Furthermore, I2PP2A/SET, a 277-aa, 39-kDa endogenous inhibitor of PP2A, which is primarily nuclear, is selectively cleaved at N175 and translocated from neuronal nucleus to the cytoplasm in the AD brain (18). Unlike I1PP2A, which inhibits PP2A activity by interacting through its amino-terminal half with the catalytic subunit of PP2A, PP2Ac (19), both the N-terminal fragment (NTF) and C-terminal fragment (CTF) of I2PP2A can interact with PP2Ac and inhibit its activity in vitro (ref. 19 and unpublished results). However, whether an etiopathogenic mechanism in which the translocation of the CTF of I2PP2A from neuronal nucleus to cytoplasm could produce AD pathology and cognitive impairment was not known.
In the present study, we employed an adeno-associated virus serotype 1 (AAV1) vector to express the C-terminal half of I2PP2A, I2CTF in the brain of Wistar rats by intracerebroventricularly infecting the newborn animals within 24 h after birth with the virus. We found a lifelong brain expression of I2CTF in AAV1-I2CTF-infected rats from 3 wk to 8 mo. There was a significant decrease in PP2A activity and increase in phosphorylation of tau at several sites studied 8 mo postinfection with AAV1-I2CTF. The tau abnormal hyperphosphorylation was associated with neurodegeneration and loss of dendritic and synaptic plasticity and spatial reference memory impairment in the AAV1-I2CTF animals. Furthermore, the AAV1-I2CTF animals also displayed an increase in the level of activated GSK-3β and enhancement of intraneuronal expression of Aβ. The I2CTF apparently induced tau hyperphosphorylation both through the inhibition of PP2A and the consequent activation of GSK-3β. Together, these data support the I2PP2A cleavage-induced etiopathogenic mechanism of AD and show the generation of a novel disease-relevant, nontransgenic rat model of AD.
MATERIALS AND METHODS
Construction of recombinant plasmids
Employing pEGFP-N3/I2PP2A (wt) generated by us previously (20) as a template, I2CTF cDNA was obtained by PCR with primer 1 (5′-GATGGATCCAAAGCCAGCAGGAAGA) and primer 2 (5′-GATCTCGAGTTAGTCATCTTCTC) (Supplemental Fig. S1). The BamHI site underlined in the primer 1 and the XhoI site underlined in the primer 2 were used to clone the fragment into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). The plasmid was verified by DNA sequencing. pcDNA 3.1- I2CTF plasmid was digested with restriction endonucleases HindIII and PmeI. The products were separated on agarose gel and the purified I2CTF (aa 176–277) DNA fragment was obtained by a gel extraction kit. The pTRUF 12 vector was digested with restriction endonucleases HindIII and EcoR V. With a blunt ligation, pTRUF 12 vector was linked with insert gene I2CTF DNA by T4 DNA ligase at room temperature for 30 min. SURE 2 supercompetent cells were transformed with 1 μl ligation reaction mixture, and aliquots of the transformation reaction were plated on lysogeny broth agar (1% tryptone, 17% NaCl, 0.5% yeast extract, and 1.5% agar) containing 50 μg/ml ampicillin, and the cultures were incubated at 37°C for 16 h. Appropriate clones were selected and cultured overnight at 37°C to obtain a saturated culture. Recombinant plasmid DNA was extracted. As a key restriction site to be packed with an adeno-associated virus, SmaI was identified in the constructed plasmids by agarose gel electrophoresis.
Vector packaging and titering
Recombinant AAV serotype 1 was generated as described previously (21). Briefly, 293-T cells were cotransfected with the pTRUF plasmids and helper plasmid pXYZ1 (22). The virus was purified by using iodixanol density gradient (Optiprep; Greiner Bio-One Inc., Monroe, NC, USA). Virus samples were concentrated and formulated into lactated Ringer's solution (Baxter Healthcare, Deerfield, IL, USA). Genome-containing particles (gcp) were determined by real-time PCR (LightCycler; Roche Diagnostics, Mannheim, Germany) and SYBR Green Taq ReadyMix (Sigma-Aldrich, St. Louis, MO, USA). Titers were calculated from a standard curve generated from pTRUF.
Cell transfection with I2CTF and treatment with lithium chloride
HEK293 cells stably transfected with human tau441 (23) were transfected with pcDNA3.1-I2CTF or, as a control, with empty vector pcDNA3.1. After 6 h of transfection, the cells were treated with GSK-3β inhibitor, 20 mM lithium chloride (24). At 12, 24, and 48 h later, the cells were washed with PBS and then lysed in 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide, 20 mM β-glycerophosphate, 50 mM NaF, 1 mM Na3VO4, 100 μg/ml phenylmethysulfonyl fluoride, and 1 μg/ml aprotinin. The lysate was bath sonicated and then centrifuged at 12,000 g for 15 min, and the supernatant was used for Western blots to study hyperphosphorylation of tau.
Animals and intracerebroventricular injection of AAV
Normal Wistar rats were purchased from Charles River Laboratories (Germantown, MD, USA) and bred and maintained in the New York State Institute for Basic Research Animal Colony. On the day of birth, designated as P 0.5, pups were individually anesthetized on ice, and 2 μl of AAV1-I2CTF was injected into each lateral ventricle with a specially designed fine 10-μl Hamilton syringe with a 30-gauge/0.5-inch/hypodermic cemented needle (Hamilton Syringe Co., Reno, NV, USA). A total of 8 × 109 AAV1 genomic equivalents in 4 μl were injected into each rat brain. Control animals were treated identically except with vector only, i.e., AAV1-GFP. Animals were housed in an animal room facility maintained at 23°C with a light-dark cycle of 12 h (lights off at 6:00 PM) and with accessible food and water ad libitum. Behavioral studies included 14 AAV1-GFP and 10 AAV1-I2CTF infected animals, and immunohistochemical and Western blot analysis employed 3 animals/group.
All procedures carried out on animals were conducted in strict compliance with protocols approved by the Animal Welfare Committee of New York State Institute for Basic Research.
Western blot analysis
Rat brain tissues, ventricular area, cerebral cortex, and hippocampus, were quickly dissected out and homogenized on ice to generate 12% (w/v) homogenate in buffer containing 50 mM Tris·HCl (pH 7.4), 8.5% sucrose, 2 mM EDTA, 10 mM β-mercaptoethanol, 0.2 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, and 2 μg/ml each of aprotinin and pepstatin A. The tissue homogenate used for Western blots also contained 20 mM β-glycerephosphate, 50 mM NaF, and 1 mM Na3VO4 to inhibit phosphatase activities. The protein concentration of each tissue homogenate was measured by modified Lowry assay (25). The tissue samples were then boiled in Laemmli's buffer in a water bath for 5 min and subjected to 10% SDS-PAGE and Western blots. Samples of HEK293/tau cell extracts were similarly analyzed. For Western blots, the primary antibodies used were pan-tau antibody, rabbit polyclonal antibody (pAb) 92e (1:5000; (26), phospho-specific tau antibodies: Tau pAb pS199 (1:1000, BioSource, Camarillo, CA, USA), Tau pAb pT205 (1:1000; BioSource), Tau pAb pT212 (1:500; BioSource), Tau pAb pS214 (1:500; BioSource), Tau pAb pT231/pS235 (1:1000; BioSource), Tau monoclonal antibody (mAb) M4 to phosphorylated Thr 231/Ser-235; (1:1500, (27), Tau pAb pS262 (1:1000; BioSource), Tau pAb pS396 (1:1000; BioSource), Tau pAb R145 to pS422 (1:3000; (9), I2CTF pAb (2 μg/ml), mAb GFP (1:1000; Rockland, Gilbertville, PA, USA), mAb β-actin (1:2000; Sigma), mAb DM1A to α-tubulin (1:1000; Sigma), and rabbit pAb βΙΙΙ tubulin (1:5000; Covance, Princeton, NJ, USA). The rabbit monoclonal antibodies used for the Western blots were rabbit mAb phospho-CREB (cyclic AMP response element binding protein) 87G3 to pSer133 (1:1000; Cell Signaling, Danvers, MA, USA), rabbit mAb 48H2 to CREB (1:1000; Cell Signaling), rabbit mAb Phospho-GSK-3β to pSer9 (1:1000; Cell Signaling), and rabbit mAb 27C10 to GSK-3β (1:2000; Cell Signaling). Immunoreactive protein bands were visualized with enhanced chemiluminescence (ECL) reagents (Pierce, Rockford, IL, USA).
