Abstract
Background
Members of the homeodomain-leucine zipper (HD-Zip) gene family encode transcription factors that are unique to plants and have diverse functions in plant growth and development such as various stress responses, organ formation and vascular development. Although systematic characterization of this family has been carried out in Arabidopsis and rice, little is known about HD-Zip genes in maize (Zea mays L.).
Methods and Findings
In this study, we described the identification and structural characterization of HD-Zip genes in the maize genome. A complete set of 55 HD-Zip genes (Zmhdz1-55) were identified in the maize genome using Blast search tools and categorized into four classes (HD-Zip I-IV) based on phylogeny. Chromosomal location of these genes revealed that they are distributed unevenly across all 10 chromosomes. Segmental duplication contributed largely to the expansion of the maize HD-ZIP gene family, while tandem duplication was only responsible for the amplification of the HD-Zip II genes. Furthermore, most of the maize HD-Zip I genes were found to contain an overabundance of stress-related cis-elements in their promoter sequences. The expression levels of the 17 HD-Zip I genes under drought stress were also investigated by quantitative real-time PCR (qRT-PCR). All of the 17 maize HD-ZIP I genes were found to be regulated by drought stress, and the duplicated genes within a sister pair exhibited the similar expression patterns, suggesting their conserved functions during the process of evolution.
Conclusions
Our results reveal a comprehensive overview of the maize HD-Zip gene family and provide the first step towards the selection of Zmhdz genes for cloning and functional research to uncover their roles in maize growth and development.
Introduction
The homeobox (HB) gene encodes a highly conserved 60–61 amino acid homeodomain (HD), which confers sequence-specific DNA binding function by folding into a characteristic three α-helix structure [1], [2]. The HB gene was first identified in a set of Drosophila genes controlling development and has been found in all investigated eukaryotes [3]. Since the discovery of Knotted-1 in maize [4], many plant HD proteins have been isolated and categorized into several different groups based on the presence of conserved domains [2]. HD-Zip protein, one group of these proteins, has only been identified in plants thus far. The characteristic of this group is the presence of a HD and an adjacent leucine zipper (Zip) acting as a dimerization motif [5], [6].
In Arabidopsis, 48 out of 110 HB genes have been identified as HD-Zip proteins [7]–[11]. Based on their structures, additional specific domains and functions, HD-Zip genes are further categorized into four classes: HD-Zip I-IV [2], [12]. Members of class I and II share the conserved HD and Zip domains and recognize similar pseudopalindromic binding sites: CAAT-N-ATTG [13]–[15]. Furthermore, HD-Zip II members possess an additional domain, known as CPSCE (based on five conserved amino acids: Cys, Pro, Ser, Cys and Glu), which is closely linked to the downstream of Zip domain and mainly responsible for redox cell state perception [16], [17]. Besides the HD and Zip domains, HD-Zip III and IV genes encode two additional common domains: a START domain with putative function in sterol binding followed by an adjacent region named SAD domain with unknown function [2], [18]–[19]. HD-Zip III proteins have a characteristic MEKHLA domain in the C-terminus, which shares significant similarity with the PAS domain and was proposed to function as a sensory domain involved in light, oxygen and redox signaling. Recent studies have indicated that the MEKHLA domain function as a negative regulator of Arabidopsis HD-ZIP III REV activity by inhibiting dimerization [2], [20]–[21].
HD-Zip encoding genes have been isolated from various plants, such as Arabidopsis [22], rice [13], tomato [23], cotton [24], maize [25] and Physcomitrella patens [26]. HD-Zip proteins have been demonstrated to participate in transcriptional regulation of a series of biological processes related to plant growth and development. HD-Zip I genes mainly participate in the regulation of abiotic stress responses, e.g. drought, extreme temperatures, osmotic and light stress [27]–[29]. Expression patterns of the ATHB5, -6, -7, and -12 are either up- or down-regulated by drought stress or by externally abscisic acid (ABA), indicating that these genes may play a crucial role in regulating plant responses to abiotic stress in an ABA-dependent pathway [22], [30]–[32]. Oshox4, a HD-Zip I gene from rice, is believed to be involved in the regulation of stem elongation and other developmental processes. Over-expression of Oshox4 leads to enhanced tolerance to drought stress [33]. Previous studies have also revealed that other members of this class, such as ATHB16 and -52, are involved in light perception signaling [27], [34]. HD-Zip II genes are mainly regulated by illumination conditions and auxin responses [35], [36]. As a negative regulator, ATHB2 can recognize its own promoter region and participate in regulating the Arabidopsis shade avoidance response [37]. Sunflower HAHB10, a HD-Zip II gene, which shares a similar gene structure and expression pattern with ATHB2, mainly regulates the plant response to light quality and quantity related to plant development [29]. Recently, two cotton HD-Zip II genes, GhHB2 and -4, were found to play essential roles in the phytohormone signaling pathway that regulates early seedling development, and expression of GhHB2 and -4 were shown to be up-regulated in hypocotyls, cotyledons and roots by external GA3 treatments [38].
The Arabidopsis genome contains five HD-Zip III genes (REV, PHB, PHV, CAN and ATHB8) [10]. Accumulating evidences have revealed that these genes play significant roles in different developmental processes. ATHB8, an Arabidopsis HD-Zip III gene, is a positive regulator of vascular cell differentiation, and ectopic expression of ATHB8 in Arabidopsis can increase the production of xylem tissue [39]. HD-Zip IV genes are generally involved in determining outer cell fates [40], [41]. OCL4 encoding a maize HD-Zip IV gene is expressed in the epidermis of the leaf blades. Functional studies showed that trichome development is inhibited in Arabidopsis transgenic plants over-expressing OCL4, but ectopic trichomes differentiation has also been observed on the edge of the leaf in OCL4 RNAi transgenic plants [25].
Maize is an important cereal crop and has become a model plant for the study of genetics, evolution and other basic biological research. The availability of the maize genome sequences has provided an excellent opportunity for whole-genome annotation, classification and comparative genomics research [42]. Although HD-Zip genes have been extensively characterized in Arabidopsis, rice and other species [8], [33], [43], a systemic analysis of the HD-Zip gene family in maize, especially for the potential stress-responsive members, has not been reported in such an important species. In this study, 55 putative HD-Zip genes were identified and characterized in the maize genome. Furthermore, we investigated the transcript levels of HD-Zip I genes in response to drought stress which severely affects maize yields. The results provide a biological reference for future studies on the functions of the HD-Zip genes and will be helpful for breeding drought-resistant maize.
