Abstract
Background
Exposure to the chemotherapeutic alkylating agent thiotepa during brain development leads to neurological complications arising from neurodegeneration and irreversible damage to the developing central nerve system (CNS). Administration of single dose of thiotepa in 7-d postnatal (P7) rat triggers activation of apoptotic cascade and widespread neuronal death. The present study was aimed to elucidate whether nicotinamide may prevent thiotepa-induced neurodegeneration in the developing rat brain.
Methodology/Principal Findings
Neuronal cell death induced by thiotepa was associated with the induction of Bax, release of cytochrome-c from mitochondria into the cytosol, activation of caspase-3 and cleavage of poly (ADP-ribose) polymerase (PARP-1). Post-treatment of developing rats with nicotinamide suppressed thiotepa-induced upregulation of Bax, reduced cytochrome-c release into the cytosol and reduced expression of activated caspase-3 and cleavage of PARP-1. Cresyl violet staining showed numerous dead cells in the cortex hippocampus and thalamus; post-treatment with nicotinamide reduced the number of dead cells in these brain regions. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) and immunohistochemical analysis of caspase-3 show that thiotepa-induced cell death is apoptotic and that it is inhibited by nicotinamide treatment.
Conclusion
Nicotinamide (Nic) treatment with thiotepa significantly improved neuronal survival and alleviated neuronal cell death in the developing rat. These data demonstrate that nicotinamide shows promise as a therapeutic and neuroprotective agent for the treatment of neurodegenerative disorders in newborns and infants.
Introduction
Neurological dysfunction is a well-known adverse effect of cancer therapeutics [1]. Chemotherapy, for example, is associated with an increased occurrence of neurodegenerative disorders that impair the development of higher mental abilities, cognitive status and academic achievements in children [2], [3], [4]. Furthermore, the toxic effects of anticancer agents can lead to neurological disorders such as cerebral infarction, seizures, leukoencephalopathy and others [5]. Chemotherapeutic toxicity has been shown to induce neuronal cell demise through the activation of two well-known apoptotic cascades [6], [7], [8]. Under the influence of some anticancer drugs, cytochrome c is released into the cytosol; in the presence of ATP, such release causes oligomerization of Apaf-1 (apoptotic protease activating factor 1) and activation of caspase-9 and caspase-3 [9], [10], [11], [12]. One such drug is thiotepa (N,N′N′-triethylenethiophosphoramide), an alkylating agent used for treatment of breast, colon, lung, brain, gastric, bladder and ovarian cancers; administration of thiotepa can also lead to poly (ADP-ribose) polymerase (PARP-1) activation [13], [14].
Nicotinamide, an amide of vitamin B3, is the precursor of coenzyme β-nicotinamide adenine dinucleotide (NAD+). NAD+ is considered to be necessary for cellular functions and metabolism [15]. Nicotinamide is well known to exhibit preclinical efficacy and to protect against neurological damage, but the exact mechanism of neuroprotection remains enigmatic. It is known that severe cellular insult leads to increased activity of PARP-1, which causes NAD+ depletion and apoptosis [14]. In the presence of nicotinamide, an essential precursor to NAD+, cellular NAD+ stores are more effectively replenished and damaged DNA is more effectively repaired [15], [16]. Nicotinamide improves neuronal survival following a variety of insults, including free radical exposure and oxidative stress [17], [18]. Its protective function is thought to be based on its numerous and diverse pharmacological effects, which include inhibition of PARP-1, prevention of ATP depletion [19], [20], lipid peroxidation, anti-inflammatory activity, and prevention of apoptosis [18], [21]. Nicotinamide also modulates mitochondrial membrane potential and the formation of pores, prevents cytochrome c release into the cytosol, and inhibits caspase-9 and caspase-3 like activities through mechanisms that are independent of those involving the mitogen-activated protein MAP kinase p38 and the c-Jun N-terminal kinases JNK [17], [18], [19], [20], [21], [22].
Chemotherapy for cancer treatment is often a necessity, and people diagnosed with cancer frequently receive chemotherapy in spite of its severe neurotoxic effects. Because thiotepa is routinely used as a chemotherapeutic agent, improvement of the neurological outcome of neonates and infants who experience neurotoxicity following treatment with this drug depends on advancing understanding of the precise molecular mechanisms triggering thiotepa-induced neurodegeneration and the development of neuroprotective therapeutics. The present study aimed to examine the protective role of nicotinamide against thiotepa-induced neurodegeneration in developing rats. The results show that nicotinamide effectively inhibits thiotepa-induced apoptotic neurodegeneration in developing rats. However, more comprehensive research and clinical trials will be required to determine whether nicotinamide can be used in conjunction with chemotherapy for prophylaxis of neurodegeneration or for the treatment of anticancer drug-induced neurodegeneration.
Results
Effect of nicotinamide on thiotepa-induced upregulation of Bax mRNA expression in the developing rat brain
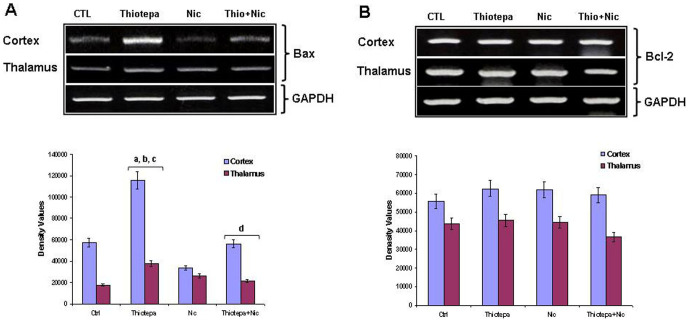
Overexpression of Bax leads to mitochondrial dysfunction and cell death [23]. RT-PCR analysis was performed to examine whether the thiotepa-induced upregulation of Bax mRNA levels could be reversed by nicotinamide. After administration of a single dose (30 mg/kg) of chemotherapeutic alkylating agent (thiotepa) [1] to developing rats after 4 h, a significant increase in Bax mRNA levels was found in the cortex and thalamus; no such change was seen in the hippocampus (data not shown). Treatment of the animals with nicotinamide shortly after thiotepa administration significantly decreased Bax mRNA expression level in the cortex and thalamus (Fig. 1A). No marked change was observed in the Bcl-2 mRNA levels in thiotepa-exposed developing rat cortex, hippocampus and thalamus either with or without nicotinamide treatment (Fig. 1B).
Figure 1. Co-treatment effect of nicotinamide (Nic) on thiotepa-induced Bax and Bcl-2 mRNA levels.
Representative RT-PCR analysis shows expression levels of (A) Bax and (B) Bcl-2 mRNA in the cortex and thalamus of 7-day-old rat pups. Treatment with thiotepa (30 mg/kg for 4 h) increased Bax mRNA levels in the cortex and thalamus. Treatment with (1 mg/g) of nicotinamide for 4 h significantly reduced thiotepa-induced up-regulation of Bax mRNA levels in the cortex and thalamus, as shown. Detailed procedures are described in the Materials and Methods section. The mRNA bands of RT-PCR were quantified using Sigma gel software; these differences are represented in the graph. GAPDH was used as mRNA loading control. Density values are expressed as mean ± SEM (n = 5 animals/group). The density values on the Y-axis are expressed as arbitrary units (AU). Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.01 and thalamus P<0.01]; bSignificantly different from Nicotinamide, [cortex P<0.001 and thalamus P<0.05]; cSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01 and thalamus P<0.05]; dSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.05]).