Immunoprecipitation
Immunoprecipitation was carried out on 16,000-g extracts of cerebral cortex, ventricular area, and hippocampus from control AAV1-GFP and experimental rats (AAV1-I2CTF) at 9 wk and 8 mo post-AAV infection. Briefly, tissues were homogenized in lysis buffer containing 50 mM Tris·HCl (pH 7.4), 100 mM NaCl, 1 mM EGTA, 2 mM EDTA, 1% Triton X-100, 0.2 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, and 2 μg/ml each of aprotinin and pepstatin A. After centrifugation at 16,000 g for 15 min, the lysates (300 μg of protein) were immunoprecipitated with anti-PP2Ac rabbit polyclonal antibody R123d (28), followed by protein A Sepharose (20333; Pierce). Eluted proteins were divided into two parts: one part was analyzed by Western blots developed with anti-PP2Ac mouse monoclonal 1D6 (Millipore, Billerica, MA, USA), and the other part was used for PP2Ac activity assay.
PP2Ac activity assay
An ELISA was used to assay PP2A activity in proteins immunoprecipitated by anti-PP2Ac rabbit polyclonal antibody R123d (28). Briefly, a 96-well plate was coated at 4°C overnight with 30 μl of 8.0 μg/ml solution of synthetic tau phosphopeptide p17 corresponding to tau194–207, to which 3 lysines were added at the C-terminal end to enhance its binding to the microtiter plate (Ac-Arg-Ser-Gly-Tyr-Ser-Ser(OPO32−)-Pro-Gly-Ser-Pro-Gly-Thr-Pro-Gly-Lys-Lys-Lys-NH2). For blocking, the coating solution was removed, and the wells were blocked with 190 μl of protein-free blocking buffer (37571; Pierce) for 1 h at room temperature. The plates were then washed 4 times for 30 min with 190 μl/well 50 mM Tris·HCl (pH 7). The enzymatic reaction was performed with 34 μl/well of the immunoprecipitated protein solution resuspended with reaction buffer containing 50 mM Tris·HCl (pH 7), 20 mM β-mercaptoethanol, 2 mM MnCl2, and 0.1 mg/ml BSA. The plates were incubated for 90 min at 30°C in a moist chamber. To stop the enzyme reaction, the mixture was immediately removed, and the wells were washed with blocking buffer containing 50 mM NaF. The plates were washed 4 times for 5 min with 190 μl/well 50 mM Tris, 0.05% Tween-20 (pH 7.6), and each well was then incubated with 37 μl of the monoclonal antibody Tau-1 to tau unphosphorylated at Ser-198/199/202 (1:25000) (3, 29) overnight at 4°C. The plates were washed 4 times and incubated with secondary antibody (peroxidase-linked goat anti-mouse IgG, 1:1,200) for 1 h at room temperature. Finally, after the plates were washed 5 times with TBS (pH 7.6), the color reaction was performed using 50 μl of tetramethylbenzidine (TMB) per well, and the development was monitored at 650 nm in the microtiter plate reader.
Immunohistochemistry and double-labeling immunofluorescence staining
For immunohistochemical studies, anesthetized rats were perfused through the aorta with 100 ml 0.9% NaCl followed by 100 ml phosphate buffer containing 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde overnight then equilibrated in a cryoprotectant solution of 30% sucrose/PBS and stored at 4°C. Coronal sections (40 μm thick) were cut using a freezing sliding microtome. After incubation in 0.3% Triton-X100-PBS for 30 min, free floating sections were blocked with 5% goat serum containing 0.1% Tween 20 in PBS for 30 min at room temperature. Sections were then incubated overnight at 4°C with primary antibodies. The primary antibodies used were mAb Aβ17–24 (4G8, 1:300; Covance), pAb Aβ34–40 (1:500; Invitrogen), pAb Aβ36–42 (1:500; Invitrogen), mAb GFP (1:500; Rockland), Tau pAb pS199 (1:500; BioSource), Tau pAb pS262 (1:500; BioSource), and Tau pAb pS396 (1:5000; BioSource), pAb I2CTF (1:1000), mAb SMI52 to MAP2a,b (1: 1000; Covance), mAb synaptophysin (1:200; Chemicon, Temecula, CA, USA), and pAb synapsin-1 (1:500; Stressgen, Ann Arbor, MI, USA). This was followed by incubation with secondary antibodies: Alexa 488-conjugated goat anti-mouse antibody (1:1000) and Cy3 goat anti-rabbit antibody (1:1,000) (Jackson Immunoresearch, West Grove, PA). Finally, the sections were washed in TBS and mounted on glass slides with ProLong Gold antifade reagent (Invitrogen).
Image analysis and semiquantification of immunofluorescence
The sections were subjected to confocal microscopy (PCM 2000 Confocal Imaging System; Nikon, Melville, NY, USA) for quantitative analysis using a ×40 objective. Area of interest was outlined, and maximum projection images were then generated based on confocal z stacks. The antibody staining was semiquantitated by measuring mean fluorescence intensities (MFIs) with NIH Image J software (U.S. National Institutes of Health, Bethesda, MD, USA). Six to 10 images of sections stained for MAP2, synaptophysin, or synapsin were obtained per hippocampal CA1 and CA3 region, respectively. MFI per square micrometer area was calculated by dividing the MFI units by the area of outlined regions.
Fluoro-Jade B labeling
Tissue sections were mounted on 2% gelatin-coated slides and then dried at room temperature overnight. Slides were briefly rinsed in distilled water, followed by 3-min incubation in 100% alcohol, 1 min in 70% alcohol, 1 min in 30% alcohol, and a 1-min rinse in distilled water. The sections were then treated with a solution of 0.06% potassium permanganate for 15 min with gentle shaking and rinsed in distilled water for 1 min. The staining solution was prepared to contain a 0.001% Fluoro-Jade B (Chemicon) in 0.1% acetic acid. After incubating for 30 min in the staining solution, the sections were rinsed for 1 min in each of three distilled water washes and dried. The dry sections were washed 3 times in xylene 2 min each before mounting with DPX (Fluka, Milwaukee, WI, USA). Images were captured using a Nikon 90i microscope equipped with epifluorescence optics.
Behavioral procedures
Open-field activity
Anxiety and exploratory activity were evaluated by allowing rats to freely explore an open field for 20 min. The testing apparatus was a classic open field (i.e., a PVC square arena of 100×100 cm2, with 70-cm-high walls). The open field was placed in a part of the room separated from the experimenter and the control station with a black opaque curtain. Rats were individually submitted to a single 20-min session. Because for rodents the middle of a nonfamiliar arena is anxiogenic, anxiety was studied by analyzing the percentage of time spent in the middle of the arena. To assess exploratory activity, the total distance that the animals covered in the arena was tracked and measured. Data collection was performed using tracking files of the experiment recorded with SMART 2.0.14 software (Pan Lab/San Diego Instruments, San Diego, CA, USA).
