Abstract
A Japanese male developed deafness, pyramidal signs and ataxia at age 50. A cerebrospinal fluid examination showed elevated levels of iron, transferrin and ferritin. Brain MRI showed atrophy of the cerebellum and pons as well as potential iron deposits on the surface of the brain. At autopsy, the brain weighed 1090 g and showed severe atrophy and necrosis of the cerebellum. No vascular malformation was observed. Extensive deposits of hemosiderin that were well stained with Berlin blue and ferritin immunohistochemistry were present at the surface and in the superficial layers of the cerebrum, brainstem, cerebellum and spinal cord. In these regions, numerous AT8 (p-τ)-immunopositive deposits were present in neurons and glia. In addition, phosphorylated α-synuclein-immunopositive Lewy bodies and neurites were observed in the brainstem nuclei. In the present report, the authors derive the novel insight that superficial siderosis is a distinctive entity associated with tauopathy and synucleinopathy.
Background
Superficial siderosis (superficial hemosiderosis) is a neurodegenerative disorder characterised clinically by a classic triad (cerebellar ataxia, sensorineural deafness, and myelopathy) as well as neuropathologically by deposition of hemosiderin, an iron-storage complex, in the subpial regions of the cerebrum and cerebellum.1 Ever since superficial siderosis was first reported in 1908, approximately 200 English articles on the disorder have been published. Most cases have been diagnosed based on the classic clinical symptoms and neuroradiological findings. However, only a few neuropathologists have reported neuropathologic analyses of superficial siderosis.2–11 These authors reported numerous hemosiderin deposits in the subpial regions of the cerebrum and cerebellum, and pathologic alteration of the cerebellum was particularly severe in many cases. The pathomechanism underlying the susceptibility of the cerebellum may be that Bergmann glia serve as conduits for heme. In addition, the eighth cranial nerves are also susceptible to superficial siderosis, presumably because they consist of central nervous system axons, myelin and neuroglial tissue along their subarachnoid course.11
One of the neuropathologic hallmarks of Alzheimer’s disease is abnormal aggregation of phosphorylated τ protein in neurons. The abnormal aggregation of the presynaptic protein phosphorylated α-synuclein is a cardinal neuropathologic change of Parkinson’s disease. In both pathologic conditions, several studies have indicated an association between oxidative stress and abnormal accumulation of τ or synuclein.12–14 In particular, iron, hemosiderin and ferritin may contribute to the production of free radicals and neurodegeneration.15
In this paper, we report unique neuropathologic findings of superficial siderosis at autopsy. The patient showed numerous sites of τ and α-synuclein accumulation in multiple regions of the central nervous system. These sites may be associated with oxidative stress due to hemosiderin deposition.
Case presentation
A Japanese male developed mild cognitive impairment, dysarthria, deafness, pyramidal signs and cerebellar ataxia at age 50. Although he had difficulty speaking, he was able to continue his work as an operator of a fire station without any trouble. At age 54, he was examined by an orthopedist because of lower back pain. According to the patient’s medical records, MRI had shown mild degeneration of a lumbar disc. Around the same time, there were several claims about his speech that were difficult to understand over the phone. A neurological examination at age 54 revealed mild cognitive impairment (total intelligence quotient, 65; verbal intelligence quotient, 67; performance intelligence quotient, 70; Hasegawa dementia scale-revised, 25 out of 30). The patient exhibited severe dysarthria, moderate hearing loss, dysdiadochokinesis, dysmetria in the upper extremities, hyperreflexia in the upper and lower extremities and ataxic gait. Neither urinary incontinence nor anosmia was recorded. Laboratory tests showed a mild elevation of serum ferritin (256–512×; normal range, 16–128×). An examination of the cerebrospinal fluid (CSF) revealed normal cell counts, total protein levels, and glucose levels in the absence of red blood cells. The level of iron was 16 μg/dl (normal range: 0.01–0.02 μg/dl), that of transferrin was 3.0 mg/dl (0.024–0.04 mg/dl) and that of ferritin was 189 ng/ml (normal range: less than 1 ng/ml). Electromyogram and nerve conduction tests were normal. Pure-tone audiometry results were consistent with sensorineural deafness on both sides. An intravenous olfactory test was normal. Brain MRI showed diffuse cerebellar atrophy in T1-weighted images. MRI with T2- and T2*-weighted images showed hypointense rims surrounding the brainstem, cerebellum and cerebrum (figure 1). The patient was diagnosed as having superficial hemosiderosis without a definite causative disorder. His anamnesis revealed otitis media at age 12, appendicitis at age 17 and a motor vehicle accident at age 19. However, he had no history of surgery on the central nervous system.