Results
Identification and annotation of HD-Zip genes in maize
The consensus protein sequences of the HD Hidden Markov Model (HMM) profile were employed as a query to search against the maize genome database with BlastP program. Through this approach, a total of 99 HD-containing sequences were identified. To confirm putative HD-Zip genes in the maize genome, the amino acid sequences of all the 99 proteins were searched for the presence of both HD and Zip domains by Pfam and SMART. As results of an extensive search for HD-Zip genes, 55 non-redundant HD-Zip genes (named Zmhdz1 to Zmhdz55) were confirmed from the original data. The total number of HD-Zip genes identified in maize (55) is a little greater than that in either Arabidopsis (48) or rice (48) (Table 1) [8]–[11], [44]. We aligned all the Zmhdz genes with Arabidopsis and rice HD-Zip genes to generate a phylogenetic tree for classification of maize HD-Zip genes. Based on their relationships with Arabidopsis and rice HD-Zip genes, the 55 Zmhdz genes were divided into four distinct classes, including class I (17 genes), class II (18 genes), class III (5 genes) and class IV (15 genes) (Table 1).
Table 1. Numbers of HD-Zip genes in the maize, rice and Arabidopsis genomes.
| Category | Maize | Arabidopsis | Rice |
| Class I | 17 | 17 | 14 |
| Class II | 18 | 10 | 13 |
| Class III | 5 | 5 | 9 |
| Class IV | 15 | 16 | 12 |
| Total number | 55 | 48 | 48 |
Although all of the HD-Zip genes encode the conserved HD and Zip domains, their sequences elsewhere are highly diverse. The HD-Zip III and IV proteins are in general longer (600–950aa) than those of the HD-Zip I and II proteins (200–400aa). Furthermore, the molecular weights of these deduced Zmhdz proteins have a range from 23.8 kDa to 103.56 kDa. Similar to that reported in Arabidopsis and rice [2], [33], the Zmhdz genes stand out from a large number of other plant gene families in their bimodal distribution of exons. Class I and II Zmhdz genes are generally found to have two to four exons, whereas class III and IV contain multiple exons, ranging from seven to eighteen (Table S1).
Phylogenetic and structural analysis of Zmhdz genes
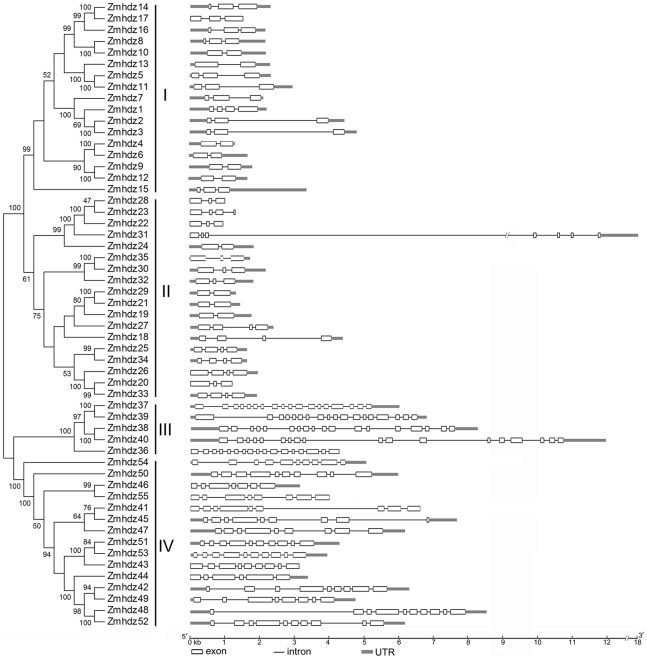
The predicted protein sequences of all the Zmhdz genes were used to generate an unrooted phylogenetic tree (Figure 1). The unrooted tree categorized the Zmhdz genes into four major groups (class I, II, III and IV) with well-supported bootstrap values. Fifty-five of the Zmhdz genes formed 18 gene pairs, and all of them had strong bootstrap support (>94%), with the exception of Zmhdz28/23, Zmhdz41/45 and Zmhdz51/53. We subsequently performed an exon-intron structure analysis to support the phylogeny reconstruction. The schematic structures revealed that each coding sequence of Zmhdz gene is disrupted by one or more introns. Based on those results, the Zmhdz genes can be divided into four groups as shown in figure 1. The HD-Zip genes within the same groups of the phylogenetic tree all showed similar exon-intron structures. Similar to that reported in Arabidopsis and rice, the numbers of introns are quite different across the four classes. Class III HD-Zip genes contain the most number of introns, while class I and II genes contain the fewest of introns.
Figure 1. Phylogenetic relationship and gene structure of the 55 predicted maize HD-Zip proteins.
NJ tree (the part of left side): The unrooted tree was generated with MEGA4.0 program using the full-length amino acid sequences of the 55 maize HD-Zip proteins. The bootstrap values are indicated at the branches in black numbers, and the proteins were named according to their gene codes (see Table S1). Gene structure (the part of right side): Exons and introns are indicated by white boxes and single lines, respectively. Thick gray lines represent the untranslated regions (UTRs). The length of each HD-Zip gene can be estimated using the scale at the bottom.