Effect of nicotinamide on thiotepa-induced upregulation of Bax protein in the developing rat brain
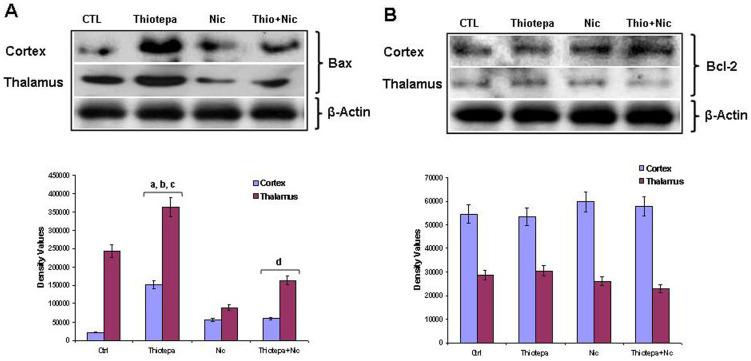
To explore the molecular mechanisms of thiotepa-induced apoptotic neurodegeneration and protection by nicotinamide, we monitored the change in the expression of apoptotic proteins in the mitochondrial pathway. The Bcl-2 family members, pro-apoptotic Bax and anti-apoptotic Bcl-2, are the critical regulators of the apoptotic pathway in mitochondria [24]. Therefore, we measured the levels of Bax and Bcl-2 protein in the cortex, hippocampus and thalamus of young rats after 4 h of thiotepa treatment with and without nicotinamide. Bax levels in the cortex and thalamus showed an upregulation on thiotepa treatment compared to those of control animals. Treatment with nicotinamide significantly reversed thiotepa-induced upregulation of Bax (Fig. 2A), while no marked difference was observed in the levels of Bax in hippocampus after nicotinamide treatment alone (data not shown). In agreement with the results of a previous investigation [25], the expression level of Bcl-2 in the cortex, hippocampus and thalamus of the drug-treated groups was unchanged as compared to control (Fig. 2B). These results show that treatment with thiotepa for 4 h results in an increase in Bax/Bcl-2 ratio, favoring neuroapoptosis, while nicotinamide supplementation significantly reduced Bax/Bcl-2 ratio, favoring neuroprotection.
Figure 2. Co-treatment effect of nicotinamide (Nic) on thiotepa-induced expression of Bax and Bcl-2 protein levels.
Representative Western blot analysis shows the expression levels of (A) Bax and (B) Bcl-2 protein in the cortex and thalamus of a 7-day-old rat. Treatment with (30 mg/kg) of thiotepa for 4 h increased Bax protein levels in the cortex and thalamus. Treatment with (1 mg/g) of nicotinamide for 4 h inhibited thiotepa-induced upregulation of Bax protein levels in the cortex and thalamus, as shown. The protein bands of the Western blot were quantified using Sigma gel software; their differences are represented in the graph. Actin was used as a protein loading control. Density values, expressed as mean ± SEM (n = 5–6 animals/group), of Bcl-2 and Bax proteins are presented. The density values on the Y-axis are expressed as arbitrary units (AU). Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.001 and thalamus P<0.05]; bSignificantly different from Nicotinamide, [cortex P<0.01 and thalamus P<0.001]; cSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01 and thalamus P<0.05]; dSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.05]). The cortex and thalamus Bcl-2 panels are excluded from this article's CC BY license. See the accompanying retraction notice for more information.
Effect of nicotinamide on thiotepa-induced cytochrome c release from mitochondria into cytosol
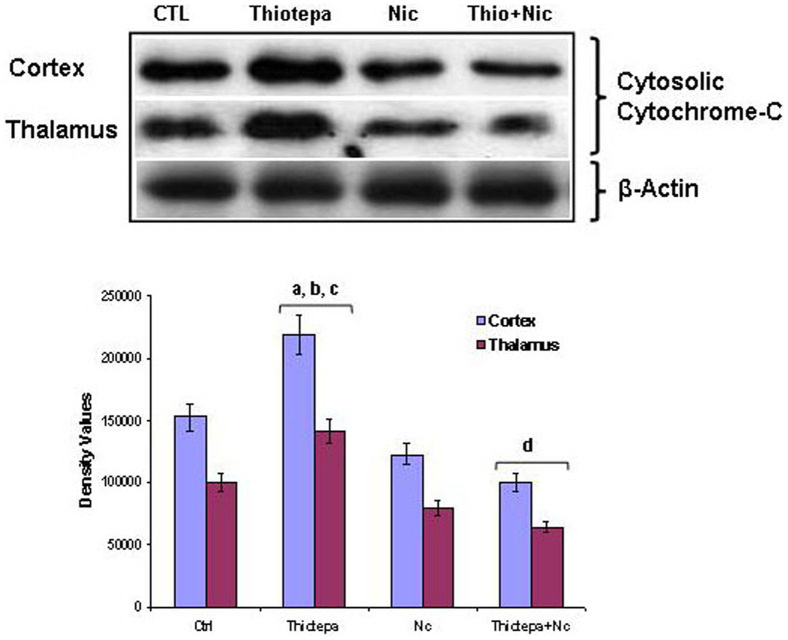
The mitochondrial apoptotic cascade requires the release of the inter-mitochondrial membrane protein cytochrome c into the cytosol; an increased cytosolic level of cytochrome c induces activation of caspase-9 and caspase-3 and, subsequently, neuronal death [12]. Protection by nicotinamide against thiotepa-induced neurodegeneration might occur as a result of the inhibition of cytochrome c release from mitochondria into the cytosol. Because cytosolic cytochrome c is a marker for the activation of the mitochondrial apoptotic cascade, we measured the levels of cytosolic cytochrome c in the cortex, hippocampus and thalamus. Four hours after administration of thiotepa, we found that in the cortex and thalamus, increased levels of Bax correlated with increases in cytosolic cytochrome c levels; no significant difference in the cytosolic level of cytochrome c was observed in the hippocampus (data not shown). Administration of nicotinamide with thiotepa treatment resulted in significantly reduced cytochrome c release from mitochondria into cytosol compared to that in animals treated with thiotepa alone (Fig. 3).
Figure 3. Co-treatment effect of nicotinamide on thiotepa-induced cytosolic cytochrome c levels.
Representative Western blot analysis of cytochrome c levels in the cortex and thalamus of 7-day-old rats. Significant increases in the cytosolic level of cytochrome c in the cortex and thalamus after administration of thiotepa are shown. Treatment with nicotinamide for 4 h significantly reduced thiotepa-induced cytosolic cytochrome c levels in cortex and thalamus, as shown. The protein bands of the Western blot were quantified using Sigma gel software analysis; their differences are represented in the graph. Actin reactivity was used as a protein loading control. Density values, expressed as mean ± SEM (n = 5–6 animals/group), for cytochrome c and activated caspase-9 are presented. The density values on the Y-axis are expressed as arbitrary units (AU). Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.05 and thalamus P<0.05]); bSignificantly different from Nicotinamide, [cortex P<0.05 and thalamus P<0.05]; cSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01 and thalamus P<0.01]; dSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.01]). The thalamus cytosolic cytochrome-C and β-actin panels are excluded from this article's CC BY license. See the accompanying retraction notice for more information.