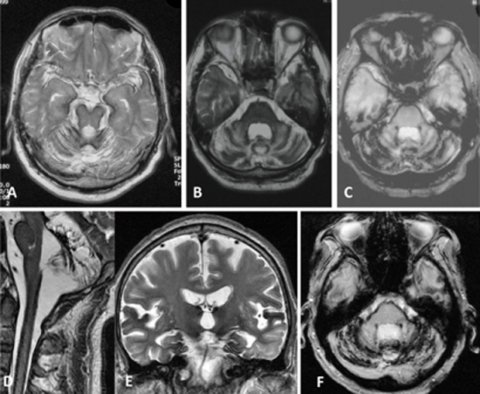
Figure 1.
Brain MRI images of the patient described in this report. (A) T2-weighted image (T2WI; 1.5 T) at age 54. (B–F) T2WI (B, D, E) and T2*-weighted images (T2*WI) (C, F) obtained with a 3-T MRI scanner at ages 58 (B, C), 63 (D), 64 (E), and 66 (F). T2WI and T2*WI show hypointense rims surrounding the brainstem, cerebellum, and temporal lobes as well as the spinal cord. Severe atrophy of the cerebellum is also evident. Mild progressive atrophy of the cerebellum may have occurred over the course of the12-year period.
Treatment
Although the patient was given taltirelin hydrate (a thyrotropin-releasing hormone analogue) for his cerebellar ataxia, his neurological condition showed no improvement.
Outcome and follow-up
At age 56, the patient started to use a cane, although his general neurological condition was stable. At age 60, decreased tendon reflexes were recorded in the extremities. At age 63, he was admitted to our hospital for respite care. A neurological examination showed no marked alterations in comparison to the test results at age 60. In addition, there were no obvious changes across repeated MRI exams at ages 54, 58, 62, 63 and 64 (figure 1). Repeated MRI images showed no subdural hematoma up to age 64.The MRI exam at age 63 revealed the presence of hemosiderin deposits around the spinal cord. After the patient was affected with community-acquired pneumonia at age 64, he developed disuse syndrome and needed to use a wheelchair. His condition deteriorated; ultimately, he became bedridden and remained so until his death at age 66.
Neuropathology
Gross neuropathology
The brain weighed 1090 g. There was diffuse and moderate atrophy of the cerebrum, as well as severe atrophy of the brainstem and cerebellum. The surface of the brain exhibited a diffusely brownish discolouration that was particularly conspicuous at the basilar surface, brainstem and cerebellum. In particular, the anterior portion of the cerebellar vermis showed severe tissue degradation with brownish discolouration, suggesting hemosiderin deposition. The same discolouration was also observed at the olfactory, optic, oculomotor, trigeminal, facial and acoustic nerves. There were thin, brown neo-membranes inside the right dura mater. This finding is consistent with a chronic subdural hematoma. There was minimal atherosclerosis of the major cerebral arteries, and no anomalies were present. Neither aneurysms nor vascular malformations were present. In coronal slices, there was no parenchymal haemorrhage evident in the cerebrum or brainstem. The basilar surface of the cerebrum showed a brownish colour. There was a brown rim surrounding the brainstem and spinal cord. It was difficult to identify the upper surface of the cerebellar cortex because of severe deterioration of the parenchyma.
Microscopic neuropathology
Hemosiderosis
The most remarkable finding was the extensive deposition of hemosiderins (figure 2), which are brown pigments in macrophages easily identified by the use of Berlin blue stain. The hemosiderins were present in the subarachnoid space, subpial regions and parenchyma. In the cerebrum, they were more prominent at the basilar surface, including the basilar surfaces of the frontal and temporal cortices, where the hemosiderins were observed in the upper cortical layers. They were also conspicuous in the perivascular spaces of small parenchymal vessels. The upper cortical layers displayed vacuolar changes and gliosis.
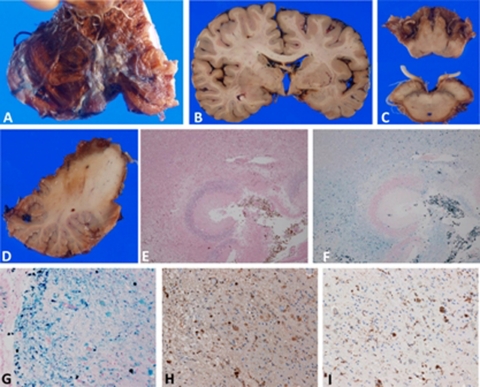
Figure 2.