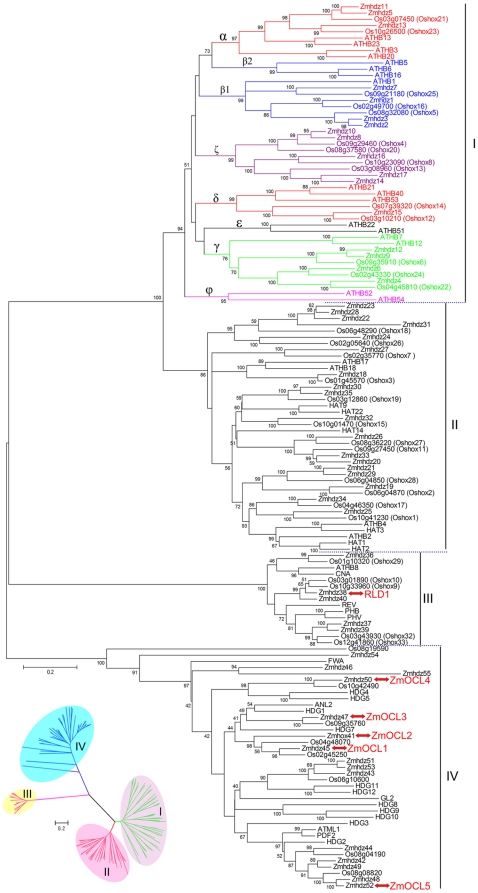
To examine the phylogenetic relationships of HD-Zip genes from different species, a combined phylogenetic tree was constructed from alignment of the full-length sequences of maize, rice and Arabidopsis HD-Zip proteins (Text S1). Our results showed that the Arabidopsis 48 HD-Zip genes fell broadly into four distinct classes, which contained representative maize and rice HD-Zip genes (Figure 2). In Arabidopsis, the HD-Zip I genes have been further divided into six monophyletic subclasses, including α, β, γ, δ, ε and ϕ [8]. In this study, all of the maize and rice HD-Zip I genes can be grouped together with their Arabidopsis counterparts except for one clade, which contained nine members of Zmhdz10, -8, -16, -17, -14 and Oshox4, -20, -8 and -13 with high bootstrap support (>95%). Thus, we assigned these nine sequences to the now maize and rice specific ζ clade according to the nomenclature previously proposed by Agalou et al. (2008) [33]. Furthermore, Arabidopsis ε and ϕ clades did not group with maize or rice HD-Zip genes. As previously reported in rice, the original β clade was also separated into two clades in our NJ tree, β1 and β2 clades. Clade β1 comprised of four maize and three rice homologs whereas clade β2 did not contain any maize or rice HD-Zip genes. The phylogenetic relationships based on the MP or ML trees were largely consistent with those results (Figure S1 and S2).
Figure 2. Phylogenetic relationships of maize, rice and Arabidopsis HD-Zip proteins.
The tree was generated with MEGA4.0 program using the NJ method. Bootstrap values are indicated at the branches in black numbers. The unrooted tree in the lower-left corner was constructed using the same method.
The clade of class II genes could be further divided into several subclasses as described by Ciarbelli et al. (2008) [9]. However, a novel subclass, including Zmhdz23, -28, -22, -31 -24 and Oshox18 and -26, was shown to evolve separately from the Arabidopsis homologs. The NJ tree also showed that Zmhdz18 and Oshox3 were closely related to ATHB17 and -18. Although some maize or rice HD-Zip genes could be grouped with their Arabidopsis counterparts, the bootstrap values in the tree nodes were low. For example, the bootstrap value of the HAT14 containing clade was only 51%. In addition, Zmhdz27 and Oshox7 formed a sister pair and existed as a single clade.
In plants, class III HD-Zip genes are very conserved, not only in the sequences of HD, Zip and START domains but also in the number. Although class III consisted of just 15 HD-Zip genes from Arabidopsis, rice and maize, six sister pairs were found in the NJ tree. Two sister pairs (Zmhdz37/39 and Oshox32/33) showed close relationship with the PHB and PHV sister pair. Unlike HD-Zip I and III genes, the phylogenetic relationships of class IV genes were unclear and the bootstrap values were low in some branches. This phenomenon was more significant in the outer lineage-expansion clades, such as the ANL2 containing clade. Moreover, some independently evolved singletons were also found in this group. Remarkably, Zmhdz50 and Os10g42490 formed a sister pair and closely related to the HDG4 and -5.
Representatives of the six published HD-Zip genes of class III and IV were also confirmed in the tree. As shown in figure 2, no Arabidopsis representatives were grouped with the Zmhdz38 (RLD1) containing clade. In contrary to RLD1, ZmOCL4 showed a close relationship with HDG4 and -5, and this observation was also strengthened by the fact that ZmOCL4 and HDG4 and -5 showed strong expression in flower organs [11]. It should be noted that ZmOCL1 and -2 clustered in the same clade, suggesting that genes of this clade may play important roles in outer cell layer development, such as root and floral meristems [45]–[47].
Investigation conserved motifs in Zmhdz genes
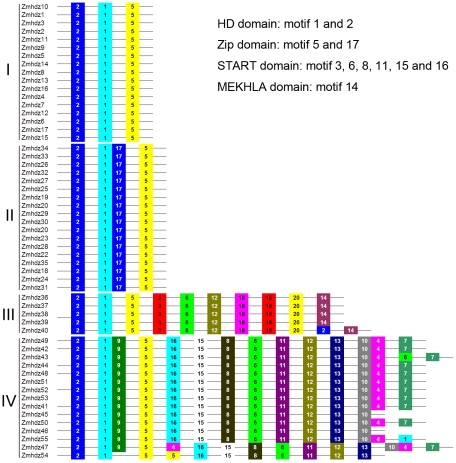
Using the MEME web server, 20 conserved motifs were identified in the maize HD-Zip proteins (Figure 3). Each of the putative motifs obtained from MEME was annotated by searching Pfam and SMART. Based on the distribution of the 20 predicted motifs, we categorized the 55 Zmhdz genes into four classes, completely consistent with the classifications from the phylogenetic analysis. Motifs 1 and 2 encoding the HD domain were found in all the 55 Zmhdz genes. All of the 55 Zmhdz genes had either motif 5 or 17 encoding the Zip domain. The conserved motifs 3, 6, 8, 11, 15 and 16 specifying the START domain were identified in class III and IV genes. The MEKHLA domain, represented by motif 14, was found to be distributed in the C-terminus of each class III gene. In addition, some subfamily-specific motifs were also found with unknown functions, indicating that these motifs are likely required for subfamily-specific functions. The conserved domains and highly conserved amino acid residues encoding subfamily-specific motifs were also confirmed from alignment of the protein sequences of the four classes (Figure S3). The detailed information on conserved amino acid sequences and lengths of the 20 motifs are shown in table 2.
Figure 3. Distribution of 20 putative conserved motifs in maize HD-Zip proteins.
Motifs of HD-Zip proteins were identified by MEME web server. Note that the length of each box in the proteins does not represent the actual motif size, and the colored boxes were ordered manually according to the results of the MEME analysis. The conserved amino acid sequences and length of each motif are shown in table 2.