Effect of nicotinamide on thiotepa-induced expression of active caspase-3 in the developing rat brain
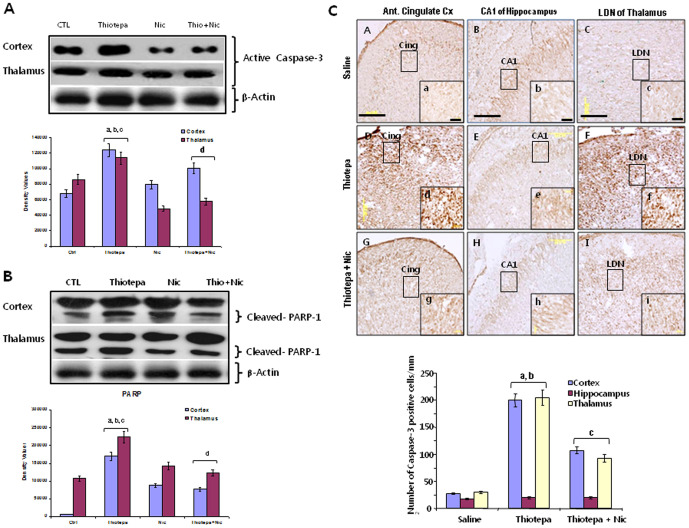
Caspases are cysteine proteases that play a key role in apoptosis [26]. We therefore examined whether the prevention of mitochondrial membrane depolarization and cytochrome c release by nicotinamide occurs at the level of downstream cellular pathways such as the inhibition of activated caspase-3. We measured the levels of activated caspase-3 in the cortex, hippocampus and thalamus by western blotting. Compared to controls, levels of activated caspase-3 increased significantly in the cortex and thalamus after thiotepa administration (Fig. 4A); no marked difference was observed in the level of activated caspase-3 in the hippocampus (data not shown). Because we detected low levels of activated caspase-3 in the control group also, tissue extraction may induce low levels of caspase-3 activation; however, the levels were significantly lower than those in the experimental groups. Nicotinamide treatment with thiotepa significantly decreased the level of activated caspase-3 in both brain regions compared to that in animals treated with thiotepa alone.
Figure 4. Co-treatment effect of nicotinamide on thiotepa-induced expression of active caspase-3 and cleavage of PARP-1.
(A) Representative Western blot analysis of cleaved caspase-3 levels in the cortex and thalamus of rats. Thiotepa administration resulted in a significantly enhanced level of expression of active caspase-3. Treatment with nicotinamide significantly reduced thiotepa-induced activated caspase-3 levels in both cortex and thalamus as compared to control group. Detailed procedures are described in the Materials and Methods section. The protein bands of Western blots were quantified using Sigma gel software, and their differences are represented in the graph. Actin reactivity was used as a protein loading control. Density values, expressed as mean ± SEM (n = 5–6 animals/group), of the caspase-3 protein are presented. The density values on the Y-axis are expressed as arbitrary units (AU). Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.05 and thalamus P<0.05]; bSignificantly different from Nicotinamide, [cortex P<0.05 and thalamus P<0.01]; cSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.05 and thalamus P<0.05]; dSignificantly different from Thiotepa, [cortex P<0.05 and thalamus P<0.05]). (B) Representative Western blot analysis of cleaved PARP-1 levels in the cortex and thalamus of rats. Thiotepa administration resulted in significantly enhanced expression levels of active caspase-3, and caspase-3 mediated a high level of cleaved 89-kDa apoptosis-related fragment of PARP-1. As shown, treatment with nicotinamide significantly reduced thiotepa-induced activated caspase-3 and cleaved PARP-1 levels in both the cortex and thalamus when compared to control group. Density values, expressed as mean ± SEM (n = 5–6 animals/group), for 89 Kda caspase-3 cleaved PARP-1 proteins are presented. Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.001 and thalamus P<0.01]; bSignificantly different from Nicotinamide, [cortex P<0.01 and thalamus P<0.05]; cSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01 and thalamus P<0.05]; dSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.05]). (C) Representative Photomicrographs showing immunohistochemical analysis 24 h after treatment with 30 mg/kg thiotepa in the absence or presence of nicotinamide. Light micrographs show active caspase-3-positive neurons in cingulate cortex and LDN of thalamus (Dd and Ff) after thiotepa treatment, but none in hippocampus (Ee). The arrows indicate dead neuronal cells with high active caspase-3 expression. Treatment of nicotinamide (1 mg/g) with thiotepa resulted in a marked reduction in the number of active caspase-positive neurons in the cortex and thalamus (Gg and Ii) compared to controls (Aa and Cc). Images are representative of staining obtained in sections (3–5/group) prepared from at least 5–6 animals/group. (a–i = 40×) are magnified views from panels (A–I = 20×), Scale bar = 50 µm. Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.001 and thalamus P<0.001]; bSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.05 and thalamus P<0.01]; cSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.01]). The β-actin panel is excluded from this article's CC BY license. See the accompanying retraction notice for more information.
Effect of nicotinamide on thiotepa-induced cleavage of PARP-1 in the developing rat brain
Despite its function in DNA repair, overactivation of PARP-1 in neuronal excitotoxicity induces cell death [27]. In our experiments, western blot analysis was used to determine whether the elevated levels of activated caspase-3 observed in the cortex and thalamus of developing rats treated with thiotepa led to the cleavage of PARP-1 as occurs during apoptosis. The level of cleaved PARP-1 in the cortex and thalamus of thiotepa-treated animals was significantly enhanced compared to that in the control and nicotinamide-treated groups. Nicotinamide treatment after thiotepa resulted in a remarkable decrease in the level of full-length (116 Kda) PARP-1 and a concomitant decrease in its 89 Kda caspase-3 cleaved products in the cortex and thalamus (Fig. 4B). Taken together, our results demonstrate that an overall increase in PARP-1 cleavage occurs in the cortex and thalamus of the developing rat brain after exposure to thiotepa and that nicotinamide significantly reduces the levels of thiotepa-induced cleaved PARP-1 in both of these brain regions, such that the levels remain similar to those observed in control animals.
Effect of nicotinamide on caspase-3 expression in the developing rat brain 24 h after thiotepa treatment
Increased expression of active caspase-3 protein contributes to neuronal cell death. In order to investigate the role of activated caspase-3 in the pathogenesis of neuronal death after thiotepa treatment, RT-PCR and western blot findings were supplemented by immunohistochemical analysis. Expression of active caspase-3 was analyzed in the cortex, hippocampus and thalamus after 24 h of thiotepa treatment. In the cingulate cortex and LDN of thalamus of thiotepa-treated rats, numerous injured and dead neurons with strong active caspase-3 immunoreactivity were observed (Fig. 4C, panels Dd and Ff); however, no active caspase-3 immunoreactivity was observed in the CA1 region of hippocampus (Fig. 4C, panel Ee). The number of active caspase-3-positive cells in the cortex and thalamus progressively decreased when nicotinamide was administered with thiotepa (Fig. 4C, panels Gg and Ii).