Neuropathology of hemosiderosis. (A–D) Gross neuropathology. (A, D) Severe degeneration and brownish discolouration, indicating hemosiderosis, were observed at the anterior part of the cerebellum. Part of the left cerebellar hemisphere was removed and stored at –80°C for future analysis. (B, C) Hemosiderosis was present at the basal part of the cerebrum as well as the surface of the brainstem and cranial nerve roots. (E, F) Microscopic neuropathology. Severe necrotic lesions and hemosiderin deposits were observed in the cerebellar cortex (E, H&E stain; F, Berlin blue stain). (G) Severe iron deposits contained in macrophages were detected in the parahippocampus. (H) Some free iron deposits were also observed. Some ovoid bodies were present, which were also immunopositive for ferritin. (G, Berlin blue stain; H, ferritin immunohistochemistry; I, CD68 (for macrophages) immunohistochemistry) (E and F, 10× objective; G–I, 40× objective).
Similar alterations were observed on the surface of the brainstem and spinal cord. In addition to hemosiderin deposition, the parenchymal tissue at the anterior part of the cerebellum was lost. Hemosiderin deposits and loss of myelinated fibres were evident in the cranial nerve roots.
Although most hemosiderins were observed in macrophages, some were also detected in glia and in the cerebral parenchyma as free deposits. Hemosiderins were immunoreactive for polyclonal antibodies raised against ferritin. In the cerebral parenchyma, there were moderate numbers of pale oval bodies (ovoid bodies). These bodies were also positive for the Berlin blue stain and ferritin immunohistochemistry. In some instances, the glia were positive for Berlin blue, and ferritin immunohistochemistry revealed immunopositive deposits in the cell somata and dendrites of glial cells. There was no strong evidence of an active subarachnoid haemorrhage because only a few red cells were observed in the subarachnoid space.
There was mild thickening of the vessel walls of the basal ganglia. A monoclonal antibody raised against the synthetic peptide Aβ11-28 (IBL, Maebashi Japan) rarely stained amyloid deposits in the parenchymal and leptomeningeal vessel walls at the level of the temporal lobe. No amyloid angiopathy was detected in other regions such as the cerebellum, brainstem and spinal cord. In addition, haemorrhagic changes were absent in the brain and spinal cord. Aβ11-28–immunopositive diffuse plaques were sparse in the neocortex. A chronic subdural hematoma was present in the convexity of the dura mater. Histological examinations revealed thin membranes with hemosiderin deposits.
Tauopathy
Abundant τ deposits were notable in the present case. Neurofibrillar tangles (NFTs) and neuropil threads (NTs) were observed in the hippocampus, subiculum and entorhinal regions when the tissue was stained with a monoclonal antibody raised against phosphorylated tau (AT8, Innogenetics, Temse, Belgium). The distribution of NFTs and NTs was consistent with stage III of Braak’s classification.16 In addition, AT8-immunopositive pretangles and NFTs were observed in the cerebellar dentate nucleus and locus coeruleus, respectively (figure 3). In the neurons of the locus coeruleus, NFTs and Lewy body were occasionally co-localised in the same neurons (figure 4). It is notable that τ-immunopositive deposits were observed in astrocytes and neurites (figure 3). These deposits were apparently associated with areas in the parahippocampus and occipital cortex in which hemosiderin deposits were abundant (figure 3). In the cerebellar cortex, τ-immunopositive deposits were observed in Bergmann glia (figure 3). τ-immunopositive neurites were also observed in the cranial nerve roots.
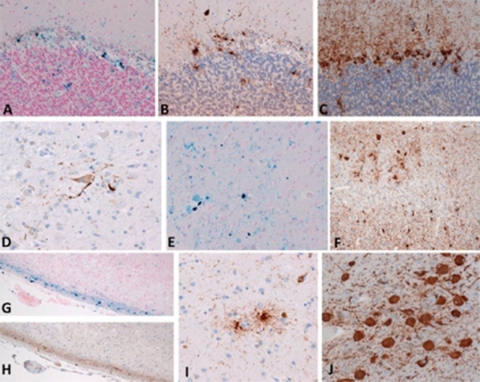
Figure 3.
