Abstract
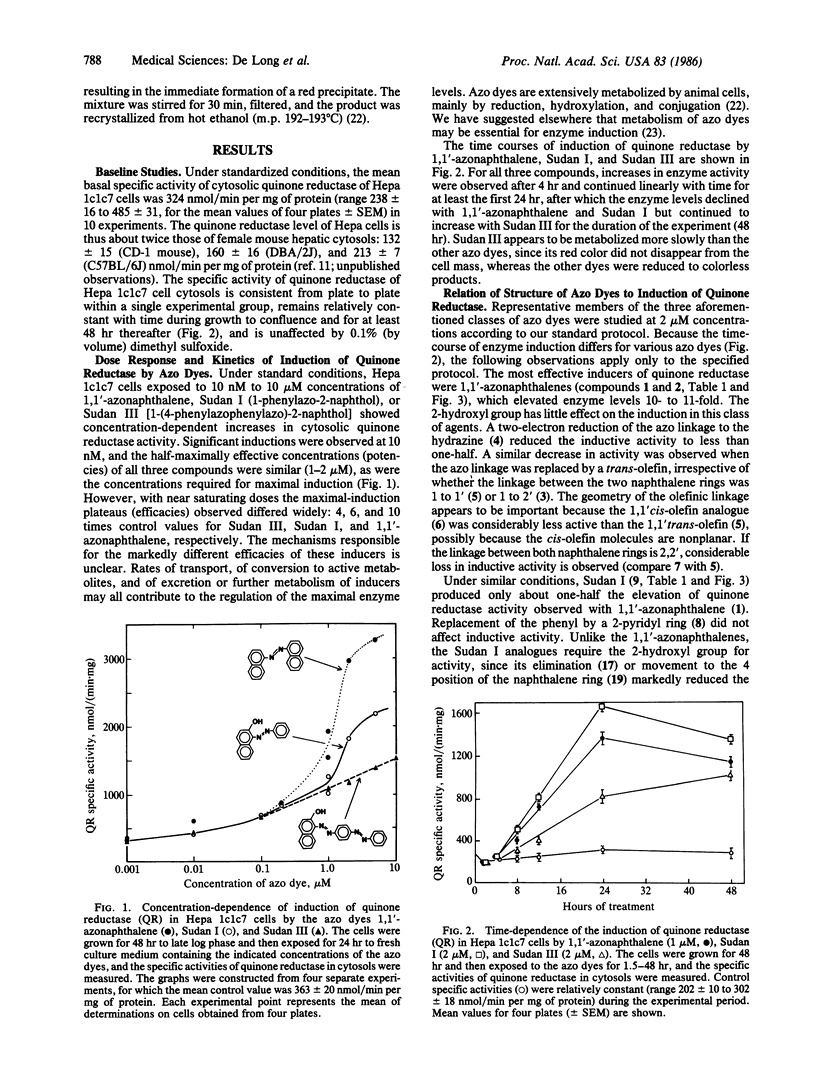
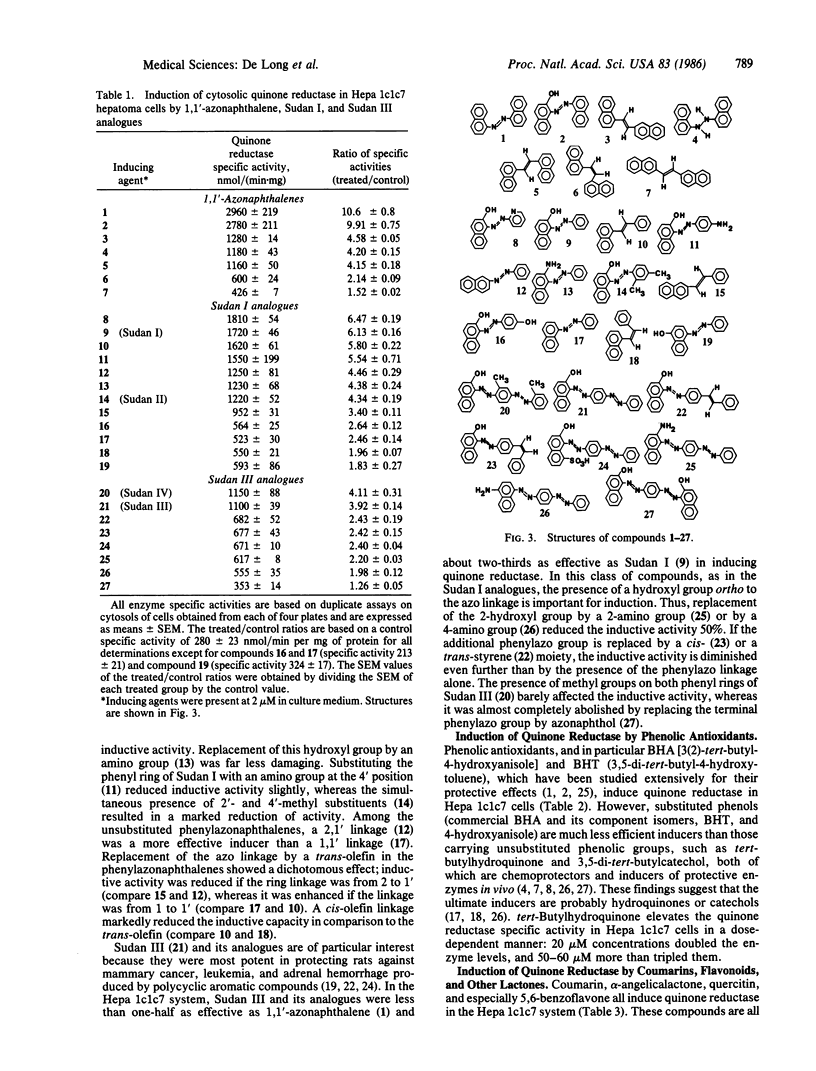
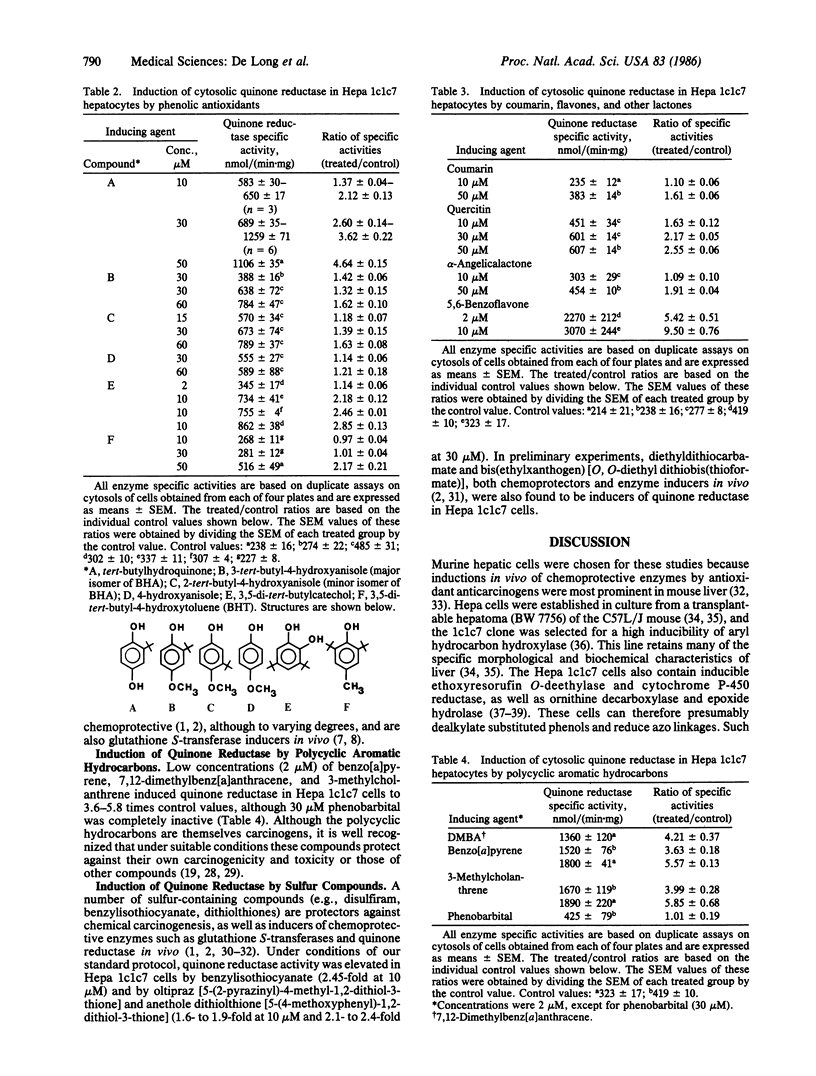
Exposure of murine hepatoma (Hepa 1c1c7) cells to a variety of chemical agents known to protect animals against the neoplastic, mutagenic, and other toxic effects of chemical carcinogens results in dose- and time-dependent inductions of NAD(P)H:quinone reductase (EC 1.6.99.2). This enzyme protects against quinone toxicity by promoting obligatory two-electron reductions that divert quinones from oxidative cycling or direct interactions with critical nucleophiles. Quinone reductase levels are stable in culture, are easily measured, and are useful markers for the inductive effects of chemoprotective agents. The Hepa 1c1c7 system responds to chemoprotective compounds such as phenolic antioxidants (e.g., BHA [3(2)-tert-butyl-4-hydroxyanisole], BHT (3,5-ditert-butyl-4-hydroxytoluene), and tert-butylhydroquinone), lipophilic azo dyes belonging to the 1,1'-azonaphthalene, Sudan I (1-phenylazo-2-naphthol), and Sudan III [1-(4-phenylazophenylazo)-2-naphthol] families, polycyclic aromatic hydrocarbons, coumarin and various other lactones, flavonoids, and certain sulfur compounds (e.g., benzylisothiocyanate, dithiolthiones, and dithiocarbamates), all of which are recognized enzyme inducers and chemoprotectors in vivo. Quinone reductase induction in Hepa 1c1c7 cells therefore provides a simple, versatile, and reliable system for the evaluation of the potency, kinetics, and mechanism of action of anticarcinogens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansher S. S., Dolan P., Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983 Nov-Dec;3(6):932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Gielen J. E., Owens I. S., Niwa A., Bebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. IV. Stimulation of the enzyme activity in established cell lines derived from rat or mouse hepatoma and from normal rat liver. Biochem Pharmacol. 