Effect of nicotinamide on thiotepa-induced neurodegeneration in the developing rat brain
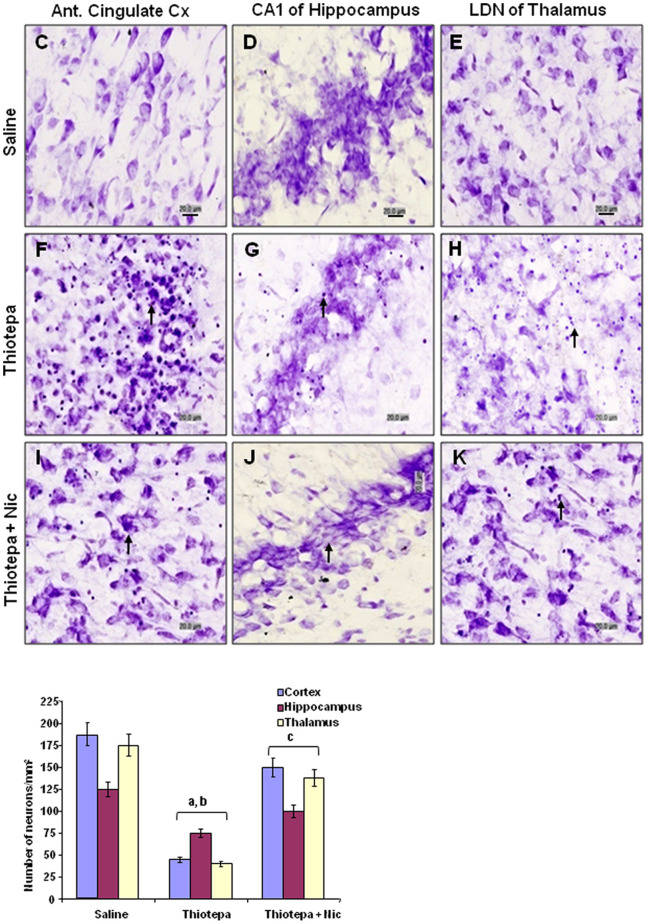
Nissl staining was performed after 24 h to examine the extent of neuronal cell death induced by thiotepa and to assess protection by nicotinamide in the cortex, hippocampus and thalamus of the developing rat brain. Nissl staining identifies all structures, particularly the nucleus and nucleic acids, which appear violet, while neurons appear faintly blue. This method allowed clear identification of dead neuronal cells (i.e., those with large or small condensed, fragmented and dark nuclei and apoptotic bodies) in the cingulate cortex, CA1 of hippocampus and LDN of thalamus. Significantly increased vacuolization, neuronal loss, and tissue breakdown were seen in the anterior cingulate cortex, CA1 of hippocampus and LDN of thalamus of thiotepa-treated animals (Fig. 5, panels F, G and H) as compared to control animals; in control animals these brain regions appeared morphologically normal (Fig. 5, panels C, D and E). The number of degenerated neurons was significantly higher in the cortex and thalamus than in the hippocampal region, which showed a low level of cell death. Treatment of nicotinamide with thiotepa was associated with significantly less vacuolization and neuronal loss in these brain regions (Fig. 5, panels I, J and K).
Figure 5. Co-treatment effect of nicotinamide on thiotepa-induced neuronal cell death.
Light micrographs of cresyl violet-stained neurons in tissue sections of developing rat brain after thiotepa with nicotinamide. The majority of thiotepa-induced degenerating neuronal cells (generally shrunken in appearance, as is commonly observed) are present in the anterior cingulate cortex, CA1 of hippocampus and LDN of thalamus (F, G, and H). The arrows indicate shrunken and damaged neurons. Almost complete protection was observed (I, J and K), when nicotinamide was administered with thiotepa. Images are representative of staining obtained in sections (3–5/group) prepared from at least 5–6 animals/group. (C–K) of Nissl-stained brain tissue at higher magnification with 40× objective field, Scale bar = 20 µm. Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.001, hippocampus P<0.05 and thalamus P<0.001]; bSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01, hippocampus P<0.05 and thalamus P<0.01]; cSignificantly different from Thiotepa, [cortex P<0.01, hippocampus P<0.05 and thalamus P<0.01]). Figure 5G and 5H are excluded from this article's CC BY license. See the accompanying retraction notice for more information.
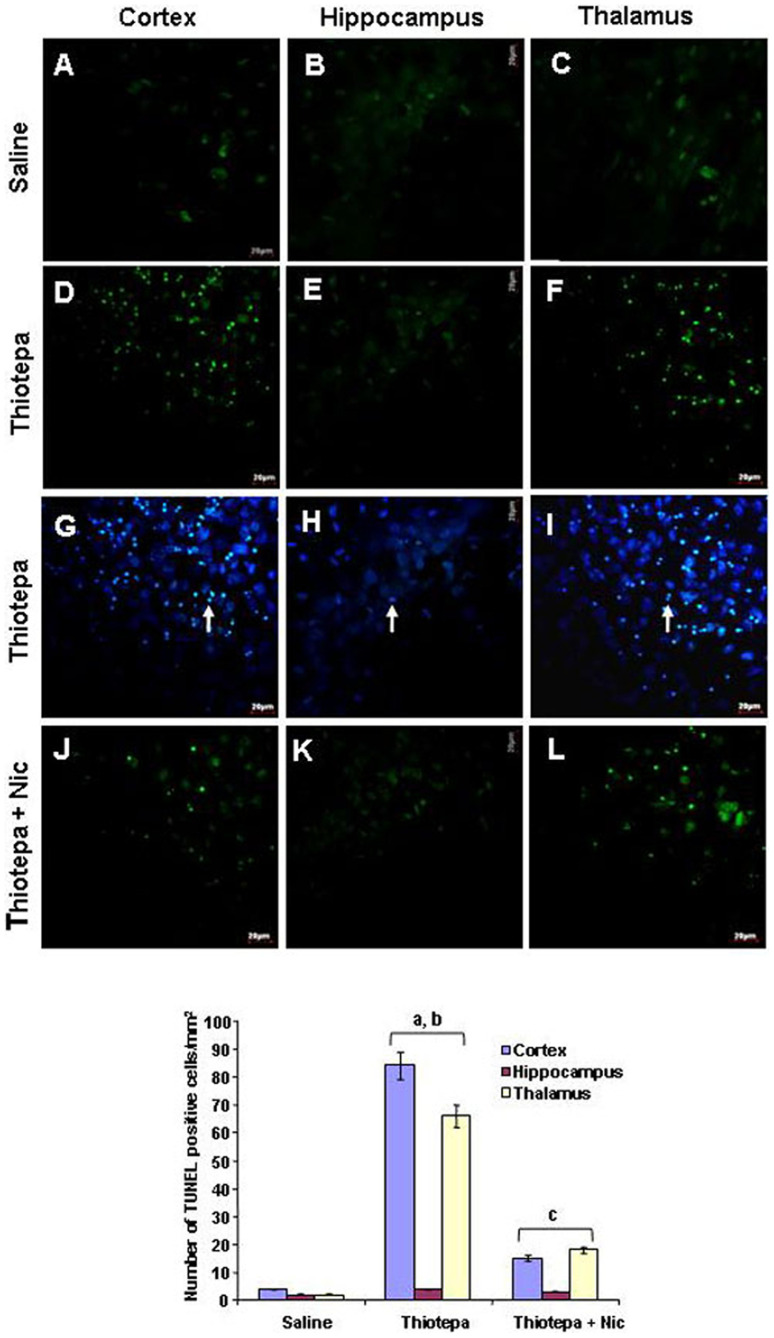
DNA damage is one of the hallmarks of apoptosis. Visualization of DNA damage is possible using TUNEL staining, an assay for DNA breaks based on enzymatic labeling of free 3′ DNA ends. The nuclear morphology of brain tissue was evaluated with TUNEL and DAPI staining. Thiotepa-induced neuroapoptosis in developing rat brain cortex and thalamus resulted in the presence of apoptotic bodies and nuclear fragmentation revealed by TUNEL and DAPI staining. Compared to control animals (Fig. 6, panels A and C), treatment with thiotepa significantly increased the number of TUNEL-positive shrunken cells with small multi-DNA masses (typical features of apoptosis) in the cortex and thalamus (Fig. 6, panels D and F) counterstained with DAPI (Fig. 6, panels G and I). TUNEL-positive cells were not detected in CA1 of hippocampus of thiotepa-treated rats (Fig. 6, panels E and H). Most of the TUNEL-positive cells were in the cortex and thalamus, and overlapping of TUNEL staining with DAPI staining showed apoptotic neuronal death (Fig. 6, panels G and I). Nicotinamide treatment with thiotepa significantly reduced the number of TUNEL-positive cells in these brain regions (Fig. 6, panels J and L).