Water maze
Spatial reference learning and memory were evaluated by the Morris water maze procedure. The test required that rats used a spatial navigational strategy based on a spatial representation of the environment to find a fixed submerged escape platform. The procedure was performed in a 180-cm-diameter circular tank. The pool was filled with water (21±1°C) made opaque by adding white nontoxic paint. Acquisition started with the escape platform (13-cm diameter submerged 1 cm below water surface) in the northwest quadrant, and each animal was given 90 s to find the platform. If the rat did not find the platform in 90 s, it was gently guided to it. At the end of each trial, the rat was left on the platform for 20 s, then dried and returned to its home cage until the next trial. Four such acquisition trials were given on each day for 3 consecutive days. Three months later, rats were submitted to a similar training, but the platform location was changed from northwest to southeast, as well as the spatial environment of the experimental room was modified. Twenty-four hours after completion of the 3 training days, a transfer test was performed with changed platform location. Animals received 3 training trials for the transfer test. Finally, 30 d after the transfer test, rats were tested for this latest platform location and spatial environment performing 3 trials.
Rat behavior in the water maze was monitored by a Samsung digital camera (SDC 4304; Samsung, Seoul, South Korea) mounted to the ceiling and tracked and timed by SMART 2.0.14 software.
Statistical analysis
Statistical analyses were performed using Student's t test for Western blot analyses and measurement of anxiety in the open field. Two-way ANOVAs were performed for exploration in the open field and training in the water maze. Statistical significance was accepted at the 95% confidence level (P<0.05). Data are expressed as means ± se.
RESULTS
The AAV1-induced brain expression of GFP and I2CTF after intraventricular injection in newborn rats is long lasting
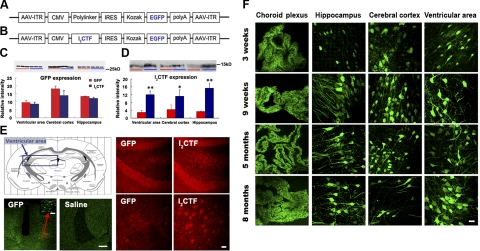
Viral expression systems offer a simple, direct, and a relatively inexpensive method, as compared with the generation of transgenic rodent models for the expression or inhibition of a targeted gene, and are being increasingly found to be a valuable research tool (see Verma and Weitzman, ref. 30). To test the hypothesis that the cleavage of I2PP2A found by us in AD brain previously could be an etiopathogenic mechanism of AD, we employed AAV1 to induce the expression of I2CTF in rat brain in the present study (Fig. 1A, B). We bilaterally injected AAV1 encoding enhanced green fluorescent protein (GFP) and I2CTF or, as control, AAV1-GFP alone into the cerebral lateral ventricles of P 0.5 Wistar rats. To investigate the level of AAV1-induced expression, we performed Western blots using mAb GFP to detect GFP and pAb I2CTF to detect I2CTF in the cerebral cortex, hippocampus, and ventricular area. GFP expression in AAV1-I2CTF-injected rats was not significantly different from that in AAV1-GFP-injected rats, suggesting a similar level of transduction between the experimental and the control animals (Fig. 1C). However, the level of I2CTF in AAV1-I2CTF animals was markedly higher than in AAV1-GFP controls, confirming the expression of I2CTF in the AAV1-I2CTF animals (Fig. 1D). Immunohistochemically, GFP expression was detected by green fluorescence at 3 wk to 8 mo after injection. As a control, one litter of animals received an intraventricular injection of saline. As expected, these saline-injected neonates did not show fluorescent signals in any brain region (Fig. 1E). Next, we studied the expression of I2CTF immunohistochemically. Anti-I2CTF staining was widely found in the neuronal cytoplasm in the cerebral cortex, hippocampus, and ventricular areas (Supplemental Fig. S2). The expression of I2CTF was especially marked in dentate gyrus and CA1 of the hippocampus (Fig. 1E).
Figure 1.
AAV1-induced gene expression in brain is stable and long lasting when newborn rats are infected intracerebroventricularly. A, B) Linear maps of the AAV vector plasmids (based on pTRUF12) used in the present study. With the exception of the inverted terminal repeats (ITR), all viral genes had been removed and replaced with GFP (A), or I2CTF and GFP (B). CMVe-CBA (cytomegalovirus enhancer-chicken beta actin promoter); IRES (internal ribosomal entry site) from poliovirus. C) Western blots developed with mAb to GFP showed a similar level of expression of GFP between AAV1-I2CTF and AAV1-GFP rats. D) In contrast, blots developed with pAb to I2CTF showed a marked increase in the brain level of I2CTF in AAV1-I2CTF as compared with AAV1-GFP animals. E) AAV1-I2CTF animals (13 wk) showed an increase in the expression of I2CTF over endogenous I2PP2A seen in AAV1-GFP rats; cytoplasmic staining of neurons of the dentate gyrus hilus (anti-I2CTF, red); fluorescence of GFP (anti-GFP, green); ventricular area studied (framed). Animals injected with saline as expression control showed only background staining. F) Immunohistofluorescence using mAb GFP showed that the AAV1-induced expression was long lasting in the neuronal cell layers of choroid plexus, hippocampus, cerebral cortex, and ventricular area. Scale bars = 400 μm (E); 80 μm (E, inset); 25 μm (F).
Intracranial delivery of AAV serotype 1 to neonatal rats has been shown to result in widespread and long-lasting neuronal expression of transgenes (31). To investigate how extensive and how long expression was induced by the AAV infection of newborn rats, we carried out GFP immunohistochemistry at different periods after infection. As expected, GFP expression was observed in the choroid plexus, cerebral cortex, hippocampus, and ventricular area at 3 wk, 9 wk, 5 mo, and 8 mo after AAV injection studied (Fig. 1F). These data showed that AAV1-I2CTF infection can induce a widespread neuronal transduction of the packaged gene with a long-lasting expression.
PP2A activity in AAV1-I2CTF-treated rats is decreased
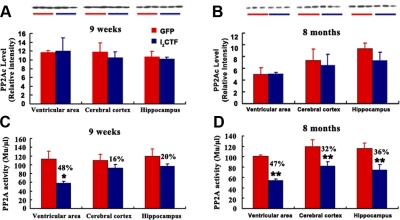
It has been demonstrated that I2PP2A inhibits PP2A and does not affect the activities of PP1, PP2B, and PP2C in vitro (17). In a recent study, we have discovered that I2CTF interacts with PP2Ac and inhibits its activity (unpublished results). However, whether I2CTF inhibits PP2A activity in vivo was not known. In the present study, we investigated the effect of the expression of I2CTF on PP2A inhibition using ELISA and Western blots. We found that there was no significant change in the level of PP2Ac in the brain either in 9-wk-old or 8-mo-old AAV1-I2CTF rats when compared with AAV1-GFP control group (Fig. 2A, B). However, the activity of PP2A was markedly decreased in all brain areas of 8-mo I2CTF rats studied (Fig. 2C, D). The decrease in PP2A activity was also observed as early as in 9-wk I2CTF rats in all brain areas studied but reached statistical significance only in the ventricular area at this age. These data suggested that I2CTF probably inhibited PP2A activity.
Figure 2.
Infection with AAV1-I2CTF results in the decrease of PP2A activity without any significant change in its level. Coimmunoprecipitation was carried out on lysates of cerebral cortex, ventricular area, and hippocampus from I2CTF and, as control, GFP rats at 9 wk (A, C) and 8 mo (B, D) after infection with AAV. Lysates were immunoprecipitated with anti-PP2Ac rabbit polyclonal antibody R123d. Immunoprecipitates were divided into two parts: one part was employed for Western blots probed with anti-PP2Ac mouse monoclonal 1D6 (A, B), while the other part was used for assaying PP2Ac activity (C, D); the PP2A activity was decreased in AAV1-I2CTF in comparison to AAV1-GFP animals. *P < 0.05; **P < 0.01.