1973 Nov 1;22(21):2766–2769. doi: 10.1016/0006-2952(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Benson A. M., Cha Y. N., Bueding E., Heine H. S., Talalay P. Elevation of extrahepatic glutathione S-transferase and epoxide hydratase activities by 2(3)-tert-butyl-4-hydroxyanisole. Cancer Res. 1979 Aug;39(8):2971–2977. [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard H. P., Darlington G. J., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973 Nov;35(1):83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- Chesis P. L., Levin D. E., Smith M. T., Ernster L., Ames B. N. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL J. W. The excretion and metabolism of edible food colors. Toxicol Appl Pharmacol. 1962 Sep;4:572–594. doi: 10.1016/0041-008x(62)90085-6. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Bernhard H. P., Miller R. A., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells. J Natl Cancer Inst. 1980 Apr;64(4):809–819. [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Tissue-specific induction patterns of cancer-protective enzymes in mice by tert-butyl-4-hydroxyanisole and related substituted phenols. Cancer Res. 1985 Feb;45(2):546–551. [PubMed] [Google Scholar]
- Duthu G. S., Hankinson O. The defects in all classes of aryl hydrocarbon hydroxylase-deficient mutant of mouse hepatoma line, Hepa-1, are restricted to activities catalyzed by cytochrome P-450. Cancer Lett. 1983 Oct;20(3):249–254. doi: 10.1016/0304-3835(83)90021-6. [DOI] [PubMed] [Google Scholar]
- Duthu G. S., Nestor M. S., Berliner J. A., Philpot R. M., Hankinson O. Characterization of NADPH-cytochrome P-450 reductase in a mouse hepatoma cell line. Cancer Lett. 1983 Apr;18(3):237–243. doi: 10.1016/0304-3835(83)90231-8. [DOI] [PubMed] [Google Scholar]
- Fujita S., Suzuki M., Suzuki T. Structure-activity relationships in the induction of hepatic drug metabolism by azo compounds. Xenobiotica. 1984 Jul;14(7):565–568. doi: 10.3109/00498258409151449. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., FUKUNISHI R. INDUCED PROTECTION OF ADRENAL CORTEX AGAINST 7,12-DIMETHYLBENZ(ALPHA)ANTHRACENE. INFLUENCE OF ETHIONINE. INDUCTION OF MENADIONE REDUCTASE. INCORPORATION OF THYMIDINE-H3. J Exp Med. 1964 Jan 1;119:923–942. doi: 10.1084/jem.119.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., PATAKI J. AROMATIC AZO DERIVATIVES PREVENTING MAMMARY CANCER AND ADRENAL INJURY FROM 7,12-DIMETHYLBENZ(A)ANTHRACENE. Proc Natl Acad Sci U S A. 1965 Apr;53:791–796. doi: 10.1073/pnas.53.