Figure 6. Co-treatment effect of nicotinamide on thiotepa-induced apoptotic neurodegeneration.
Representative photomicrographs of TUNEL staining show apoptotic dead neuronal cells after thiotepa administration with nicotinamide. The arrows indicate thiotepa-induced TUNEL stained apoptotic dead neurons (D and F) counterstained with DAPI (G and I) in the cortex and thalamus. Nicotinamide treatment effectively blocked thiotepa-induced apoptosis, as evident from the lack of TUNEL-positive cells (J and L). Images are representative of staining obtained in sections (3–5/group) prepared from at least 5–6 animals/group. (A–L) of TUNEL-stained brain tissue at higher magnification with 40× objective field, Scale bar = 20 µm. Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. (aSignificantly different from CTL, [cortex P<0.001 and thalamus P<0.001]; bSignificantly different from Thiotepa + Nicotinamide, [cortex P<0.01 and thalamus P<0.01]; cSignificantly different from Thiotepa, [cortex P<0.01 and thalamus P<0.01]).
Discussion
Neurotoxicity is a common and often dose-limiting complication of chemotherapy [28]. Despite intensive efforts at management of the neurological side effects of chemotherapy in patients and the development of neuroprotective agents, there is no generally accepted therapy at the present time [29]. The use of neuroprotective agents can reduce neurotoxicity resulting from anticancer chemotherapy. Intensive research is therefore currently being focused on the identification and understanding of molecular and cellular mechanisms of neuroprotectants against such toxicity [30]. A mitochondria- dependent apoptotic cascade plays an important role in thiotepa-induced neurodegeneration [31], [32].Specifically, the normal functions of the central nervous system depend on mitochondrial activity for regulating calcium homeostasis, which itself serves as a regulator of several enzymatic activities and cell processes including apoptosis [33].
Due to its high metabolic rate and relatively reduced capacity for cellular regeneration, the brain is particularly susceptible to the damaging effects of oxidative stress [34]. The chemotherapeutic alkylating agent thiotepa has neurodegenerative effects in the infant rat brain that appear to be associated with disruption of mitochondrial energy metabolism, oxidative stress, and activation of cell death cascades [1]. Our results show that the neuronal cell death induced by single dose of alkylating agent thiotepa in the developing rat brain is apoptotic, as revealed by the occurrence of apoptotic bodies, nuclear fragmentation and activation of caspases. Apoptotic signals amplify through mitochondria due to changes in mitochondrial membrane permeability, which facilitate the release of the apoptogenic factor cytochrome c, regulated by the Bax and Bcl-2 proteins [35]. Activation of PARP-1 is a critical event that directs toxic signaling towards apoptosis or necrosis. Its cleavage facilitates DNA alteration and nuclear disassembly and ensures completion of the energy-dependent cell death process [36]. Activated caspase-3 cleaves PARP-1, which is an important protein in DNA repair, thus promoting apoptosis; in contrast, excessive activation of PARP-1 depletes NAD+ and ATP, resulting in necrosis [27], [37]. Our results show that after 4 h of thiotepa treatment, the level of expression of anti-apoptotic Bcl-2 was not changed; this confirms the results of a previous study [25]. In contrast, thiotepa induced upregulation of Bax and release of cytochrome c into the cytosol. Activation of caspase-3 and cleavage of PARP-1 by caspase-3 were analyzed in order to assess the involvement of the mitochondrial apoptotic pathway [31] in the neuronal degeneration caused by thiotepa. The expression of Bax and Bcl-2 proteins exhibited trends similar to those of the corresponding mRNA levels which is control by p53 [38]. Thiotepa-induced insult has been shown to increase mitochondrial membrane permeability and lead to the release of cytochrome c, the activation of caspase-9 and the subsequent activation of caspase-3, all of which play key roles in apoptosis [12], [26], [39].
Nicotinamide is a necessary nutrient that acts as a protective agent against neurodegeneration induced by a variety of insults including oxidative stress [40], [41], [42]. In developing rats, its systemic use provides significant protection against thiotepa-induced apoptotic and necrotic cell death. The results presented here show that treatment with nicotinamide inhibits thiotepa-induced activation of the apoptotic cascade through downregulation of proapoptotic Bax, thus counteracting thiotepa-induced increases in Bax levels. As a result, mitochondrial membrane potential is stabilized, release of cytochrome c is inhibited, and the levels of active caspase-3 and the cleavage of PARP-1 are reduced, which are involved in the activation of DNAses and the formation of apoptotic bodies [43], [44]. Our results clearly show that the protective effect of nicotinamide against thiotepa involves the maintenance of mitochondrial integrity and the concomitant repression of Bax, inhibition of the mitochondrially-mediated release of cytochrome c into the cytosol, inhibition of activated caspase-3 and maintenance of NAD+ levels, all of which contribute to the inhibition of apoptotic and possible necrotic cell death in the developing rat brain (Fig. 7).
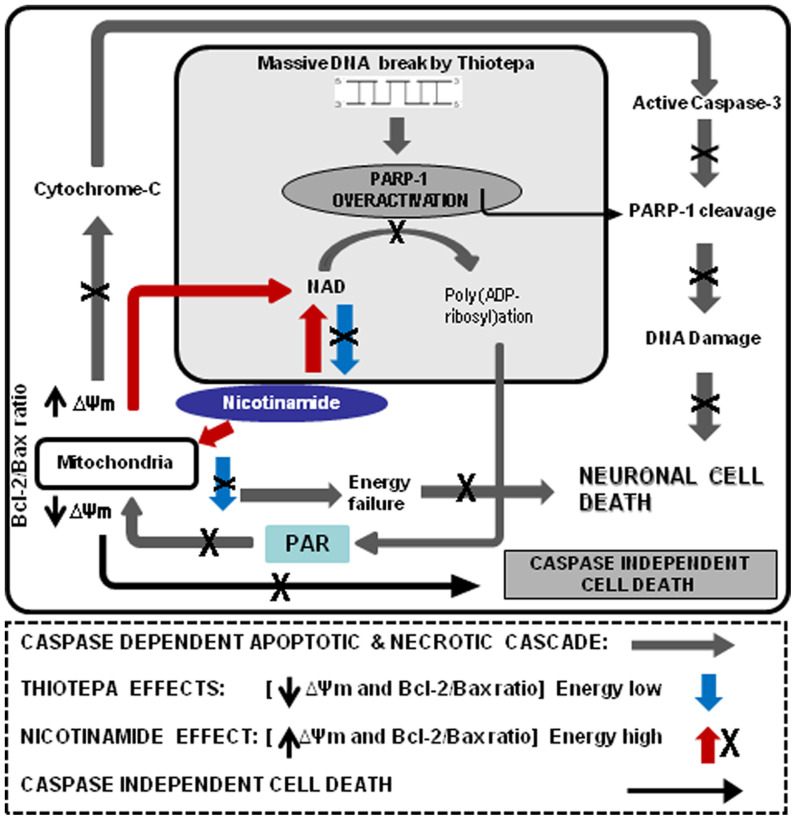
Figure 7. A schematic diagram representing the hypothetical mechanism by which nicotinamide protects against thiotepa-induced neurodegeneration in the brain of the developing rat.