Expression of I2CTF induces tau hyperphosphorylation
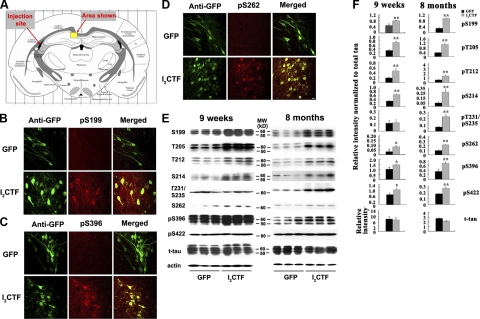
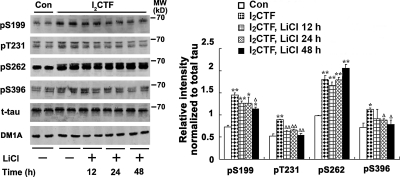
PP2A is a major regulator of tau phosphorylation and accounts for ∼70% of all phosphoserine/phosphothreonine phosphatase activity in human brain (6, 8, 32–37). Unlike I2PP2A full-length, I2CTF is small, i.e., ∼14 kDa, it can freely translocate from the neuronal nucleus to the cytoplasm where both PP2A and tau are localized. Thus, inhibition of PP2A would be expected to lead to abnormal hyperphosphorylation of tau. We looked for the abnormal hyperphosphorylation of tau, both by immunohistochemical staining and by Western blots. The distribution and expression of I2CTF and tau phosphorylation were detected via double immunostaining in the brain. Because AAV1-I2CTF rats expressed simultaneously I2CTF and GFP, we used GFP as a reporter protein. In both AAV1-GFP and AAV1-I2CTF rats, we observed strong immunofluorescence in the hippocampus (selected area of interest is shown in Fig. 3A, representative pictures are presented in Fig. 3B–D), the cerebral cortex, (not shown) and the ventricular area (not shown). These data show that targeted genes GFP and I2CTF were expressed after viral infection and concomitantly tau was abnormally phosphorylated at pS199, pS262 and pS396 epitopes in the hippocampus, the cerebral cortex (not shown) and the ventricular area (not shown) of I2CTF, but not GFP rats. I2CTF colocalized with hyperphosphorylated tau. These data suggested that I2CTF overexpression led to tau hyperphosphorylation at Ser-199, Ser-262, and Ser-396 studied in AAV1-I2CTF rats.
Figure 3.
I2CTF induces abnormal hyperphosphorylation of tau. A–D) At 9 wk postinfection, an immunohistochemical staining in a selected area of interest (A) shows expression in GFP and I2CTF rats; pS199 (B), pS396 (C), and pS262 (D) show only background in AAV1-GFP rat brain and abnormal hyperphosphorylation of tau in AAV1-I2CTF rat brain and a colocalization with I2CTF in I2CTF- but not in GFP-rats. Scale bars = 25 μm. E) Western blots show increase in abnormal hyperphosphorylation of tau in 9-wk and 8-mo I2CTF rats. F) Quantitative analysis of the blots showing levels of phosphorylated taus normalized with total tau levels (left panel) shows significant increase in tau hyperphosphorylation at several sites studied, and this increase was further enhanced from 9 wk to 8 mo (right panel) at several sites. *P < 0.05; **P < 0.01.
Using Western blot analysis, we found that 9 wk and 8 mo postinfection with AAV1-I2CTF, there was an increase of tau hyperphosphorylation at Ser-199, Thr205, Thr212, Ser-214, Thr231/Ser-235, Ser-262, and Ser-396 in the hippocampus, the cerebral cortex and the ventricular area of AAV1-I2CTF rats when compared with AAV1-GFP control animals (Fig. 3E and Supplemental Fig. S2). To learn the effect of the expression of I2CTF on tau phosphorylation, levels of phosphorylated tau at above-mentioned sites were normalized by the level of total tau. Consistent with neurodegeneration (see below) in AAV1-I2CTF rats, a small decrease in total tau level was observed (Fig. 3F and Supplemental Fig. S3). Expression of I2CTF caused hyperphosphorylation of tau at all of the sites and areas of the brain studied both in 9-wk and 8-mo rats (Fig. 3F and Supplemental Fig. S3). The increase in tau phosphorylation in AAV1-I2CTF rats compared to AAV1-GFP controls was higher at 8 mo of age than at 9 wk at most of the sites studied (Fig. 3F). This is consistent with the inhibition of PP2A activity from 9-wk- to 8-mo-old AAV1-I2CTF rats compared to the AAV1-GFP control animals. These data further suggested that, in AAV1-I2CTF rats, the abnormal phosphorylation of tau was most likely due to the inhibition of PP2A activity.
Expression of I2CTF induces loss of dendritic and synaptic plasticity and neurodegeneration
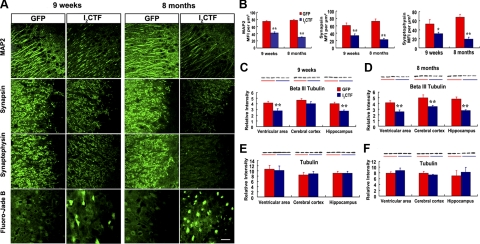
Loss of dendritic (38) and synaptic plasticity (39) and neuronal degeneration occurs in brains of AD patients and constitutes a key neurobiological basis of dementia. We, therefore, investigated whether I2CTF-induced inhibition of PP2A activity, and the resulting abnormal hyperphosphorylation of tau could produce loss of dendritic and synaptic plasticity and neurodegeneration. We determined the effect of I2CTF expression on neuronal integrity, by examining in brains of AAV1-I2CTF and AAV1-GFP rats, levels of the dendritic marker MAP2 and synaptic markers synaptophysin and synapsin. The semiquantitative results showed that mean fluorescence intensities (MFIs) of MAP2 immunoreactivity in the pyramidal neurons of CA1 region of the hippocampus was strongly decreased in 9-wk and 8-mo AAV1-I2CTF rats (by 43 and 61%, respectively) compared to AAV1-GFP controls (Figs. 4A, B). Similarly, MFIs of synaptophysin and synapsin were significantly decreased in the CA3 subfield of the hippocampus in both 9-wk (40 and 45%, respectively) and 8-mo (71 and 69%, respectively) AAV1-I2CTF compared with AAV1-GFP rats. Altogether, these data suggested that I2CTF overexpression resulted in dendritic and synaptic loss.
Figure 4.
I2CTF induces neurodegeneration and loss of dendritic and synaptic plasticity. A, B) As compared with GFP rats, I2CTF rats show a marked decrease in the expression of somato-dendritic marker MAP2 and synaptic markers synapsin and synaptophysin in the hippocampus, and this decrease was enhanced from 9 wk to 8 mo post-AAV1-I2CTF infection (B). Fluoro Jade staining shows a selective neurodegeneration in the AAV1-I2CTF rat hippocampus (A). C–F) Levels of the neuron-specific βΙΙΙ tubulin (C, D) and not total tubulin (E, F) were selectively decreased in ventricular area of 9-wk and all areas of the 8-mo AAV1-I2CTF rats studied. *P < 0.05; **P < 0.01.
Next, we studied neurodegeneration, both by Fluoro-Jade B staining and by determining by Western blot analysis the level of the neuron-specific microtubule protein, β III tubulin. Fluoro-Jade B has been reported to be a sensitive and reliable marker of neuronal degeneration (40). We found a significant Fluoro-Jade B staining in the hippocampus of AAV1-I2CTF, but not AAV1-GFP rats (Fig. 4A). Furthermore, there was a significant decrease of neuron-specific β III tubulin staining in the ventricular area and in the hippocampus of 9-wk AAV1-I2CTF-infected rats, and in the ventricular area, the cerebral cortex and the hippocampus of 8-mo AAV1-I2CTF rats compared with AAV1-GFP control animals (Fig. 4C, D). However, no alteration of total tubulin by the DM1A staining was observed (Fig. 4E, F).