Table 2. Major MEME motif sequences in maize HD-Zip proteins.
| Motif no.a | Width | Conserved amino acid sequences |
| 1 | 29 | LARQLGLQPRQVKVWFQNRRARWKTKQTE |
| 2 | 21 | KKYHRHTKEQIQFLEDCFKEC |
| 3 | 113 | ETLTEFMSKATGTAIDWVQMPGMKPGPDSVGIIAISHGCRGVAARACGLVNLEPTKVIEILKDRPSWYRDCRSMEVYHVIPTGNGGTIELIYMQMYAPTTLAPPRDFWTLRYT |
| 5 | 15 | LTEENDRLQKEIDEL |
| 6 | 21 | PSGCLIQDMPNGYCKVTWVEH |
| 8 | 37 | STGVAGNYNGALQLMYMEFQVPSPLVPTRECYFLRYC |
| 11 | 23 | SGLAFGAHRWVATLQRQCEYLAI |
| 14 | 76 | FANQAGFDMLETTLVNIQDLTLEKIFDEQGRKALYAEIPKIMEQGYAYLPGGVCMSGMGRHVSYEQAVAWKVLGDD |
| 15 | 29 | SGVVIMTHVSLVEIFMDVNKWMEMFPCIV |
| 16 | 29 | DKPMIVELAVAAMDELIRMAQMDEPLWIP |
| 17 | 11 | VDCEYLKRCCE |
Motifs with unknown functions are not shown in this table. The motif numbers are consistent with the numbers in figure 3.
Chromosomal locations and gene duplication
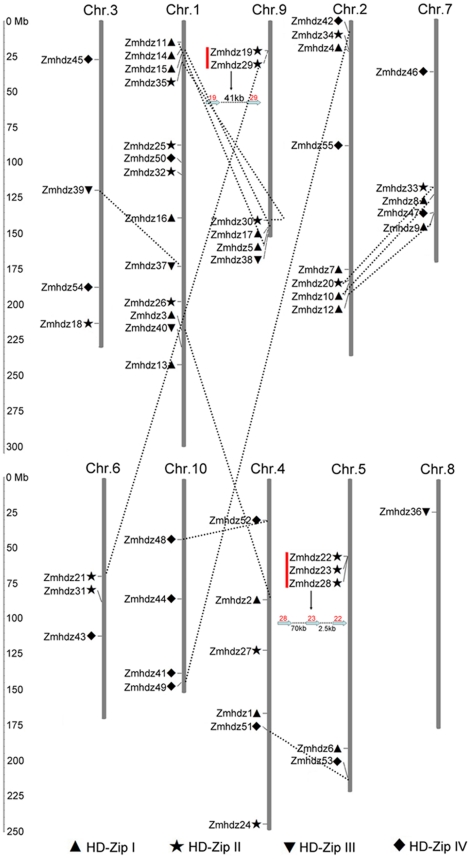
The 55 HD-Zip genes were found to be distributed unevenly across all the ten chromosomes in the maize genome (Figure 4). The number of Zmhdz genes appearing on each chromosome had a wide range. Chromosome 1 contains the maximum number of genes (thirteen), followed by chromosome 2 (eight). By contrast, chromosome 8 has only one HD-Zip gene. Relatively high densities of Zmhdz genes were found in some chromosomal regions, such as the top of chromosome 1 and the bottom of chromosome 9. In addition, two gene clusters including five genes (Zmhdz22/23/28 and Zmhdz19/29) were detected on chromosomes 5 and 9, respectively.
Figure 4. Chromosomal locations of maize HD-Zip genes on all 10 chromosomes.
Markers next to the gene names represent the classes to which each HD-Zip gene belongs (HD-Zip I, ▴; HD-Zip II, ★; HD-Zip III, ▾; HD-Zip IV,  ). Chromosome numbers are shown at the top of each vertical gray bar, and the names on the left side of each chromosome correspond to the approximate locations of each HD-Zip gene. Genes involved in segmental duplication are joined by dashed lines, and the red rectangle indicates the gene cluster on each chromosome. The scale is in megabases (Mb).
). Chromosome numbers are shown at the top of each vertical gray bar, and the names on the left side of each chromosome correspond to the approximate locations of each HD-Zip gene. Genes involved in segmental duplication are joined by dashed lines, and the red rectangle indicates the gene cluster on each chromosome. The scale is in megabases (Mb).
In this study, gene duplication events, including tandem and segmental duplications, were investigated with the purpose of elucidating the expanded mechanism of the Zmhdz gene family that are thought to have occurred during the process of evolution [48], [49]. Based on the phylogenetic analysis and the chromosomal distribution of the Zmhdz genes, 12 gene pairs (Zmhdz14/17, Zmhdz8/10, Zmhdz5/11, Zmhdz2/3, Zmhdz9/12, Zmhdz35/30, Zmhdz29/21, Zmhdz20/33, Zmhdz37/39, Zmhdz51/53, Zmhdz42/49 and Zmhdz48/52) were identified to be involved in the segmental duplication events (Figure 4). Among the 12 segmental duplication events, the high frequency of segmental duplication occurred between chromosomes 1 and 9, which contained three segmental duplication events, as well as between chromosomes 7 and 2. According to the B73 maize genome annotation, the clustered genes of Zmhdz19 and -29 were spaced by a 41 kb genomic distance and separated by only one gene on the chromosome. The locations of Zmhdz28, 22 and 23 were adjacent to each other on the chromosome. Since the fact that genes in the same cluster have high sequence similarity and share close evolutionary traits, all the five genes involved in the two clusters were considered as tandem duplicated genes.
It is noteworthy that among the 18 sister pairs, five gene pairs (Zmhdz4/6, Zmhdz25/34, Zmhdz38/40, Zmhdz46/55 and Zmhdz41/45) (Zmhdz28 and -23 were found to be involved in tandem duplication) were located just outside the segmental duplication regions [50]. However, their gene structure and phylogenetic relationships indicated that they share a similar evolutionary history and are closely related to each other (Figure 1). Therefore, the five gene pairs were determined as putative segmental duplications.