Thiotepa-induced neurodegeneration is caused by the overactivation of mitochondria-dependent apoptosis, beginning with the down-regulation of Bax, an increase in cytochrome c release from mitochondria to cytosol, expression of activated capase-3, cleavage of PARP-1 and necrosis by overactivation and cleavage of PARP-1 and depletion of NAD+ (blue arrow). PARP-1 activation and formation of Poly ADP-ribose PAR in the nucleus, which translocates to the cytosol to induce caspase-independent cell death (black arrow). Nicotinamide, as indicated by the X sign, inhibits several key elements in the apoptotic cascade beginning with the down-regulation of Bax, a decrease in cytochrome c release from mitochondria to cytosol, inhibition of activated caspase-3 and cleavage of PARP-1 and necrosis by the inhibition of PARP-1 activation and prevention of ATP and NAD+ depletion (red arrow), resulting in protection against thiotepa-induced apoptotic and possible necrotic cell death.
Modulation of the PARP-1 cascade is a well-known strategy used by cells to prevent ATP and NAD+ depletion; such modulation protects the developing brain against ischemia and excitotoxic insults involving DNA damage. Pharmacological inhibition or genetic disruption of PARP-1 can markedly reduce cell death resulting from oxidative stress [45], [46]. Under normal physiological conditions, PARP-1 is thought to play a key role in DNA repair, thereby contributing to the maintenance of cellular genomic integrity [47]. However, high metabolic and oxidative stress activates PARP-1 and depletes intracellular NAD+ stores [48], [49]. Rapid depletion of NAD+ and ATP is a suicide response that can result in cell death by apoptosis or necrosis if broken DNA strands are not repaired [27], [50], as degree of reduction in the level of ATP that represents the threshold between apoptotic and necrotic cell death [51], [52], [53]. Treatment of developing rats with nicotinamide after thiotepa inhibits induction of the apoptotic cascade due to inhibition of caspase-3, which is responsible for cleavage of PARP-1; such treatment thereby interferes with NAD+ depletion and may inhibits necrosis (Fig. 7) and other non-apoptotic cell death mechanisms [20], [54], [55].
Immunohistochemical and TUNEL findings were consistent with the molecular results; both showed that thiotepa treatment significantly increased expression of active caspase-3 in the cortex and thalamus while there was no significant change in the hippocampus. Histomorphological analysis by Nissl staining showed low levels of neuronal cell death in the hippocampal region as well. The exact reason for this discrepancy is unknown; one possible explanation is the involvement of neuronal apoptosis inhibitory protein (NAIP), in the hippocampal region [56], [57]. Whether or not NAIP is involved in the inhibition of alkylating agent-induced degeneration of developing brain in hippocampus remains to be determined. Histomorphological analysis also showed that the cell death induced by thiotepa is more pronounced and severe in the cortex and thalamus than in the hippocampus, indicating that, in the developing rat, these brain regions are more sensitive to thiotepa-induced apoptotic insult. Here, we speculate that thiotepa-induced cell death in the cortex and thalamus is mainly apoptotic and caspase-dependent, while in the hippocampus, caspase-independent or non-apoptotic cell death processes might be involved (Fig. 6). A relationship between histomorphological neuronal death and cognitive deficits could not be established; however, it is reasonable to suggest that protection by nicotinamide against thiotepa might be beneficial for mental abilities, cognitive skills, and academic achievements as well as for other behavioral outcomes.
In the present study, we found that nicotinamide treatment of the developing rat brain with thiotepa was associated with a decrease in thiotepa-induced apoptotic and necrotic cell death and improved brain histomorphological outcomes. Because nicotinamide can easily reach the brain [58], [59], it might be able to function as a critical protective agent in various neurodegenerative paradigms in which the mitochondrial apoptotic pathway is involved. Our data suggests that stabilization of the mitochondrial apoptotic pathway by nicotinamide may protect developing neurons treatment with anticancer drugs or other forms of excitotoxic insult. These findings open new avenues for examining the role of nicotinamide as a promising and safe neuroprotective agent for the treatment of neurodegenerative disorders [59]. Other neuroprotectants that have similar modes of action might also be developed to stabilize the apoptotic cascade caused by various toxic agents in infants.
Conclusions
Our results support the hypothesis that nicotinamide treatment protects against thiotepa-induced neurodegeneration in the developing rat brain, interfering with apoptotic cell death. Nicotinamide could be a potential remedy for neurodegenerative conditions caused by toxic effects of various neurotoxic drugs in newborns or infants. More work is clearly needed to comprehensively assess the neuroprotective role of nicotinamide.
Materials and Methods
Animals
Seven-day-old Sprague-Dawley rat pups (average body weight 15 g) were used in all experimental paradigms and were equally distributed into four different groups (control, thiotepa, nicotinamide, and thiotepa + nicotinamide). All efforts were made to minimize the number of animals used and their suffering. All the experimental procedures were approved (Approval ID: 125) by the animal ethics committee (IACUC) of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University South Korea.
Drug treatment
Protective effect of nicotinamide against thiotepa was observed at different time points i.e. (2 h) pre-treatment of thiotepa, Co-treatment of thiotepa and (2 h) post-treatment of thiotepa. At all time periods nicotinamide showed its effects but the strongest effect was observed when used with thiotepa treatment. In our experiments developing rats were injected subcutaneously with (30 mg/kg) thiotepa with (1 mg/g) nicotinamide in 0.9% saline solution. Animals were sacrificed 4–24 h after drug treatment. Saline injections of equal volume were used as controls.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
The cerebral cortex, hippocampus and thalamic cortex were rapidly collected, separated and snap-frozen in liquid nitrogen. Samples were kept at −80°C until further processing. Total cellular RNA was isolated by acidic phenol/chloroform extraction. The RNA was aliquoted and stored at −80°C until further use. Then, 2 µg of total RNA was reverse-transcribed to single-stranded cDNA using Oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA, USA) with M-MLV reverse transcriptase (Promega, Madison, WI, USA). For PCR reactions, 4 µl of cDNA was incubated with 20 pmol each of the forward and reverse primers (Bioneer Corporation, Seoul, South Korea), and GoTaq®Green Master Mix 2× containing PCR buffer, 25 mM magnesium chloride, 10 mM dNTP mix and Taq enzyme (Promega, Madison, WI, USA) and amplified. The primers of each transcript were as follows: Bcl-2, 5/-CGACGACTTCTCCCGCCGCTACCGC-3/(forward) and 5/-CCGCATGCTGGGGCCGTACAGTTCC-3/(reverse), Bax, 5/-GTGCACCAAGGTGCCGGAC-3/ (forward) and 5/-TCAGCCCATCTTCTTCCAGA-3/ (reverse), and β-actin (as loading control), 5/-GTGGGGCGCCCCAGGCACCA-3/(forward) and 5/CTCCTTAATGTCACGCACGATTTC-3/ (reverse). The conditions for PCR were: initial denaturation at 94°C for 5 min; 25 cycles of denaturation at 94°C for 1 min, annealing for 1 min (Bcl-2, 68°C; Bax, 53°C; Caspase-3, 55°C; β-actin, 63°C), elongation at 72°C for 1 min and a final extension step at 72°C for 10 min on a PC-812 Thermal Cycler (Astec, Fukuoka, Japan). The PCR products were electrophoresed in 1% agarose gels containing ethidium bromide for 25 min and exposed to UV light for photography of the bands. The molecular sizes of the amplified products were determined by comparison with molecular weight markers (100 bp DNA ladder, Promega, Madison, WI, USA) run in parallel with the PCR products. The densities of the mRNA bands were analyzed using Molecular Analyst™, version 1.4.1 (Bio-Rad, Hercules, CA, USA).