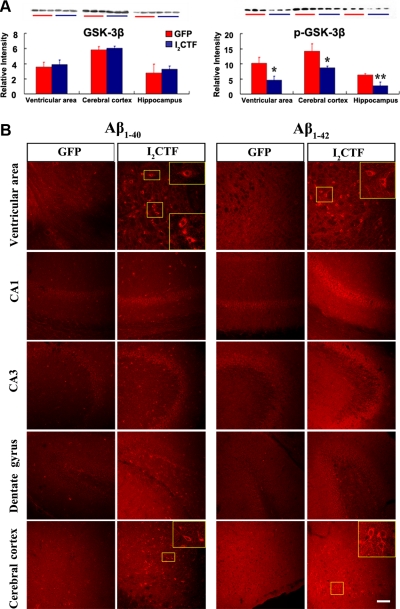
I2CTF induces increase in the level of activated GSK-3β and the expression of intraneuronal Aβ
PP2A is known to regulate the phosphorylation of tau both directly (6) and by regulating the activities of GSK-3β and several other tau protein kinases (7, 9–12, 41, 42). GSK-3β, which is up-regulated and associated with Alzheimer neurofibrillary pathology at all Braak stages (43), is also known to promote the amyloidogenic processing of βAPP by forming a complex with presenilins and promoting their γ-secretase activities (44, 45). Furthermore, inhibition of the GSK-3 expression by siRNA has been shown to inhibit Aβ generation (46). We, therefore, investigated both the level of the activated GSK-3β and the expression of Aβ in AAV1-I2CTF animals. We found that while the level of total GSK-3β was unchanged, that of its inactivated form, pSer-9 GSK-3β, was markedly decreased in 8-mo AAV1-I2CTF as compared with the AAV1-GFP control animals (Fig. 5A). Concomitant with this increase in GSK-3β activity, we found an increase in the expression of intraneuronal Aβ in the AAV1-I2CTF rats (Fig. 5B and Supplemental Fig. S4), suggesting that I2CTF-induced decrease in PP2A activity can lead to Aβ pathology.
Figure 5.
I2CTF induces a selective increase in GSK-3β activity by decreasing the level of the inactive form pSer GSK-3β and an increase in the intraneuronal expression of Aβ. A) Western blots and levels of total GSK-3β and the inactive form pSer9 GSK-3β determined from the blots. Decrease in the pSer GSK-3β but not in total GSK-3β in I2CTF rats suggest an increase in the activity of this kinase. B) Immunohistochemical staining with rabbit polyclonal antibodies to Aβ34–40 and to Aβ36–42 showing a selective increase in the intraneuronal expression of Aβ. Scale bar = 100 μm.
I2CTF leads to abnormal hyperphosphorylation of tau both by the inhibition of PP2A and the consequential activation of GSK-3β
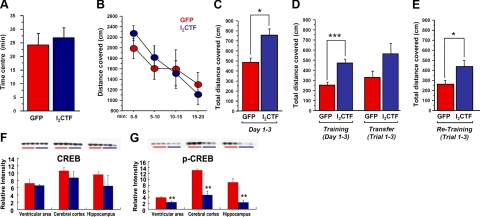
To learn the possible mechanism by which I2CTF induced neurodegeneration, we investigated whether, in addition to PP2A, GSK-3β was also involved in the abnormal hyperphosphorylation of tau. We studied the phosphorylation of tau at Ser-199, Thr231, and Ser-396, which is regulated by both GSK-3β and PP2A, and at Ser-262, which is regulated by PP2A but not GSK-3β (33). We found that transfection of tau stably transfected HEK293 cells with I2CTF, but not vector alone, induced phosphorylation of tau at all the 4 sites examined. In the presence of 20 mM LiCl, which inhibits GSK-3β (24), there was a significant decrease in tau phosphorylation at Ser-199, Thr231, and Ser-396 but not at the non-GSK-3β site, Ser-262 (Fig. 6). These findings suggest that I2CTF probably causes abnormal hyperphosphorylation of tau both by inhibiting PP2A and indirectly via up-regulation of GSK-3β activity and possibly other tau kinases, the activities of which are regulated directly or indirectly by PP2A.
Figure 6.
I2CTF-induced tau hyperphosphorylation involves both PP2A and GSK-3β. HEK293/tau cells were transfected with pcDNA3.1-I2CTF or, as a control, with pcDNA3.1. After 6 h of transfection, the cells were treated with 20 mM lithium chloride to inhibit GSK-3 for 12 h, 24 h, and 48 h. Western blots of lysates of these cells were analyzed for phosphorylation of tau at Ser-199, Thr231, Ser-262, and Ser-396. A partial inhibition of tau phosphorylation at Ser-199, Thr231, and Ser-396, the GSK-3β-acting sites and no inhibition at Ser-262, a PP2A, but not GSK-3β site, demonstrates involvement of both PP2A and GSK-3β activities in I2CTF-induced abnormal hyperphosphorylation of tau. *P < 0.05, **P < 0.01 vs. control; ΔP < 0.05, ΔΔP < 0.01 vs. I2CTF.
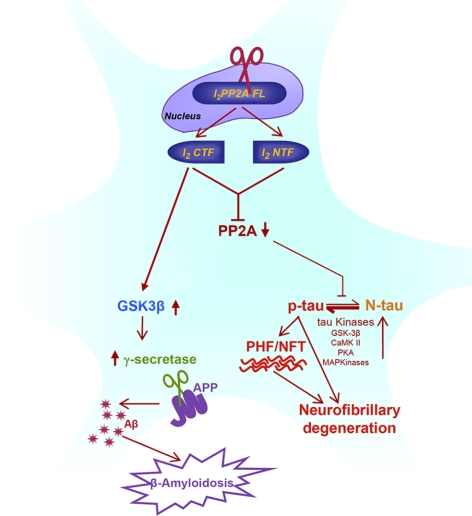
AAV1-I2CTF impairs spatial learning and memory in rats
Because hippocampal-dependent impairments are specifically the earliest cognitive symptoms detected in AD, we tested AAV1-I2CTF and control rats in a classic spatial reference memory task, which highly involves the hippocampal integrity. Animals were first tested at 5 mo of age. Before the water-maze learning and memory test, anxiety and exploratory activity levels were evaluated in the open field. AAV1-I2CTF rats displayed similar patterns of anxiety and exploratory activity as AAV1-GFP control animals (Fig. 7A,B; P=0.646 and P=0.814, respectively). However, assessment of learning and memory functions in the spatial reference memory task in water maze revealed a marked deficit in AAV1-I2CTF rats compared to AAV1-GFP controls (Fig. 7C; P=0.003). Although performance of AAV1-I2CTF rats increased across days of training, levels remained significantly lower than for the control group. These results revealed that AAV1-I2CTF rats were not able to properly encode the spatial coordinates of the submerged platform, suggesting a deficit in building a precise spatial representation of the environment, an hippocampal impairment.
Figure 7.
Expression of I2CTF impairs spatial learning and memory, and this impairment is associated with a decrease in the brain level of pSer133-CREB. A–D) While, as compared with the GFP rats (control group), the I2CTF rats showed no change in anxiety (A) or exploration (B) in the open field, these animals were impaired in spatial learning and memory at age 5 mo; P < 0.005 (C) and 8 mo; P < 0.01 (D, left bars) in Morris water maze during training but not in the transfer test (D, right bars). E) I2CTF animals also showed impairment in the consolidation of memory on retesting 1 mo after the previous water maze test; P < 0.05. F, G) These impairments in I2CTF rats were associated with a selective decrease in the brain level of pSer133-CREB.
The same animals were tested when they turned 8 mo of age. To suitably assess de novo the hippocampal function of these animals, we changed both the spatial environment of the testing room, as well as the geographic localization of the submerged platform in the pool. As represented in Fig. 7D, AAV1-I2CTF rats were impaired compared to AAV1-GFP controls (P=0.005) during the training of the task. We performed an additional session (i.e., transfer test) where we changed the platform location to assess hippocampal flexibility. Surprisingly, although global performance of AAV1-I2CTF rats was worse than control animals, the difference did not reach significance (Fig. 7D; P=0.344). These results suggested that AAV1-I2CTF rats were not impaired in the transfer test and can relocate the location of the platform in the environment as easily as control animals. We finally tested these groups of animals 1 mo later, i.e., at age of 9 mo, to evaluate long-term retention of the learned information. We observed that AAV1-I2CTF rats were impaired compared to AAV1-GFP animals (Fig. 7E; P=0.041). Overall, these results suggested that I2CTF overexpression induced hippocampal-dependent cognitive impairment, as well as difficulties to maintain or reactivate the long-term memory trace of given spatial information.