Identification of putative stress-related cis-elements in the promoters of Zmhdz I genes
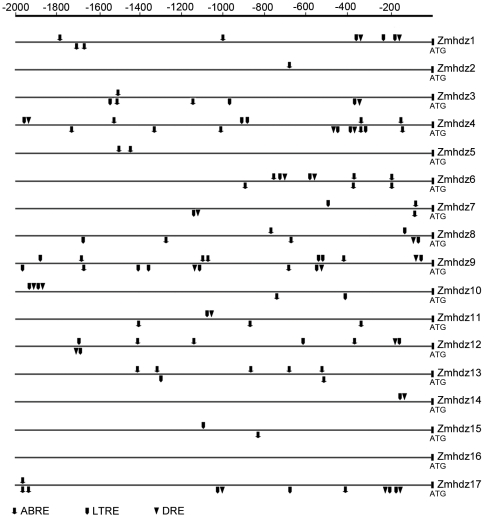
To elucidate the possible regulatory mechanism of Zmhdz I genes in various stress responses, we identified putative stress-related cis-elements in the 2 kb promoter regions upstream of the transcription start site (ATG) of the stress-responsive Zmhdz I genes. Three types of cis-elements, which are directly related to the ABA-responsive element (ABRE) [51], [52], low temperature-responsive element (LTRE) [52] and dehydration-responsive element (DRE) [51], were detected by searching the prompter sequence against the PLACE database. Surprisingly, we found that all the Zmhdz I genes except for Zmhdz16 contained the putative ABRE, LTRE or DRE in their promoter regions (Figure 5). Moreover, several members, such as Zmhdz4, -9 and -12, were enriched with multiple ABRE, LTRE and DRE in their promoters. By comparing the distribution of the three regulatory elements in the promoter regions, the six sister pairs of HD-Zip I genes were found to exhibit significant differences in their promoter sequences, suggesting that the duplicated genes may not have some regulatory features in common, but rather under similar regulatory pathway in some respects. For example, each of the duplicated genes contained at least one ABRE element in their promoter regions.
Figure 5. Distribution of major stress-related cis-elements in the promoter sequences of the 17 Zmhdz I genes.
Putative ABRE, LTRE and DRE core sequences are represented by different symbols as indicated. The cis-elements distributed on the sense strand and reverse strand are indicated above and below the black lines, respectively.
Expression patterns of Zmhdz I genes under drought stress
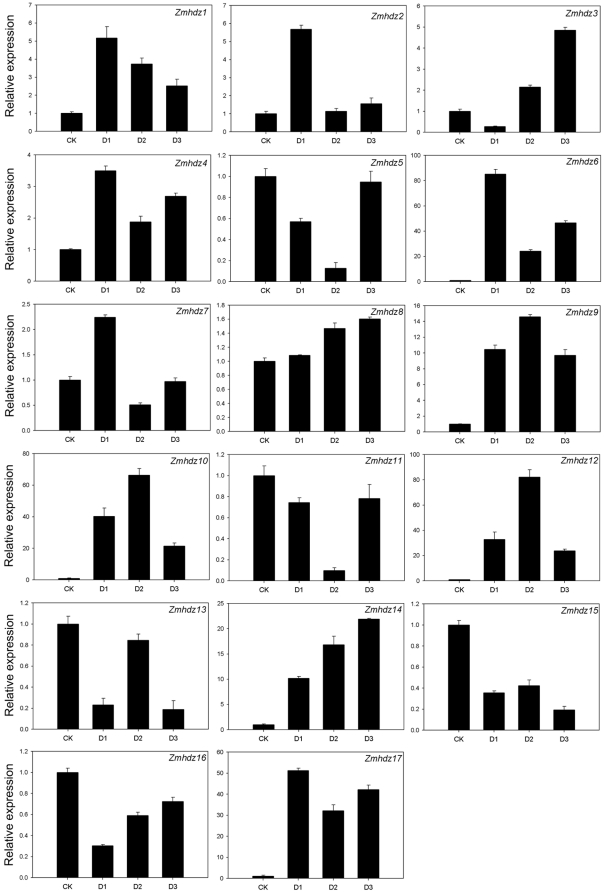
The HD-Zip I genes have been demonstrated to play important roles in abiotic stress. In this study, we investigated the expression patterns of the Zmhdz I genes in three-week-old leaves under drought stress, one of the serious environmental stresses affecting maize production. As shown in figure 6, the expression levels of all the 17 HD-Zip I genes responsive to slight, moderate and severe drought stress were compared with normal plants. Of the 17 HD-Zip I genes, the expressions of 12 Zmhdz I genes were obviously up-regulated in response to drought stress, whereas those of the other 5 were down-regulated. It should be noted that 9 out of 12 up-regulated Zmhdz I genes showed major expression changes (scales from 0 to 4 up 0 to 100), including Zmhdz1, -2, -3, -6, -9, -10, -12, -14 and -17, while the other 3 genes (Zmhdz4, -7 and -8) showed minor changes (scales from 0 to 4 and lower). The differential expression patterns were also found among different subclasses based on phylogenetic classification. Drought stress resulted in a down-regulation of Zmhdz11, -5 and -13 of subclass α. By contrast, the expression levels of the subclass γ genes, including Zmhdz12, -9, -6 and -4, were strongly induced by more than 2-fold compared to the control. The drought treatment also induced the expressions of Zmhdz7, -1, -3 and -2 of the subclass β1. Interestingly, besides Zmhdz16, all genes of the maize-specific ζ clade exhibited significantly up-regulated of their expression levels, especially for Zmhdz10 and -17. In addition, the expression of Zmhdz15 was greatly down-regulated by drought stress.
Figure 6. Expression patterns of the 17 Zmhdz I genes under drought stress.
Relative expressions of Zmhdz I genes were examined by qRT-PCR and normalized by Actin 1 expression. The Y axis is the scale of the relative expression level. The X axis is the time course of drought stress treatment. CK, normal plant, D1 (slight stress), leaves sampled at 3 h after treatment, D2 (moderate stress), leaves sampled at 6 h after treatment and D3 (severe stress), leaves sampled at 12 h after treatment. Date represent mean ± SD.
The expression profiles of the six duplicated gene pairs were also compared. The results revealed that the duplicated genes within a sister pair exhibited the similar expression patterns upon drought treatment. The different expression patterns between the two duplicated genes were also observed. For example, the highest expression level of Zmhdz2 was observed at 3 h (D1) after drought treatment, while Zmhdz3 was observed at 12 h (D3).