Western blot analysis
In order to assess whether changes in the mRNA expression profiles of Bax and Bcl-2 were matched by corresponding changes in protein levels in the developing rat pups, the amounts of these proteins were determined in the control, thiotepa, nicotinamide and thiotepa-plus- nicotinamide treated animals. Seven-day-old rats were killed after treatment with (1 mg/g) nicotinamide immediately after administration of (30 mg/kg) of thiotepa for 4 h; the brains were rapidly removed, the cortex, hippocampus and thalamus were carefully dissected and the tissue was frozen in dry ice. The brain tissues were homogenized in 0.2 M PBS with protease inhibitor cocktail. The protein concentration was measured using Bio-Rad protein assay solution. Equivalent amounts of protein (40 µg per sample) were electrophoresed on 10–15% SDS-PAGE gels under reducing conditions and transferred to a polyvinylidene difluoride (PVDF) membrane (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Prestained protein markers, broad range (6–175 kDa, New England Biolabs Inc., Ipswich, MA, USA) were run in parallel for detection of the molecular weights of the proteins. The membrane was blocked with 5% (w/v) skimmed milk in order to reduce non-specific binding and immunoblotting was performed using rabbit-derived anti-Bcl-2, anti-Bax, and anti-caspase-3, anti-PARP-1 antibodies and goat-derived polyclonal anti-cytochrome c (1∶500; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-β-actin antibody (1∶500; Sigma, St. Louis, MO, USA) was used as a control to confirm uniform loading. Membranes were probed with a goat-derived horseradish peroxidase-conjugated anti-rabbit IgG (1∶1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and immunocomplexes were visualized using enhanced chemiluminescence ECL-detecting reagent (Amersham Pharmacia Biotech, Western blotting detection reagents). The X-ray films were scanned and the optical densities of the Western blots were analyzed by densitometry using the computer-based Sigma Gel, version 1.0 (SPSS, Chicago, IL, USA).
Tissue collection and sample preparation
Animals were sacrificed 24 h after drug treatment. Brain sections from control rats and rats subjected to thiotepa followed by nicotinamide for 24 h were analyzed. For tissue analysis (n = 5–6 per group), developing rat pups were perfused transcardially with 4% ice-cold paraformaldehyde followed by 1×PBS; brains were post-fixed in 4% paraformaldehyde overnight and then transferred to 20% sucrose until they sank to the bottom of the tubes. Brains were frozen in O.C.T compound (A.O. USA) and 16 µm sections were made in the coronal planes using a Leica cryostat (CM 3050C, Germany). Sections were thaw-mounted on probe-on plus charged slides (Fisher).
Immunohistochemical staining
Immunohistochemistry was performed as previously described by [60], with some modifications. The slides were washed in 0.01 M PBS, quenched for 10 min in a solution of methanol containing 3% hydrogen peroxide, and then incubated for 1 h in blocking solution (2% BSA/0.2% milk/0.1% Triton X-100 in PBS), followed by incubation overnight in rabbit anti-active caspase-3 antiserum (Cell Signaling Technology, Beverly, MA) diluted 1∶1000 in blocking solution. Following incubation with primary antiserum, the sections were incubated for 90 min in secondary antiserum (goat anti-rabbit, 1∶200 in blocking solution), and then reacted in the dark with ABC reagents (standard Vectastain ABC Elite Kit; Vector Laboratories, Burlingame, CA) for 90 min. The sections were then washed twice with PBS and incubated with VIP reagent (Vector VIP substrate kit for peroxidase, Vector Labs, Burlingame, CA) to develop a purple color. Images were viewed with a fluorescence light microscope. Active caspase-3-positive cells in the different regions of each section were counted by observers blinded to the treatment conditions.
Cresyl violet staining
Cresyl violet was used to stain tissue sections for histological examination and measurement of neuronal loss. Nissl histology of developing rat brain and the presence and absence of dead and injured neurons were analyzed on microscope slides mounted 16 µm thick brain sections. Sections derived from all investigated rat pups were defatted in ascending alcohols (70–100%), hydrated in descending alcohols (95–70%), washed in acetate buffer pH 5.0 and subsequently stained with a 0.25% cresyl violet for approximately 15 min. Section were then washed with distilled water and dehydrated in graded ethanol. Images were viewed with a fluorescence light microscope. Neurons in the different regions of each section (5–6/group) were counted manually by observers blinded to the treatment conditions.
TUNEL and DAPI staining
To detect typical features of apoptosis, nuclear DNA was stained with TUNEL (GenScript Corporation, USA) and counterstained using 4′,6-diamidino-2-phenylindole (DAPI). In situ detection of apoptotic cell death was performed using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) on cryosections (16 µm) of aggregates. TUNEL staining was performed according to supplier recommendations using the In Situ Cell Death Detection kit Fluorescein (Genescript, NJ, USA). Aggregate cryosections (16 µm) were incubated with DAPI (Molecular Probes, Eugene, OR, USA) for 10 min at room temperature and then rinsed with distilled water. Glass cover slips were mounted on glass slides with mounting medium. A DAPI filter was used to detect the DAPI staining (blue color) and an FITC filter was used was to detect TUNEL staining (green color). TUNEL-positive (green) and DAPI-positive (blue) staining patterns were acquired by use of a confocal laser scanning microscope (Fluoview FV 1000, Olympus, Japan). TUNEL-positive cells in the different regions of each section were counted by observers blinded to the treatment conditions.
Data analysis and statistics
Bands from RT-PCR and Western blots were scanned and analyzed by densitometry using the Sigma Gel System (SPSS Inc., Chicago, IL). Density values were expressed as mean ± SEM. Statistical difference was determined using one-way analysis of variance (ANOVA) followed by Student's t-test. P values less than 0.05 were considered significant.
Acknowledgments
We thank Moon Seok Park for statistical analysis.