The AAV1-I2CTF -induced impairment in reference memory is associated with a decrease in pSer-133 CREB
Transcription factor CREB is known to regulate synaptic plasticity and learning and memory (47). We therefore investigated the levels of the activated pSer133 CREB and total CREB in AAV1-I2CTF animals. We found that the level of phospho-CREB (Ser-133 in 8-mo AAV1-I2CTF rats was markedly decreased compared with matched AAV1-GFP animals in ventricular area, cerebral cortex, and hippocampus separately (Fig. 7G), while total CREB level had a trend to decrease, but there were no significant difference between the two groups of animals (Fig. 7F).
DISCUSSION
AD is a major public health problem in modern society. Over 5 million people in the United States and 30 million people worldwide are affected by this single major cause of dementia in middle- to old-age individuals. By 2050, the number of AD cases could reach >16 million and 100 million in the United States and the world, respectively, unless drugs are available that can prevent or cure the disease. A first key step for a rational drug design is the elucidation of the different etiopathogenic mechanisms of this multifactorial disorder and the development of animal models based on these mechanisms. Previously, we showed that in AD brain I2PP2A/SET, which is primarily nuclear, is cleaved into I2NTF and I2CTF and translocated from the neuronal nucleus to the cytoplasm (18). Employing AAV1-induced expression of I2CTF in the brain, the present study shows that I2CTF inhibits PP2A activity, which leads to activation of GSK-3β, abnormal hyperphosphorylation of tau, intraneuronal accumulation of Aβ, neurodegeneration, loss of dendritic and synaptic plasticity, a decrease in the level of pSer133-CREB, and a hippocampal-based impairment in reference memory in the rat. These findings suggest a novel etiopathogenic mechanism of AD, which is initiated with the cleavage and the translocation of I2PP2A/SET from the neuronal nucleus to the cytoplasm. Furthermore, the present study simultaneously offers a novel nontransgenic animal model of AD, which recapitulates key features of the human disease and is relatively simple, rapid, and inexpensive and can be reproduced easily in other laboratories.
In the present study, along with the inhibition of PP2A, we found a decrease in the brain level of pSer9 GSK-3β, which indicates an increase in GSK-3β activity, in AAV1-I2CTF rats. This is consistent with a previous study that showed an antagonistic relationship between PP2A and GSK-3β through the mediation of I2PP2A activity by an alteration in the expression of heterogeneous ribonucleoprotein A18 in vivo (41). GSK-3β is known to form a complex with presenilin and enhance its γ-secretase activity and consequently promote β-amyloidosis (48). Thus, inhibition of PP2A and activation of GSK-3β by expression of I2CTF in AAV1-I2CTF animals would also be expected, as shown in the present study, to promote not only neurofibrillary degeneration of abnormally hyperphosphorylated tau but also stimulate β-amyloidosis through increased amyloidogenic processing of APP. Inhibition of GSK-3 activity has been shown to reduce Aβ production (46, 49). Moreover, the activities of both PKA and CaMKII, which are known to phosphorylate and modulate processing of APP, are regulated by PP2A (see ref. 50). Inhibition of PP2A activity by okadaic acid was previously shown to increase APP processing in PC12 cells (51). Thus, inhibition of PP2A by I2CTF might promote amyloidogenic processing of APP both through its phosphorylation by protein kinases that are regulated by PP2A and through modulation of the γ-secretase activity.
The exact nature of the protease that cleaves I2PP2A at N175 into I2CTF and I2NTF is at present not known. A recent study has reported that I2PP2A/SET can be cleaved at N175 by a lysosomal cysteine protease, asparagine endopeptidase (52). If this or a similar protease is found to be responsible for the generation of I2CTF from I2PP2A/SET in AD brain, inhibition of this protease activity could be a very promising therapeutic approach.
Consistent with previous studies that showed the role of PP2A as a major regulator of tau phosphorylation (6) and that the nonfilamentous/cytosolic abnormally hyperphosphorylated tau inhibits microtubule assembly (53), in the present study, we found abnormal hyperphosphorylation of tau and neurodegeneration in AAV1-I2CTF rats. Up to ∼9 mo post-AAV1-I2CTF infection, we observed the abnormal hyperphosphorylation but no aggregation of tau into neurofibrillary tangles in rat brain. Intronic mutations that alter the alternative splicing of tau, which changes the 3R:4R ratio, are known to produce tau pathology and FTDP-17 (54). Unlike adult humans, who express both 4R and 3R taus, adult rats express mostly 4R tau, and because of this difference in the isoform composition of this protein, it might require much longer than 9 mo for the AAV1-I2CTF rats to form any tangles. Like in AD, the AAV1-I2CTF animals showed loss of dendritic and synaptic plasticity as measured by levels of MAP2, synaptophysin, and synapsin-1.
Despite its abnormal hyperphosphorylation, the level of total tau in the I2CTF animals was found to be slightly decreased. This decrease in tau level is consistent with neurodegeneration, observed as a decrease in the level of neuron-specific βΙΙΙ tubulin, and increase in Fluoro-Jade staining.
The AAV1-I2CTF rats showed impairment in the acquisition of a spatial reference memory task and so was their ability to consolidate or reactivate a memory after a long delay. These impairments are characteristic of hippocampal dysfunction and mimic the early cognitive deficits observed in AD. The impairment of acquisition reflects difficulties to build a cognitive representation and the long-term deficit of retention reflects retrograde amnesia, which are specific symptoms of hippocampal dysfunction. These impairments were associated with a decreased level of pSer133-CREB, a known marker of memory specifically crucial for information processing in the hippocampus (55). Overall, AAV1-I2CTF rats displayed a behavioral phenotype matching with hippocampal alterations specifically observed in animal models of AD-like pathology and AD.
Finally, AAV1-I2CTF rat model, although it did not have any detectable neurofibrillary tangles or Aβ plaques by 8 mo, displayed many key features of AD, i.e., decrease in PP2A activity, abnormal hyperphosphorylation of tau, intraneuronal accumulation of Aβ, neurodegeneration, loss of dendritic and synaptic plasticity, decrease in the level of pSer133-CREB and cognitive impairment that is associated with the hippocampal function. These effects of inhibition of PP2A are probably both direct and indirect.
PP2A is not only a major tau phosphatase but also regulates the activities of several protein kinases, including cyclic AMP-dependent protein kinase, calcium calmodulin-dependent protein kinase II, and mitogen-activated protein kinases (6, 7, 11, 56, 57). Thus, the abnormal hyperphosphorylation of tau produced by inhibition of PP2A by I2CTF in the present study could be both due to a decrease in tau phosphatase and an increase in tau kinase activities. In the present study, we found the involvement of GSK-3β in the I2CTF-induced abnormal hyperphosphorylation of tau in stably tau-transfected HEK293 cells. The molecular mechanisms by which I2CTF caused a decrease in pSer9 GSK-3β and pSer133 CREB are currently not understood but most likely involve indirect effects through activation of other Ser/Thr phosphatases such as PP1 and PP2B. I2PP2A binds the catalytic subunit of PP1 under physiological concentrations of Mn2+ and stimulates its activity many fold (58). PPI probably dephosphorylates PSer9 GSK-3β and pSer133 CREB (59, 60). GSK-3β is also known to inhibit CREB activity by phosphorylating it at Ser-129 (61).