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O., Andersen R. D., Birren B. W., Sander F., Negishi M., Nebert D. W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985 Feb 10;260(3):1790–1795. [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins C. B., Ueda N., Russo A. Azo dyes prevent hydrocarbon-induced leukemia in the rat. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4524–4527. doi: 10.1073/pnas.75.9.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Kahl R. Synthetic antioxidants: biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology. 1984 Dec;33(3-4):185–228. doi: 10.1016/0300-483x(84)90038-6. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Egner P. A., Trush M. A., Bueding E., Groopman J. D. Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis. 1985 May;6(5):759–763. doi: 10.1093/carcin/6.5.759. [DOI] [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Lind C. Relationship between the rate of reduction of benzo(a)pyrene-3,6-quinone and the formation of benzo(a)pyrene-3,6-quinol glucuronides in rat liver microsomes. Biochem Pharmacol. 1985 Mar 15;34(6):895–897. doi: 10.1016/0006-2952(85)90772-5. [DOI] [PubMed] [Google Scholar]
- Lind C., Vadi H., Ernster L. Metabolism of benzo(a)pyrene-3,6-quinone and 3-hydroxybenzo(a)pyrene in liver microsomes from 3-methylcholanthrene-treated rats. A possible role of DT-diaphorase in the formation of glucuronyl conjugates. Arch Biochem Biophys. 1978 Sep;190(1):97–108. doi: 10.1016/0003-9861(78)90256-4. [DOI] [PubMed] [Google Scholar]
- Lubet R. A., Connolly G., Kouri R. E., Nebert D. W., Bigelow S. W. Biological effects of the Sudan dyes. Role of the Ah cytosolic receptor. Biochem Pharmacol. 1983 Oct 15;32(20):3053–3058. doi: 10.1016/0006-2952(83)90248-4. [DOI] [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A., BROWN R. R., MACDONALD J. C. On the protective action of certain polycyclic aromatic hydrocarbons against carcinogenesis by aminoazo dyes and 2-acetylaminofluorene. Cancer Res. 1958 May;18(4):469–477. [PubMed] [Google Scholar]