A previous study showed up-regulation in expression levels of maize PSY2 and -3 under drought stress [53]. Therefore, we investigated the expression pattern of these two genes under drought stress as a control for the drought treatment in this study. Consistent with results of the earlier report, the expression levels of PSY2 and -3 were significantly increased under drought conditions (Figure S4A). Furthermore, expression level of Actin 1, a traditional housekeeping gene, was compared with a stably expressed gene (GRMZM2G027378_T01) reported by Sekhon et al. (2011) under the same drought conditions to examine the reliability of the expression results [54]. As shown in figure S4B, expression of each housekeeping gene did not decrease or in some case increased only to a small extent in the four cDNA samples, suggesting that the expression levels of the two genes were relatively stable under the drought treatment adopted in this study.
Discussion
Segmental duplication plays a major role in expansion of the HD-Zip gene family
In the present study, a total of 55 non-redundant HD-Zip genes were identified in the maize genome. However, the number of HD-Zip genes from our search was lower than that previously reported (70) [7]. This difference may be attributed to the fact that the identification of HD-Zip genes in this study was based on the amino acid sequences using BlastP program, whereas the analysis in the previous report was based on the nucleotide sequences using TblastN searches. The P-values might affect the number of candidate sequences identified in this step. Moreover, the maize genome database used in our study was the most current with updated sequence assemblies, this may be another reason caused the different number of the HD-Zip genes. Additionally, maize genome contains extensive regions of chromosomal duplication [50]. To exclude potentially redundant sequences from our candidate HD-Zip genes, the criterion used in the two studies could not be completely consistent.
Although the maize genome size is approximately 18 times (2300 Mb: 130 Mb) and the gene number is about 1.3 times (32,000: 25,000) that of Arabidopsis, the number of Zmhdz genes was found surprisingly to be only seven more than in Arabidopsis [8]–[11], [42]. It is believed that tandem and segmental duplications resulted in a substantial expansion in gene family members during the process of genome evolution. Like Arabidopsis and rice [55]–[58], the maize genome has undergone several rounds of genome duplication early in its evolution [59], [60]. A total 12 sister pairs of close paralogs were found lying on the segmental duplicated regions among the 55 Zmhdz genes, and the other 5 sister pairs were detected as putative segmental duplicated genes. These genes represented approximately 62% of Zmhdz genes that have evolved from duplicated chromosomal regions. By contrast, merely five genes (Zmhdz28, -23, -22, -19 and -29) belonging to the HD-Zip II subfamily were found to involve in tandem duplication. Significantly, the number of Zmhdz genes involved in segmental duplication was much larger than that those arranged in tandem duplication. On the other hand, the results indicated that segmental duplication was the main contributor to the expansion of maize HD-Zip genes.
At least 75% of Arabidopsis HD-Zip genes are involved in segmental duplication, and no obviously clustered HD-Zip genes have been detected on the chromosomes [8]–[11]. The results suggested that the segmental duplication of HD-Zip genes in Arabidopsis is more prevalent than in the maize (62%) and rice (54%) genomes [44]. From the results mentioned above, it is clear that segmental duplication was largely responsible for the expansion of HD-Zip gene family in these three species. In general, segmental duplications are thought to occur regularly in more slowly evolving gene families, e.g. MYB gene family [48], [61]. The prevalence of segmental duplication might dominate the slow evolutionary rate of the plant-specific HD-Zip gene family throughout the evolutionary process, resulting in the similar number of HD-Zip genes in the maize, rice and Arabidopsis genomes.
Phylogenetic analysis and evolution of HD-Zip family genes
We have performed a detailed phylogenetic analysis of maize, rice and Arabidopsis HD-Zip genes and thus divided Zmhdz genes into four distinctly classes. Six monophyletic subclasses of Arabidopsis HD-Zip I genes described by Henriksson et al. (2005) were shown in the phylogenetic tree (Figure 2). However, no maize or rice homologs were found in the clades ε and ϕ. The phylogenetic analysis suggested that the six subclasses of HD-Zip I genes share a common origin that may existed in early organisms [8], suggesting that the lineages of clades ε and ϕ genes have been lost in maize and rice [33]. Conversely, an additional clade (ζ) was shown to be specific for maize and rice, implying that it is unique to the grass genomes. A specific subclass of maize and rice class II genes (including Zmhdz23, -28, -22, -31, -24 and Oshox18 and -26) was also identified. The phylogenetic analysis suggested that the genes of this subclass may have diverged from their common ancestral genes predating the monocots and dicots divergence and evolved separately in the grass genomes. Additionally, some branches with low bootstrap values were also found. This phenomenon should be due to the expansion of HD-Zip genes of some clades along with the genome evolution following the monocot-dicot divergence, thus resulting in the sequence divergence of some members. The prevalence of Zmhdz-Oshox ortholog pairs in the phylogenetic tree indicated that maize HD-Zip genes are more closely allied with rice homologs than Arabidopsis representatives, which is consistent with the evolutionary relationships among maize, rice and Arabidopsis.
The results of the gene structure analysis not only supported the phylogenetic reconstruction, but also revealed that the HD-Zip genes were highly conserved in evolution. The Zmhdz genes within the same class or subclass share similar exon-intron structure, and the domains defined by motif distribution are highly conserved within a class. The conserved evolution of HD-Zip genes was also reflected in the gene duplication. Previous studies revealed that the maize genome experienced several rounds of genome duplication, including an ancient genome duplication that occurred before the divergence of the grass genomes between 50 to 70 million years ago (Mya), resulting in the ancient tetraploid ancestry, an additional whole genome duplication about 5 Mya after the divergence of maize and sorghum and a recent duplication event [42], [50], [62], [63]. Date from the investigation of Arabidopsis genome indicated that genome duplication resulted in the expansion of the HD-Zip members was thought to have occurred approximately between 20 to 60 Mya [8], [64], [65]. It is believed that a common ancestor of each clade must have existed prior to the divergence of monocots and dicots, because of all the four clades were found to have representatives from rice, maize and Arabidopsis [44]. Thus, pairs of paralogous HD-Zip genes in maize derive from genome duplication were estimated to have taken place in the period of the ancient tetraploid ancestry and the divergence of maize and sorghum. Most orthologs showed a close relationship than paralogs between maize and rice HD-Zip genes, indicating that these genes formed the ortholog pairs might have originated from their common ancestor, in which ancient duplication events occurred predating maize-rice divergence. Those observations combined with the prevalence of segmental duplication of the HD-Zip genes implied that this gene family is a conserved and slowly evolving family in plant genomes.