In summary, the present study suggests a novel etiopathogenic mechanism of AD in which the generation of I2CTF by the cleavage of I2PP2A/SET initiates a cascade of events, a key feature of which is the inhibition of PP2A activity (Fig. 8). The decreased PP2A activity leads to neurofibrillary degeneration of abnormally hyperphosphorylated tau by a decrease in dephosphorylation and by an increase in hyperphosphorylation through an increase in the activity of GSK-3β and probably other tau kinases, such as MAP kinase, C-Jun kinase, PKA, and CaMKII, the activities of which are known to be regulated by PP2A. Phosphorylation of APP by GSK-3β, PKA, and CaMKII is known to modulate the amyloidogenic processing of APP. GSK-3β is also known to enhance the γ-secretase activity of presenilin. Increase in the intraneuronal expression of Aβ in AAV1-I2CTF rats might have resulted from the involvement of all these processes. Furthermore, the AAV1-I2CTF rat model recapitulates many key features of AD and, thus, provides one of the most relevant animal models of sporadic AD. This animal model is simple, less labor intensive, and inexpensive, and can be easily replicated in other laboratories. Furthermore, it represents a feasible approach in which one has temporal and spatial control over transgene expression.
Figure 8.
A proposed novel etiopathogenic mechanism of AD, which is initiated by the cleavage of I2PP2A/SET into I2NTF and I2CTF.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Jian-Zhi Wang (Tongji Medical College, Wuhan, China) for a kind gift of HEK293/tau cells; to Drs. Fei Liu, Bin Li, and Guo-Hua Fan for helpful technical suggestions during the conduct of this study; to Dr. Ezzat El-Akkad for preparation of digital images; and to Ms. Janet Murphy for secretarial assistance.
This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and a research grant from the National Institutes of Health/National Institute of Aging, grant AG019158, and a 1-yr fellowship to Dr. Xiaochuan Wang from the National Natural Science Foundation of China (30871259) and the Education Ministry of China (NCET-07-0332). Author contributions: X.W. performed most of the experiments and analyses; J.B. carried out some of the immunohistochemical and all behavioral studies and analyses; E.K., N.C., and R.M.L. generated recombinant AAV1 and did the vector packaging and tittering; A.R. directed the generation of the I2CTF recombinant plasmids; I.G.I. directed and analyzed all immunohistochemical and immunochemical studies and the preparation of the manuscript; K.I. designed and oversaw the research project and directed most of the experiments, analyses, and writing.
REFERENCES
- 1. Iqbal K., Flory M., Khatoon S., Soininen H., Pirttila T., Lehtovirta M., Alafuzoff I., Blennow K., Andreasen N., Vanmechelen E., Grundke-Iqbal I. (2005) Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann. Neurol. 58, 748– 757 [DOI] [PubMed] [Google Scholar]
- 2. Alafuzoff I., Iqbal K., Friden H., Adolfsson R., Winblad B. (1987) Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. (Berl.). 74, 209– 225 [DOI] [PubMed] [Google Scholar]
- 3. Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U. S. A. 83, 4913– 4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261, 6084– 6089 [PubMed] [Google Scholar]
- 5. Bennecib M., Gong C. X., Grundke-Iqbal I., Iqbal K. (2000) Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 485, 87– 93 [DOI] [PubMed] [Google Scholar]
- 6. Gong C. X., Lidsky T., Wegiel J., Zuck L., Grundke-Iqbal I., Iqbal K. (2000) Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J. Biol. Chem. 275, 5535– 5544 [DOI] [PubMed] [Google Scholar]
- 7. Bennecib M., Gong C. X., Grundke-Iqbal I., Iqbal K. (2001) Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 490, 15– 22 [DOI] [PubMed] [Google Scholar]
- 8. Liu F., Grundke-Iqbal I., Iqbal K., Gong C. X. (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 22, 1942– 1950 [DOI] [PubMed] [Google Scholar]
- 9. Tanaka T., Zhong J., Iqbal K., Trenkner E., Grundke-Iqbal I. (1998) The regulation of phosphorylation of tau in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett. 426, 248– 254 [DOI] [PubMed] [Google Scholar]
- 10. An W. L., Cowburn R. F., Li L., Braak H., Alafuzoff I., Iqbal K., Iqbal I. G., Winblad B., Pei J. J. (2003) Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am. J. Pathol. 163, 591– 607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kins S., Kurosinski P., Nitsch R. M., Gotz J. (2003) Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am. J. Pathol. 163, 833– 843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L., Sengupta A., Haque N., Grundke-Iqbal I., Iqbal K. (2004) Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 566, 261– 269 [DOI] [PubMed] [Google Scholar]
- 13. Gandy S. E., Caporaso G. L., Buxbaum J. D., de Cruz Silva O., Iverfeldt K., Nordstedt C., Suzuki T., Czernik A. J., Nairn A. C., Greengard P. (1993) Protein phosphorylation regulates relative utilization of processing pathways for Alzheimer beta/A4 amyloid precursor protein. Ann. N. Y. Acad. Sci. 695, 117– 121 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki T., Ando K., Isohara T., Oishi M., Lim G. S., Satoh Y., Wasco W., Tanzi R. E., Nairn A. C., Greengard P., Gandy S. E., Kirino Y. (1997) Phosphorylation of Alzheimer beta-amyloid precursor-like proteins. Biochemistry. 36, 4643– 4649 [DOI] [PubMed] [Google Scholar]
- 15. Oishi M., Nairn A. C., Czernik A. J., Lim G. S., Isohara T., Gandy S. E., Greengard P., Suzuki T. (1997) The cytoplasmic domain of Alzheimer's amyloid precursor protein is phosphorylated at Thr654, Ser655, and Thr668 in adult rat brain and cultured cells. Mol. Med. 3, 111– 123 [PMC free article] [PubMed] [Google Scholar]
- 16. Gong C. X., Singh T. J., Grundke-Iqbal I., Iqbal K. (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 61, 921– 927 [DOI] [PubMed] [Google Scholar]
- 17. Li M., Guo H., Damuni Z. (1995) Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 34, 1988– 1996 [DOI] [PubMed] [Google Scholar]
- 18. Tanimukai H., Grundke-Iqbal I., Iqbal K. (2005) Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer's disease. Am. J. Pathol. 166, 1761– 1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen S., Li B., Grundke-Iqbal I., Iqbal K. (2008) I1PP2A affects tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. J. Biol. Chem. 283, 10513– 10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsujio I., Zaidi T., Xu J., Kotula L., Grundke-Iqbal I., Iqbal K. (2005) Inhibitors of protein phosphatase-2A from human brain structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett. 579, 363– 372 [DOI] [PubMed] [Google Scholar]
- 21. Henckaerts E., Dutheil N., Zeltner N., Kattman S., Kohlbrenner E., Ward P., Clement N., Rebollo P., Kennedy M., Keller G. M., Linden R. M. (2009) Site-specific integration of adeno-associated virus involves partial duplication of the target locus. Proc. Natl. Acad. Sci. U. S. A. 106, 7571– 7576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zolotukhin S., Potter M., Zolotukhin I., Sakai Y., Loiler S., Fraites T. J., Jr, Chiodo V. A., Phillipsberg T., Muzyczka N., Hauswirth W. W., Flotte T. R., Byrne B. J., Snyder R. O. (2002) Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 28, 158– 167 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y. J., Xu Y. F., Liu Y. H., Yin J., Wang J. Z. (2005) Nitric oxide induces tau hyperphosphorylation via glycogen synthase kinase-3beta activation. FEBS Lett. 579, 6230– 6236 [DOI] [PubMed] [Google Scholar]
- 24. Avila J., Wandosell F., Hernandez F. (2010) Role of glycogen synthase kinase-3 in Alzheimer's disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev. Neurother. 10, 703– 710 [DOI] [PubMed] [Google Scholar]