Microscopic neuropathology associated with tauopathy. (A–C) cerebellar cortex; (E and F) visual cortex; (G and H) parahippocampus. In areas with abundant iron deposits(A, G, E), numerous τ-immunopositive deposits were observed in the glial cells of adjacent sections (B, F, H). (D) τ-immunopositive neurons were present in the dentate nucleus of the cerebellum. (I) τ-immunopositive astrocytes were detected in the caudate nucleus. (J) Numerous τ-immunopositive neurofibrillary tangles were present in the locus coeruleus. (A and G, Berlin blue stain; B–E and H–J, immunohistochemistry using antibody (AT8) raised against phosphorylated tau protein.) (A–F, I, and J, 40× objective; G and H, 20× objective).
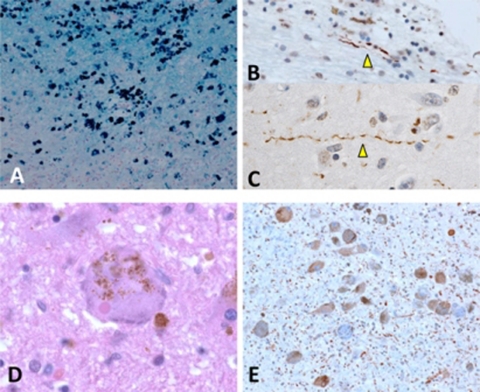
Figure 4.
Microscopic neuropathology associated with α-synucleinopathy. (A) Large numbers of iron deposits were observed in the olfactory bulb. (B, C) α-synuclein-immunopositive Lewy neurites were detected in the olfactory bulb (B) and amygdala (C). (D) A Lewy body and neurofibrillar tangles are co-localised in a neuron of the locus coeruleus. (E) α-synuclein-immunopositive Lewy neurites and Lewy bodies are observed in the locus coeruleus. (A, Berlin blue stain; B, C and E immunohistochemistry using antibody (pSyn#64) raised against α-synuclein; (D) H&E stain.) (A, 20× objective; B–D, 60× objective; E, 40× objective).
Synucleinopathy
A monoclonal antibody raised against phosphorylated α-synuclein (pSyn#64, WAKO, Japan) revealed Lewy neurites in the olfactory bulb, amygdala, parahippocampus, substantia innominata, locus coeruleus and substantia nigra (figure 4). Lewy bodies were detected in the locus coeruleus and substantia innominata. The Lewy bodies and neurites corresponded to Braak stages 2–317 and stage I (incidental Lewy body disease) of our brain bank method.18 No α-synuclein-immunopositive deposits were observed in the cerebral neocortex.
Discussion
According to a recent review article, most individuals with superficial siderosis show cerebellar ataxia (81%), sensorineural deafness (81%) and myelopathy (53%).1 The clinical presentation of our patient was clearly consistent with that of superficial siderosis. We believe that his cognitive dysfunction was directly associated with superficial siderosis and not with other neurological disorders. As mentioned below, there was no advanced Alzheimer’s disease, dementia with Lewy bodies, or cerebrovascular disorders. Although superficial siderosis is considered a progressive neurodegenerative disorder, the progression of clinical and neuroradiological conditions during a 12-year assessment period was very slow in the present patient.
The pathomechanism of superficial siderosis remains controversial. Generally, continuous or recurrent bleeding in the subarachnoid space is thought to be associated with siderosis in the central nervous system. In fact, some cases of superficial siderosis may be associated with brain tumours, dural tears, trauma, or brachial plexus trauma.1 19–23 However, no definitive lesion is identified in 50% of the cases. Although we carefully carried out an autopsy, we could not identify the occult bleeding site in the central nervous system. At the time of autopsy, a chronic subdural hematoma was observed in the convexity of the dura mater. Subdural hematomas have been reported as a rare cause of superficial siderosis.1 24 In the present case, the subdural hematoma was first recognised by MRI at age 66. That is, repeated MRI images showed no subdural hematoma up to age 64. We believe that the subdural hematoma developed in the end stage of the clinical course and was not related directly to the siderosis. In addition, the CSF analysis showed no red blood cells four years after onset (at age 54), although the iron and ferritin levels increased. Therefore, continuous subarachnoid bleeding did not cause the superficial siderosis in the present patient.