Expression analysis of Zmhdz I genes in response to drought stress
In plants, quite a few members of the HD-Zip family have been demonstrated to be regulated by dehydration [14], [22], [66]. However, no HD-Zip genes response to drought stress was reported in maize. For this purpose, we investigated the expression patterns of maize HD-Zip I genes under drought stress. The results demonstrated that the expression levels of the 17 Zmhdz I genes were either increased or repressed by drought stress. Most genes within the same subclass of the phylogenetic tree showed the similar expression patterns. Since HD-Zip genes in the same subclass share high sequence similarity, the similar expression patterns were also found by comparing the six pairs of duplicated genes, indicating that the regulatory sequences that response to the stress conditions did not diverge much along with the evolution of each gene after duplication and that the duplicated genes might have redundant functions in response to drought stress. It is noteworthy that Zmhdz I genes clustered in the maize/rice-specific subclass (ζ clade) were significantly up-regulated under drought stress, with the exception of Zmhdz16. Oshox4, a rice HD-Zip I gene of the ζ clade, has been demonstrated to play a positive role in response to drought stress [33]. Those findings suggested that genes of the ζ clade are novel to the Zmhdz gene family and more likely to play important roles in regulating drought stress. The other five down-regulated genes and three genes with minor expression changes may also have specific functions in maize under drought conditions. A few down-regulated HD-Zip genes have been reported to play positive roles in response to abiotic stress in other plants [32], [33].
In general, duplicated genes have three outcomes: neofunctionalization, subfunctionalization and pseudogenization [67]. Although Zmhdz16 was not detected as a duplicated gene in the present study, it was closely related to the duplicated genes Zmhdz14 and -17, suggesting that they probably originated from a common ancestral gene. Accordingly, we speculated that the repression of Zmhdz16 expression under drought stress might indicate its neofunctionalization or subfunctionalization during the course of evolution. Another explanation is that the promoter of Zmhdz16 does not contain any of the three types of stress-responsive cis-elements in the 2 kb promoter region. Some novel stress-responsive cis-elements should exist in the promoter region of Zmhdz16 and play an essential role in regulating stress response. Both hypotheses should be biologically examined in future studies.
Materials and Methods
In silico identification and sequence analysis of Zmhdz genes
Maize (Zea mays L. B73) genome sequences were downloaded from http://www.maizesequence.org/ index.html. The Hidden Markov Model (HMM) profile of the HD domain (PF00046) was obtained from Pfam database (http://pfam.sanger.ac.uk/) [68]. We employed this HMM profile as a query to identify all HD-containing sequences in maize by searching HD domain sequence against the maize genome database using BlastP program (P-value = 0.001). Subsequently, the Pfam and SMART (http://smart.embl-heidelberg.de/) [69] were used to determine each candidate HD protein as a member of the HD-Zip family, which contains both the conserved HD and Zip (PF02183) domains, and this step was crucial to identify the exact number of candidate HD-Zip proteins. Information regarding the number of amino acids and exons, clone number and ORF length of Zmhdz genes was obtained from the B73 maize sequence database. The molecular weight (kDa) and isoelectric point (PI) of each gene were calculated by ExPASy (http://www.expasy.org/tools/). All of the candidate HD-Zip sequences were aligned using ClustalW [70] and checked manually to exclude potentially redundant genes, and all of the non-redundant HD-Zip genes were used for further analysis. Analyses of phylogenetic relationships were conducted using MEGA 4.0 [71] with the neighbor-joining (NJ) method. A phylogenetic tree was initially constructed using the complete HD-Zip protein sequences of maize, rice and Arabidopsis HD-Zip genes with default parameters [8]–[11], [33]. Bootstrap analysis was performed using 1,000 replicates with the pairwise deletion option. Classification of the Zmhdz genes was then performed according to their phylogenetic relationships with their corresponding Arabidopsis and rice HD-Zip genes. The unrooted trees were generated using the same method.
The conserved motifs encoded by each Zmhdz gene were also investigated. Protein sequences were subjected to online MEME (Multiple Expectation Maximization for Motif Elicitation) (http://meme.sdsc.edu/meme4_3_0/intro.html) [72]. Parameters were set as follows: optimum motif width set to ≥6 and ≤200; maximum number of motifs set to 20. To predict the exon-intron structure of the Zmhdz genes, comparison of the genomic sequences and their predicted coding sequences (CDS) was performed using GSDS (http://gsds.cbi.pku.edu.cn/) [73]. The chromosome location image of Zmhdz genes was generated by MapInspect (http://www.plantbreeding.wur.nl/ uk/software_mapinspect.html) according to their starting positions in the maize chromosomes. For detection of tandem and segmental duplications, we defined two genes locating in the same clade of the phylogenetic tree as co-paralogs in the same species. Genes were designated as segmental duplication provided that they are co-paralogs and located on duplicated chromosomal blocks as proposed by Wei et al. (2007) [50], [74], [75]. Paralogs were regarded as tandem duplicated genes if two genes were separated by five or fewer genes [76].
Once the starting positions of Zmhdz genes were determined on the maize genome, the 2,000 bp genomic sequences upstream of the transcription start site (ATG) were acquired from maize B73 genomic database. PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html), an online database of plant cis-acting regulatory DNA elements (cis-elements) [77], was employed to investigate putative cis-elements in the promoter regions of the Zmhdz genes.