- Morrison H., Jernström B., Nordenskjöld M., Thor H., Orrenius S. Induction of DNA damage by menadione (2-methyl-1,4-naphthoquinone) in primary cultures of rat hepatocytes. Biochem Pharmacol. 1984 Jun 1;33(11):1763–1769. doi: 10.1016/0006-2952(84)90347-2. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Bregman H. S., De Long M. J., Talalay P. Specificity of induction of cancer protective enzymes by analogues of tert-butyl-4-hydroxyanisole (BHA). Biochem Pharmacol. 1985 Nov 1;34(21):3909–3914. doi: 10.1016/0006-2952(85)90443-5. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., De Long M. J., Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON H. L., STIER A. R., BORSOS-NACHTNEBEL E. Liver tumor inhibition and adrenal histologic responses in rats to which 3'-methyl-4-dimethylaminoazobenzene and 20-methylcholanthrene were simultaneously administered. Cancer Res. 1952 May;12(5):356–361. [PubMed] [Google Scholar]
- Smart R. C., Zannoni V. G. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984 Jul;26(1):105–111. [PubMed] [Google Scholar]
- Sparnins V. L., Chuan J., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the esophagus by phenols, lactones, and benzyl isothiocyanate. Cancer Res. 1982 Apr;42(4):1205–1207. [PubMed] [Google Scholar]
- Sparnins V. L., Venegas P. L., Wattenberg L. W. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J Natl Cancer Inst. 1982 Mar;68(3):493–496. [PubMed] [Google Scholar]
- Talalay P., Batzinger R. P., Benson A. M., Bueding E., Cha Y. N. Biochemical studies on the mechanisms by which dietary antioxidants suppress mutagenic activity. Adv Enzyme Regul. 1978;17:23–36. doi: 10.1016/0065-2571(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Talalay P., Benson A. M. Elevation of quinone reductase activity by anticarcinogenic antioxidants. Adv Enzyme Regul. 1982;20:287–300. doi: 10.1016/0065-2571(82)90021-8. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wattenberg L. W. Chemoprevention of cancer. Cancer Res. 1985 Jan;45(1):1–8. [PubMed] [Google Scholar]
- Wattenberg L. W., Coccia J. B., Lam L. K. Inhibitory effects of phenolic compounds on benzo(a)pyrene-induced neoplasia. Cancer Res. 1980 Aug;40(8 Pt 1):2820–2823. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of neoplasia by minor dietary constituents. Cancer Res. 1983 May;43(5 Suppl):2448s–2453s. [PubMed] [Google Scholar]
- Williams R. T. Comparative patterns of drug metabolism. Fed Proc. 1967 Jul-Aug;26(4):1029–1039. [PubMed] [Google Scholar]



