Abstract
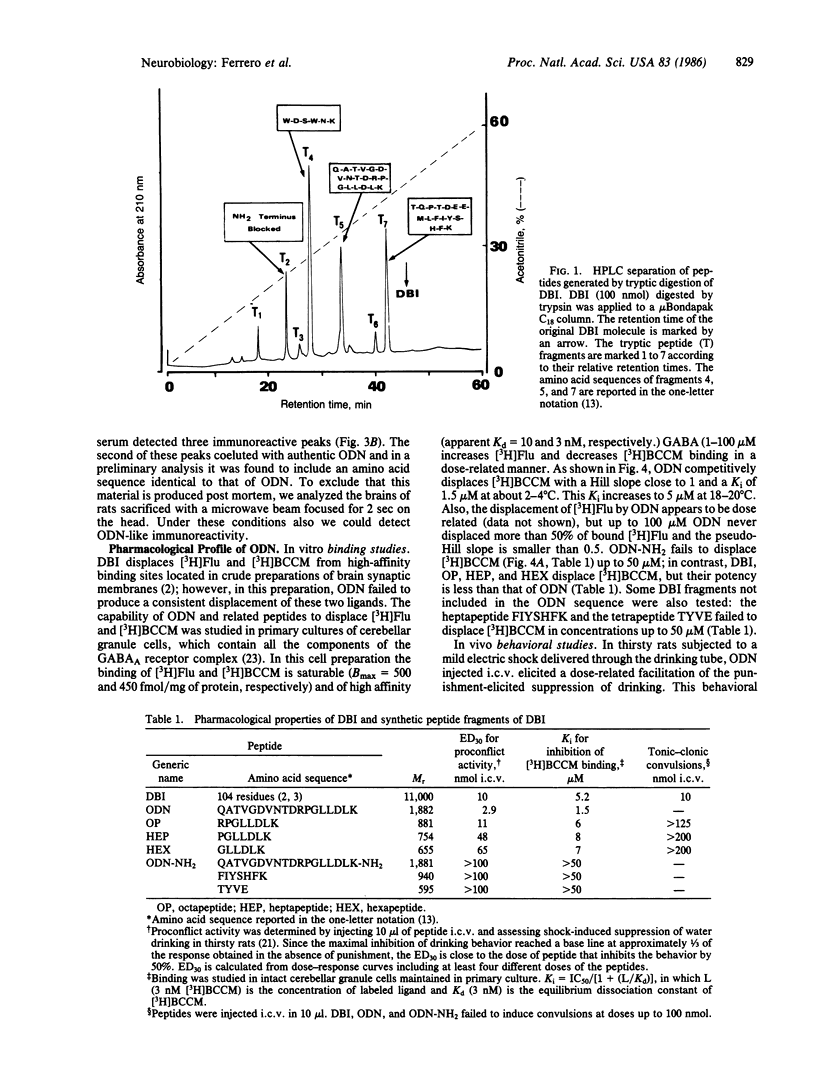
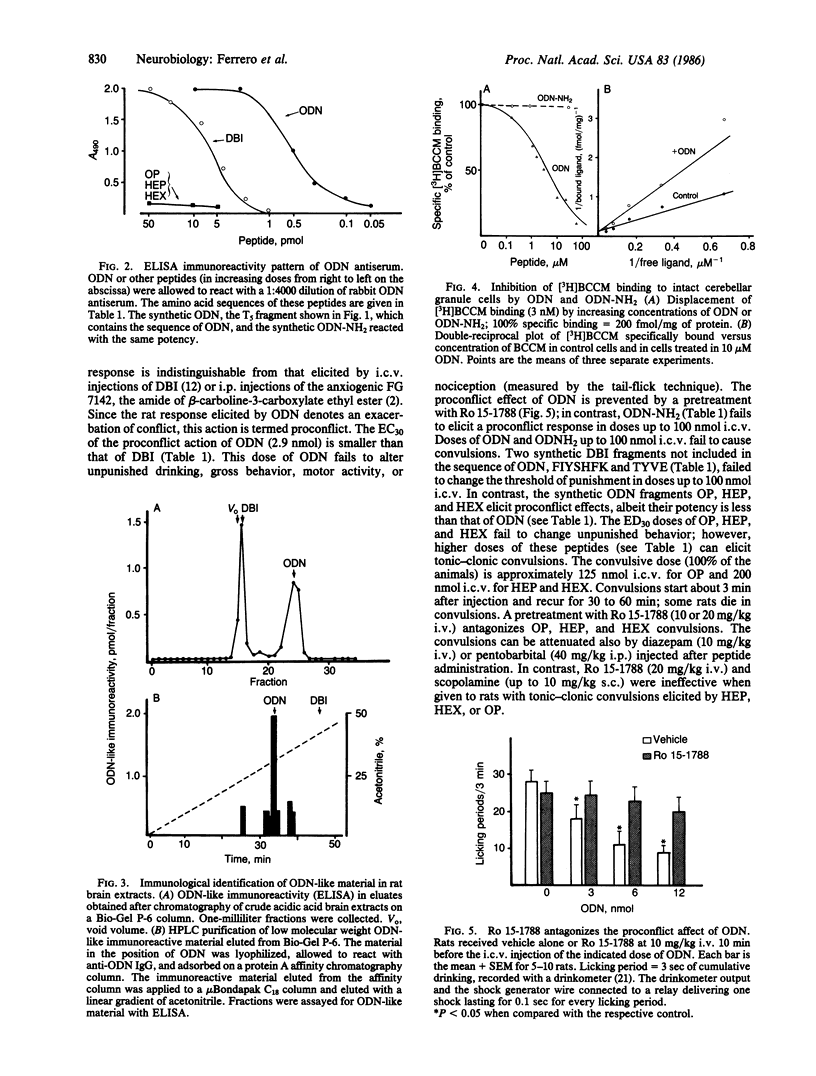
An endogenous brain neuropeptide with 104 amino acid residues that modulates gamma-aminobutyric acid receptor function was termed DBI because it displaces diazepam from its specific brain binding sites. Tryptic digestion of DBI generates an octadecaneuropeptide (ODN) that is more potent than the parent compound in the displacement of specifically bound beta-[3H]carboline-3-carboxylate methyl ester [( 3H]BCCM) and in proconflict action (Vogel test in thirsty rats). The proconflict action of ODN is antagonized by the imidobenzodiazepinone Ro 15-1788, which is a specific antagonist of beta-carboline and benzodiazepine recognition sites. The ODN amino acid sequence is Gln-Ala-Thr-Val-Gly-Asp-Val-Asn-Thr-Asp-Arg-Pro-Gly-Leu-Leu-Asp-Leu-Lys. The pharmacological properties associated with this sequence were confirmed by comparing the activity of ODN generated from tryptic digestion of DBI with that of ODN obtained by synthesis. Amidation of the terminal lysine of ODN produces a peptide (ODN-NH2) devoid of pharmacological activity. Three peptides containing the COOH-terminal segment of ODN were synthesized. All these peptides [Arg-Pro-Gly-Leu-Leu-Asp-Leu-Lys (octapeptide), Pro-Gly-Leu-Leu-Asp-Leu-Lys (heptapeptide), and Gly-Leu-Leu-Asp-Leu-Lys (hexapeptide)] express the displacing and proconflict actions of ODN. In primary cultures of cerebellar granule cells of rat, DBI, ODN, octapeptide, heptapeptide, and hexapeptide preferentially displace [3H]BCCM over [3H]flunitrazepam; moreover, they displace bound [3H]BCCM completely but [3H]flunitrazepam only by 50%. These data suggest that ODN includes a specific ligand for the gamma-aminobutyric acid receptor regulatory site occupied by beta-carbolines. Using rabbit antibodies directed against the NH2-terminal portion of ODN, we detected ODN-like material in rat brain homogenates. However, whether this material is identical to the ODN generated by tryptic digestion of DBI remains to be established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corda M. G., Blaker W. D., Mendelson W. B., Guidotti A., Costa E. beta-Carbolines enhance shock-induced suppression of drinking in rats. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2072–2076. doi: 10.1073/pnas.80.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Corda M. G., Guidotti A. On a brain polypeptide functioning as a putative effector for the recognition sites of benzodiazepine and beta-carboline derivatives. Neuropharmacology. 1983 Dec;22(12B):1481–1492. doi: 10.1016/0028-3908(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Ferrero P., Guidotti A., Conti-Tronconi B., Costa E. A brain octadecaneuropeptide generated by tryptic digestion of DBI (diazepam binding inhibitor) functions as a proconflict ligand of benzodiazepine recognition sites. Neuropharmacology. 