The basic neuropathologic findings were consistent with the well-documented pathology of superficial siderosis.6 8 11 Hemosiderin deposits undoubtedly contribute to the parenchymal degeneration of the central nervous system. However, it remains unclear why specific anatomical regions are more susceptible than others. Koeppen et al concluded that the susceptibility of the cerebellar cortex is likely due to the abundance of microglia and the presence of Bergmann glia that serve as conduits for heme.11 Revesz et al postulated that cerebellar pathology is associated with the dense capillary network of the cerebellum as well as the vulnerability of Purkinje and granule cells.10 Consistent with this, the anterior part of the cerebellar vermis and hemisphere showed severe degeneration and hemosiderin deposition in our case. However, it is still difficult to explain why the pathologic changes were also severe at the basal surface of the cerebrum, brainstem and spinal cord. A compartmentalisation of CSF flow might be important for understanding how hemosiderin preferentially accumulates in specific anatomical regions.2
τ protein is a major component of NFTs and glial tangles in many neurodegenerative disorders. In superficial siderosis, NFTs have only been reported in the locus coeruleus.6 However, extensive τ deposits have never been reported in cases of superficial siderosis.11 25 26 In addition to some NFTs in the hippocampus and parahippocampus, we found abundant glial τ accumulation in the cerebellum and in nerve roots with severe hemosiderin deposits. Importantly, two papers have mentioned increased levels of τ and phosphorylated τ protein in the CSF of individuals with superficial siderosis.27 28 An increased level of τ protein in CSF is an important biomarker for the clinical diagnosis of Alzheimer’s disease because it suggests an abnormal accumulation of τ protein in the brain parenchyma.29 Although it was impossible to evaluate the τ levels in CSF for this patient, the pathologic findings of this and other studies27 28 strongly suggest that τ accumulation in neurons and glia may be a common pathologic alteration of superficial siderosis.
The association between synucleinopathy and siderosis in the present case remains unclear. Like τ aggregation, α-synuclein aggregation might be induced under conditions of oxidative stress related to iron.13 30 In the present case, we believe that the α-synuclein accumulation in the olfactory bulb was strongly associated with oxidative stress due to severe hemosiderosis in this region. Recent studies suggest that α-synuclein accumulation may propagate along neural connections from the peripheral to the central nervous system. The olfactory bulb is one of the first anatomical regions of α-synuclein accumulation.31 It may take 10–15 years to reach Lewy body stages 2–3 based on Braak’s hypothesis.32 If the propagation hypothesis is correct, we can speculate that the condition in the patient described in the present report was reached after a comparable duration following initial accumulation of α-synuclein in the olfactory bulb. In fact, the clinical course of the disease in this patient was 16 years from onset to death.
To summarise, we present the first case of superficial siderosis associated with tauopathy and synucleinopathy. On the basis of neuropathologic analyses, we suggest that oxidative stress induced by hemosiderosis may contribute to abnormal aggregation of τ and α-synuclein protein. Given that many physicians are now using MRI in their daily clinical practice, the likelihood of identifying superficial siderosis will increase. However, only a few autopsy cases have been analysed to date. Further analyses will be important to understand the pathomechanism of superficial siderosis.
Learning points.
-
▶
Superficial siderosis is characterised clinically by sensorineural deafness, cerebellar ataxia and myelopathy.
-
▶
MRI readily identifies hemosiderosis deposits and may aid in the clinical diagnosis.
-
▶
Neuropathologic analyses showed severe degeneration of the cerebellum, brainstem and spinal cord, as well as the basal surface of the cerebrum.
-
▶
In addition to extensive hemosiderin deposition, tauopathy and synucleinopathy may be associated with the pathomechanism of the disease.