- 25. Bensadoun A., Weinstein D. (1976) Assay of proteins in the presence of interfering materials. Anal. Biochem. 70, 241– 250 [DOI] [PubMed] [Google Scholar]
- 26. Grundke-Iqbal I., Vorbrodt A. W., Iqbal K., Tung Y. C., Wang G. P., Wisniewski H. M. (1988) Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res. 464, 43– 52 [DOI] [PubMed] [Google Scholar]
- 27. Hasegawa M., Watanabe A., Takio K., Suzuki M., Arai T., Titani K., Ihara Y. (1993) Characterization of two distinct monoclonal antibodies to paired helical filaments: further evidence for fetal-type phosphorylation of the tau in paired helical filaments. J. Neurochem. 60, 2068– 2077 [DOI] [PubMed] [Google Scholar]
- 28. Pei J. J., Gong C. X., Iqbal K., Grundke-Iqbal I., Wu Q. L., Winblad B., Cowburn R. F. (1998) Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from Alzheimer's disease and control brains. J. Neural Transm. 105, 69– 83 [DOI] [PubMed] [Google Scholar]
- 29. Binder L. I., Frankfurter A., Rebhun L. I. (1985) The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101, 1371– 1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verma I. M., Weitzman M. D. (2005) Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 74, 711– 738 [DOI] [PubMed] [Google Scholar]
- 31. Lawlor P. A., Bland R. J., Das P., Price R. W., Holloway V., Smithson L., Dicker B. L., During M. J., Young D., Golde T. E. (2007) Novel rat Alzheimer's disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels. Mol. Neurodegener. 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong C. X., Grundke-Iqbal I., Iqbal K. (1994) Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience 61, 765– 772 [DOI] [PubMed] [Google Scholar]
- 33. Wang J. Z., Grundke-Iqbal I., Iqbal K. (2007) Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59– 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicolia V., Fuso A., Cavallaro R. A., Di Luzio A., Scarpa S. (2010) B vitamin deficiency promotes tau phosphorylation through regulation of GSK3beta and PP2A. J. Alzheimers Dis. 19, 895– 907 [DOI] [PubMed] [Google Scholar]
- 35. Sontag J. M., Nunbhakdi-Craig V., Montgomery L., Arning E., Bottiglieri T., Sontag E. (2008) Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A Bα subunit expression that correlate with enhanced tau phosphorylation. J. Neurosci. 28, 11477– 11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun L., Liu S. Y., Zhou X. W., Wang X. C., Liu R., Wang Q., Wang J. Z. (2003) Inhibition of protein phosphatase 2A- and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience 118, 1175– 1182 [DOI] [PubMed] [Google Scholar]
- 37. Planel E., Yasutake K., Fujita S. C., Ishiguro K. (2001) Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J. Biol. Chem. 276, 34298– 34306 [DOI] [PubMed] [Google Scholar]
- 38. Li B., Yamamori H., Tatebayashi Y., Shafit-Zagardo B., Tanimukai H., Chen S., Iqbal K., Grundke-Iqbal I. (2008) Failure of neuronal maturation in Alzheimer disease dentate gyrus. J. Neuropathol. Exp. Neurol. 67, 78– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masliah E., Terry R. D., DeTeresa R. M., Hansen L. A. (1989) Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci. Lett. 103, 234– 239 [DOI] [PubMed] [Google Scholar]
- 40. Schmued L. C., Albertson C., Slikker W., Jr (1997) Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 751, 37– 46 [DOI] [PubMed] [Google Scholar]
- 41. Liu G. P., Zhang Y., Yao X. Q., Zhang C. E., Fang J., Wang Q., Wang J. Z. (2008) Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol. Aging 29, 1348– 1358 [DOI] [PubMed] [Google Scholar]
- 42. Iqbal K., Liu F., Gong C. X., Alonso Adel C., Grundke-Iqbal I. (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 118, 53– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pei J. J., Braak E., Braak H., Grundke-Iqbal I., Iqbal K., Winblad B., Cowburn R. F. (1999) Distribution of active glycogen synthase kinase 3β (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 58, 1010– 1019 [DOI] [PubMed] [Google Scholar]
- 44. Gantier R., Gilbert D., Dumanchin C., Campion D., Davoust D., Toma F., Frebourg T. (2000) The pathogenic L392V mutation of presenilin 1 decreases the affinity to glycogen synthase kinase-3 beta. Neurosci. Lett. 283, 217– 220 [DOI] [PubMed] [Google Scholar]
- 45. Michel G., Mercken M., Murayama M., Noguchi K., Ishiguro K., Imahori K., Takashima A. (1998) Characterization of tau phosphorylation in glycogen synthase kinase-3β and cyclin dependent kinase-5 activator (p23) transfected cells. Biochim. Biophys. Acta 1380, 177– 182 [DOI] [PubMed] [Google Scholar]
- 46. Phiel C. J., Wilson C. A., Lee V. M., Klein P. S. (2003) GSK-3α regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 423, 435– 439 [DOI] [PubMed] [Google Scholar]
- 47. Davis G. W., Schuster C. M., Goodman C. S. (1996) Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron 17, 669– 679 [DOI] [PubMed] [Google Scholar]
- 48. Ho L., Qin W., Pompl P. N., Xiang Z., Wang J., Zhao Z., Peng Y., Cambareri G., Rocher A., Mobbs C. V., Hof P. R., Pasinetti G. M. (2004) Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 18, 902– 904 [DOI] [PubMed] [Google Scholar]
- 49. Rezai-Zadeh K., Douglas Shytle R., Bai Y., Tian J., Hou H., Mori T., Zeng J., Obregon D., Town T., Tan J. (2009) Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J. Cell. Mol. Med. 13, 574– 588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Small S. A., Gandy S. (2006) Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron 52, 15– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caporaso G. L., Gandy S. E., Buxbaum J. D., Ramabhadran T. V., Greengard P. (1992) Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 89, 3055– 3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Z., Jang S. W., Liu X., Cheng D., Peng J., Yepes M., Li X. J., Matthews S., Watts C., Asano M., Hara-Nishimura I., Luo H. R., Ye K. (2008) Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol. Cell. 29, 665– 678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alonso A. D., Li B., Grundke-Iqbal I., Iqbal K. (2006) Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc. Natl. Acad. Sci. U. S. A. 23, 8864– 8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 393, 702– 705 [DOI] [PubMed] [Google Scholar]
- 55. Barco A., Pittenger C., Kandel E. R. (2003) CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin. Ther. Targets 7, 101– 114 [DOI] [PubMed] [Google Scholar]
- 56. Sengupta A., Grundke-Iqbal I., Iqbal K. (2006) Regulation of phosphorylation of tau by protein kinases in rat brain. Neurochem. Res. 31, 1473– 1480 [DOI] [PubMed] [Google Scholar]
- 57. Pei J. J., Gong C. X., An W. L., Winblad B., Cowburn R. F., Grundke-Iqbal I., Iqbal K. (2003) Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer's disease. Am. J. Pathol. 163, 845– 858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katayose Y., Li M., Al-Murrani S. W., Shenolikar S., Damuni Z. (2000) Protein phosphatase 2A inhibitors, I(1)(PP2A) and I(2)(PP2A), associate with and modify the substrate specificity of protein phosphatase 1. J. Biol. Chem. 275, 9209– 9214 [DOI] [PubMed] [Google Scholar]
- 59. Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. (1992) Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell 70, 105– 113 [DOI] [PubMed] [Google Scholar]
- 60. Alberts A. S., Montminy M., Shenolikar S., Feramisco J. R. (1994) Expression of a peptide inhibitor of protein phosphatase 1 increases phosphorylation and activity of CREB in NIH 3T3 fibroblasts. Mol. Cell. Biol. 14, 4398– 4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horike N., Sakoda H., Kushiyama A., Ono H., Fujishiro M., Kamata H., Nishiyama K., Uchijima Y., Kurihara Y., Kurihara H., Asano T. (2008) AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3β and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J. Biol. Chem. 283, 33902– 33910 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.