1984 Nov;23(11):1359–1362. doi: 10.1016/0028-3908(84)90061-3. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Wise B. C., Vaccarino F., Guidotti A. gamma-Aminobutyric acid- and benzodiazepine-induced modulation of [35S]-t-butylbicyclophosphorothionate binding to cerebellar granule cells. J Neurosci. 1985 Sep;5(9):2432–2438. doi: 10.1523/JNEUROSCI.05-09-02432.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Ferrero P., Santi M. R., Alho H., Vicini S., Costa E. Gamma-aminobutyric acid and chloride channels: regulation by neuropeptides. Regul Pept Suppl. 1985;4:172–181. [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Toffano G., Costa E. An endogenous protein modulates the affinity of GABA and benzodiazepine receptors in rat brain. Nature. 1978 Oct 12;275(5680):553–555. doi: 10.1038/275553a0. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Häring P., Stähli C., Schoch P., Takács B., Staehelin T., Möhler H. Monoclonal antibodies reveal structural homogeneity of gamma-aminobutyric acid/benzodiazepine receptors in different brain areas. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4837–4841. doi: 10.1073/pnas.82.14.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Möhler H., Richards J. G., Wu J. Y. Autoradiographic localization of benzodiazepine receptors in immunocytochemically identified gamma-aminobutyrergic synapses. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1935–1938. doi: 10.1073/pnas.78.3.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Schoch P., Richards J. G., Häring P., Takacs B., Stähli C., Staehelin T., Haefely W., Möhler H. Co-localization of GABA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature. 1985 Mar 14;314(6007):168–171. doi: 10.1038/314168a0. [DOI] [PubMed] [Google Scholar]
- Sigel E., Stephenson F. A., Mamalaki C., Barnard E. A. A gamma-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J Biol Chem. 1983 Jun 10;258(11):6965–6971. [PubMed] [Google Scholar]
- Smart T. G., Constanti A., Bilbe G., Brown D. A., Barnard E. A. Synthesis of functional chick brain GABA-benzodiazepine-barbiturate/receptor complexes in mRNA-injected Xenopus oocytes. Neurosci Lett. 1983 Sep 19;40(1):55–59. doi: 10.1016/0304-3940(83)90092-7. [DOI] [PubMed] [Google Scholar]
- Vogel J. R., Beer B., Clody D. E. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21(1):1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Balázs R., Wilson J. E., Cohen J., Dutton G. R. Preparation of cell bodies from the developing cerebellum: structural and metabolic integrity of the isolated cells. Brain Res. 1976 Oct 15;115(2):181–199. doi: 10.1016/0006-8993(76)90506-0. [DOI] [PubMed] [Google Scholar]