Funding Statement
This work was supported by a grant from the Next Generation Biogreen 21 program (PJ008075) and Agenda PJ007361 of Rural Development Administration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rzeski W, Pruski S, Macke A, Felderhoff-Mueser U, Reiher AK, et al. Anticancer Agents Are Potent Neurotoxins. In Vitro and In Vivo. Ann Neurol. 2004;56:351–360. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- 2.Van Gool SW, Van Kerschaver E, Brock P, Pottel H, Hulstaert F, et al. Disease and treatment-related elevation of the neurodegenerative marker tau in children with hematological malignancies. Leukemia. 2000;14:2076–2084. doi: 10.1038/sj.leu.2401934. [DOI] [PubMed] [Google Scholar]
- 3.Brown RT, Sawyer MG, Antonious G, Tooqood I, Rice M. Longitudinal follow-up of the intellectual and academic functioning of children receiving central nervous system-prophylactic chemotherapy for leukemia. J Dev Behav Pediatr. 1999;20:373–377. doi: 10.1097/00004703-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 5.Reddy AT, Witek K. Neurologic complications of chemotherapy for children with cancer. Curr Neurol Neurosci Rep. 2003;3:137–142. doi: 10.1007/s11910-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 6.Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr Opin Pharmacol. 2003;3:101–109. [PubMed] [Google Scholar]
- 8.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 9.Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, et al. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. Cancer Chemother Pharmacol. 2002;50:343–352. doi: 10.1007/s00280-002-0522-7. [DOI] [PubMed] [Google Scholar]
- 10.Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, et al. Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia. 2000;14:1833–1849. doi: 10.1038/sj.leu.2401902. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Benedict MA, Ding L, Nunez G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1 mediated caspase-9 activation and apoptosis. EMBO J. 1999;18:3586–3595. doi: 10.1093/emboj/18.13.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 13.Chetsanga CJ, Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979;6:3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkle A. PARP-1: a regulator of genomic stability linked with mammalian longevity. Chembiochem. 2001;2:725–28. doi: 10.1002/1439-7633(20011001)2:10<725::AID-CBIC725>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 16.Ayoub IA, Lee EJ, Ogilvy C, Flint BM, Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal cerebral ischemia in wistar rats. Neurosci Lett. 1999;259:21–24. doi: 10.1016/s0304-3940(98)00881-7. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee SK, Klaidman LK, Yasharel R, Adams JD. Increased brain NAD prevents neuronal apoptosis in vivo. Eur J Pharmacol. 1997;330:27–34. doi: 10.1016/s0014-2999(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 18.Klaidman LK, Mukherjee SK, Adams JD. Oxidative changes in brain pyridine nucleotides and neuroprotection using nicotinamide. Biochim Biophys Acta. 2001;1525:136–148. doi: 10.1016/s0304-4165(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 19.Klaidman LK, Morales M, Kem S, Yang J, Chang ML, et al. Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Klaidman LK, Nalbandian A, Oliver J, Chang ML, et al. The effects of nicotinamide on energy metabolism following transient focal cerebral ischemia in Wistar rats. Neurosci Lett. 2002b;333:91–94. doi: 10.1016/s0304-3940(02)01005-4. [DOI] [PubMed] [Google Scholar]
- 21.Ungerstedt JS, Blomback M, Soderdtom T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin Exp Immunol. 2003;131:48–52. doi: 10.1046/j.1365-2249.2003.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- 23.Wisessmith W, Phansuwan-Pujito P, Govitrapong P, Chetsawan B. Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2009;46:433–440. doi: 10.1111/j.1600-079X.2009.00680.x. [DOI] [PubMed] [Google Scholar]
- 24.Chao DT, Korsmeyer SJ. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 25.Uehara N, Miki K, Tsukamoto R, Matsuoka Y, Tsubura A. Nicotinamide blocks N- methyl-N-nitrosourea-induced photoreceptor cell apoptosis in rats through poly (ADP-ribose) polymerase activity and Jun N-terminal kinase/activator protein-1 pathway inhibition. Exp Eye Res. 2006;82:488–495. doi: 10.1016/j.exer.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Rupinder SK, Gurpreet AK, Manjeet S. Cell suicide and caspases. Vascul Pharmacol. 2007;46:383–393. doi: 10.1016/j.vph.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- 28.Cavaliere R, Schiff D. Neurologic toxicities of cancer therapies. Curr Neurol Neurosci Rep. 2006;6:218–226. doi: 10.1007/s11910-006-0009-8. [DOI] [PubMed] [Google Scholar]
- 29.Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63:1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Albers J, Chaudhry V, Cavaletti G, Donehower R. Interventions for preventing neuropathy caused by cisplatin and related compounds. 2007. Cochrane Database of Systematic Reviews Issue 1. Art. No.: CD005228. DOI: 10.1002/14651858.CD005228.pub2. [DOI] [PubMed]
- 31.Debatin KM, Poncet D, Kroemer G. Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 32.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54:101–27. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Rev Neurosci. 2004;5:18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 36.Yu SW, Wang H, Dawson TM, Dawson VL. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis. 2003;14:303–317. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, Saksi J, Paetau A, et al. Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in Ischemic human stroke. Acta Neuropathol. 2009;118:541–552. doi: 10.1007/s00401-009-0559-3. [DOI] [PubMed] [Google Scholar]
- 38.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 39.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 40.Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood–brain barrier breakdown following traumatic brain injury. Brain Research. 2006;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, et al. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav. 2002a;73:901–910. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- 42.Ieraci A, Herrera DG. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006;3:547–557. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 44.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide Prevents NAD+ Depletion and Protects Neurons Against Excitotoxicity and Cerebral Ischemia: NAD+ Consumption by SIRT1 may Endanger Energetically Compromised Neurons. Neuromol Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schraufstatter IU, Hyslop PA, Hinshaw DB, Spragg RG, Sklar LA, et al. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase C. G Proc Natl Acad Sci. 1986;83:4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair: Nature. 1992;6367:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 48.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol Dis. 2000;7:225–239. doi: 10.1006/nbdi.2000.0324. [DOI] [PubMed] [Google Scholar]
- 49.Du L, Zhang X, Han YY, Burke NA, Kochanek PM, et al. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- 50.Affar EB, Shah RG, Dallaire AK, Castonguay V, Shah GM. Role of poly (ADP-ribose) polymerase in rapid intracellular acidification induced by alkylating DNA damage. Proc Natl Acad Sci. 2002;99:245–250. doi: 10.1073/pnas.012460399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol. 1998;274:315–327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 52.Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 53.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associate “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD+ Precursor Nicotinamide. Curr Med Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holcik M, Thompson CS, Yaraghi Z, Lefebvre CA, MacKenzie AE, et al. The hippocampal neurons of neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice display increased vulnerability to kainic acid-induced injury. Proc Natl Acad Sci. 2000;97:2286–2290. doi: 10.1073/pnas.040469797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davoodi J, Ghahremani MH, Es-Haghi A, Mohammad-Gholi A, Mackenzie A. Neuronal apoptosis inhibitory protein, NAIP, is an inhibitor of procaspase-9. Int J Biochem Cell Biol. 2010;42:958–64. doi: 10.1016/j.biocel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Klaidman LK, Mukherjee SK, Hutchin TP, Adams JD. Nicotinamide as a precursor for NAD+ prevents apoptosis in the mouse brain induced by tertiary-butylhydroperoxide. Neurosci Lett. 1996;206:5–8. doi: 10.1016/0304-3940(96)12446-0. [DOI] [PubMed] [Google Scholar]
- 59.Maiese K, Chong ZZ, Hou J, Shang YC. The Vitamin Nicotinamide: Translating Nutrition into Clinical Care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young C, Roth KA, Klocke BJ, West T, Holtzman DM, et al. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]