Acknowledgments
This study was in part supported by Research on Measures for Intractable Diseases (Takao, H23-nanchi-ippan-062). The authors are grateful to Katsura Suwabe, Shinichi Aoyagi and Mitsutoshi Tano for their technical assistance.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol 2007;3:54–8; quiz 59. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh JB, Holton JL, Nolan CC. Selective damage to the cerebellar vermis in chronic alcoholism: a contribution from neurotoxicology to an old problem of selective vulnerability. Neuropathol Appl Neurobiol 1997;23:355–63 [PubMed] [Google Scholar]
- 3.Heye N, Kastrup O, Terstegge K, et al. Superficial siderosis of the central nervous system. Arch Gerontol Geriatr 1994;18:181–90 [DOI] [PubMed] [Google Scholar]
- 4.Kellermier H, Wang G, Wiley C. Iron localization in superficial siderosis of the central nervous system. Neuropathology 2009;29:187–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeppen AH. Superficial siderosis of the central nervous system. J Neuropathol Exp Neurol 1971;30:135–6 [PubMed] [Google Scholar]
- 6.Koeppen AH, Barron KD. Superficial siderosis of the central nervous system. A histological, histochemical and chemical study. J Neuropathol Exp Neurol 1971;30:448–69 [DOI] [PubMed] [Google Scholar]
- 7.Koeppen AH, Borke RC. Experimental superficial siderosis of the central nervous system. I. Morphological observations. J Neuropathol Exp Neurol 1991;50:579–94 [DOI] [PubMed] [Google Scholar]
- 8.Koeppen AH, Dentinger MP. Brain hemosiderin and superficial siderosis of the central nervous system. J Neuropathol Exp Neurol 1988;47:249–70 [DOI] [PubMed] [Google Scholar]
- 9.Koeppen AH, Dickson AC. Tin-protoporphyrin prevents experimental superficial siderosis in rabbits. J Neuropathol Exp Neurol 2002;61:689–701 [DOI] [PubMed] [Google Scholar]
- 10.Revesz T, Earl CJ, Barnard RO. Superficial siderosis of the central nervous system presenting with longstanding deafness. J R Soc Med 1988;81:479–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koeppen AH, Michael SC, Li D, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol 2008;116:371–82 [DOI] [PubMed] [Google Scholar]
- 12.Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 2010;345:91–104 [DOI] [PubMed] [Google Scholar]
- 13.Li W, Jiang H, Song N, et al. Oxidative stress partially contributes to iron-induced alpha-synuclein aggregation in SK-N-SH cells. Neurotox Res 2011;19:435–42 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto A, Shin RW, Hasegawa K, et al. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 2002;82:1137–47 [DOI] [PubMed] [Google Scholar]
- 15.Quintana C, Bellefqih S, Laval JY, et al. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol 2006;153:42–54 [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Ruberu NN, Sawabe M, et al. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol 2004;63:742–9 [DOI] [PubMed] [Google Scholar]
- 19.Hoxworth JM, Patel AC, Bosch EP, et al. Localization of a rapid CSF leak with digital subtraction myelography. AJNR Am J Neuroradiol 2009;30:516–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu WC, Loevner LA, Forman MS, et al. Superficial siderosis of the CNS associated with multiple cavernous malformations. AJNR Am J Neuroradiol 1999;20:1245–8 [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N. Superficial siderosis: associations and therapeutic implications. Arch Neurol 2007;64:491–6 [DOI] [PubMed] [Google Scholar]
- 22.Leussink VI, Flachenecker P, Brechtelsbauer D, et al. Superficial siderosis of the central nervous system: pathogenetic heterogeneity and therapeutic approaches. Acta Neurol Scand 2003;107:54–61 [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez FR, Srinivasan A. Superficial siderosis of the CNS. AJR Am J Roentgenol 2011;197:W149–52 [DOI] [PubMed] [Google Scholar]
- 24.Kakeda S, Korogi Y, Ohnari N, et al. Superficial siderosis associated with a chronic subdural hematoma: T2-weighted MR imaging at 3T. Acad Radiol 2010;17:871–6 [DOI] [PubMed] [Google Scholar]
- 25.Hughes JT, Oppenheimer DR. Superficial siderosis of the central nervous system. A report on nine cases with autopsy. Acta Neuropathol 1969;13:56–74 [DOI] [PubMed] [Google Scholar]
- 26.Janss AJ, Galetta SL, Freese A, et al. Superficial siderosis of the central nervous system: magnetic resonance imaging and pathological correlation. Case report. J Neurosurg 1993;79:756–60 [DOI] [PubMed] [Google Scholar]
- 27.Ikeda T, Noto D, Noguchi-Shinohara M, et al. CSF tau protein is a useful marker for effective treatment of superficial siderosis of the central nervous system: two case reports. Clin Neurol Neurosurg 2010;112:62–4 [DOI] [PubMed] [Google Scholar]
- 28.Kondziella D, Zetterberg H. Hyperphosphorylation of tau protein in superficial CNS siderosis. J Neurol Sci 2008;273:130–2 [DOI] [PubMed] [Google Scholar]
- 29.Schott JM, ADNI Investigators Using CSF biomarkers to replicate genetic associations in Alzheimer’s disease. Neurobiol Aging 2011. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Q, Song N, Xu H, et al. Alpha-synuclein aggregation is involved in the toxicity induced by ferric iron to SK-N-SH neuroblastoma cells. J Neural Transm 2011;118:397–406 [DOI] [PubMed] [Google Scholar]
- 31.Sengoku R, Saito Y, Ikemura M, et al. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol 2008;67:1072–83 [DOI] [PubMed] [Google Scholar]
- 32.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord 2010;16:79–84 [DOI] [PubMed] [Google Scholar]