Plant materials and drought stress treatment
In this study, three-week-old seedlings (five-leaf stage) of maize inbred line B73 were used to examine the expression patterns of HD-Zip I genes under drought stress. Plants were grown in a greenhouse at 28±2°C with a 14-h light/10-h dark photoperiod. For drought stress, three-week-old plants were treated by putting intact plants in air at 28±2°C with water-limiting conditions, and plant leaves of the seedlings were harvested at 0, 3, 6 and 12 h after drought treatment, which represented normal plants, slight stress, moderate stress and severe stress, respectively. Three replicates were conducted for each sample.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNAs were isolated from the collected samples using Trizol reagent (Invitrogen) and treated with DNase I (Invitrogen) for 20 min to remove possible contaminating genomic DNA. Total RNA was assessed on a 1.2% agarose gel and quantified by NanoDrop ND-1000 spectrophotometer. For qRT-PCR analysis, first-strand cDNAs were synthesized from 1 µg of total RNA using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed on an ABI 7300 Real-Time system (Applied Biosystems). Each reaction was done in a final volume of 25 µl containing 12.5 µl of SYBR Green Master Mix reagent (Applied Biosystems), 2.0 µl of cDNA sample, and 400 nM gene-specific primers. The specific primers of each Zmhdz I gene (Table S2) were designed to generate a size of 90–150 bp products using Primer Express 3.0 software (Applied Biosystems). The thermal cycle conditions were as follows: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. At the end of the 40 cycles, a melting curve was generated to analyze the specificity of the reactions. Each cDNA sample was tested in three replicates. Expression level of the maize Actin 1 gene was used as the endogenous control. The relative expression level was calculated as 2–ΔΔCT [ΔC T = C T, gene of interest − C T, Actin 1. ΔΔC T = ΔC T, drought treatment − ΔC T, CK (0h)]. The relative expression level (2–ΔΔCT, CK (0h)) in the normal plants without drought treatment was normalized to 1 as described previously [78]. Statistical analyses were conducted using the SDS software 1.3.1 (Applied Biosystems).
Supporting Information
MP phylogeny of maize, rice and Arabidopsis HD-Zip proteins. The MP tree was generated with MEGA5.0 program using the full-length amino acid sequences of the maize, Arabidopsis and rice HD-Zip proteins. The bootstrap analysis was performed using 1,000 replicates with the pairwise deletion option. The tree was divided into four classes, and was largely consistent with the results of the NJ tree in figure 2.
(TIF)
ML phylogeny of maize, rice and Arabidopsis HD-Zip proteins. The ML tree was generated with MEGA5.0 program using the full-length amino acid sequences of the maize, Arabidopsis and rice HD-Zip proteins. The bootstrap analysis was performed using 1,000 replicates with the pairwise deletion option. The tree was classified into four classes, and was largely consistent with the results of the NJ tree in figure 2.
(TIF)
Multiple sequence alignment of 55 maize HD-Zip proteins. The complete amino acid sequences of the 55 Zmhdz proteins were aligned using ClustalW and revised manually. The HD, Zip, START and MEKHLA domains are indicated by white box, grey box, straight line and dashed line, respectively. Due to the diverse structures of class III and IV proteins, the conserved domains were cycled by black rectangles.
(TIF)
Analysis of the expression patterns of known drought-responsive and housekeeping genes by qRT-PCR. (A) Expression levels of two drought-responsive genes in the maize B73 seedlings at the five-leaf stage were analyzed under drought stress. (B) Expression levels of the Actin 1 gene and one putative housekeeping gene were compared under the same drought treatment.
(TIF)
Sequence characteristics of 55 HD-Zip genes identified in maize.
(DOC)
Specific primers used for qRT-PCR in this study. Primer sequences used for qRT-PCR to validate the expression patterns of Zmhdz I genes under drought stress.
(XLS)
Amino acid sequences of maize, rice and Arabidopsis HD-Zip proteins used for phylogenetic analysis.
(TXT)
Acknowledgments
We are grateful to the members of the Key Laboratory of Crop Biology of Anhui Province for their assistance in this study. We thank Dr. Guomin Han for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The research work was supported by grants from the National Key Technologies R & D Program of China during the Eleventh Five-Year Plan Period (2009BADA6B) (http://finance.most.gov.cn) and Research Fund for the Doctoral Program of Higher Education of China (20103418120001) (http://finance.most.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanes SD, Brent R. DNA specificity of the bicoid activator protein is determined by homeodomain recognition helix residue 9. Cell. 1989;57:1275–1283. doi: 10.1016/0092-8674(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 2.Ariel FD, Manavella PA, Dezar CA, Chan RL. The true story of the HD-Zip family. Trends Plant Sci. 2007;12:419–426. doi: 10.1016/j.tplants.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Schena M, Davis RW. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci U S A. 1992;89:3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350:241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- 5.Ruberti I, Sessa G, Lucchetti S, Morelli G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991;10:1787–1791. doi: 10.1002/j.1460-2075.1991.tb07703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayda E, Tornero P, Conejero V, Vera P. A tomato homeobox gene (HD-zip) is involved in limiting the spread of programmed cell death. Plant J. 1999;20:591–600. doi: 10.1046/j.1365-313x.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee K, Brocchieri L, Burglin TR. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 2009;26:2775–2794. doi: 10.1093/molbev/msp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, et al. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005;139:509–518. doi: 10.1104/pp.105.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, et al. The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Mol Biol. 2008;68:465–478. doi: 10.1007/s11103-008-9383-8. [DOI] [PubMed] [Google Scholar]
- 10.Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, et al. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura M, Katsumata H, Abe M, Yabe N, Komeda Y, et al. Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis. Plant Physiol. 2006;141:1363–1375. doi: 10.1104/pp.106.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee K, Brocchieri L, Burglin TR. A Comprehensive Classification and Evolutionary Analysis of Plant Homeobox Genes. Mol Biol Evol. 2009;26:2775–2794. doi: 10.1093/molbev/msp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijer AH, Scarpella E, van Dijk EL, Qin L, Taal AJ, et al. Transcriptional repression by Oshox1, a novel homeodomain leucine zipper protein from rice. Plant J. 1997;11:263–276. doi: 10.1046/j.1365-313x.1997.11020263.x. [DOI] [PubMed] [Google Scholar]
- 14.Frank W, Phillips J, Salamini F, Bartels D. Two dehydration-inducible transcripts from the resurrection plant Craterostigma plantagineum encode interacting homeodomain-leucine zipper proteins. Plant J. 1998;15:413–421. doi: 10.1046/j.1365-313x.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 15.Sessa G, Morelli G, Ruberti I. The Athb-1 and -2 HD-Zip domains homodimerize forming complexes of different DNA binding specificities. EMBO J. 1993;12:3507–3517. doi: 10.1002/j.1460-2075.1993.tb06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan RL, Gago GM, Palena CM, Gonzalez DH. Homeoboxes in plant development. Biochim Biophys Acta. 1998;1442:1–19. doi: 10.1016/s0167-4781(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 17.Tron AE, Bertoncini CW, Chan RL, Gonzalez DH. Redox regulation of plant homeodomain transcription factors. J Biol Chem. 2002;277:34800–34807. doi: 10.1074/jbc.M203297200. [DOI] [PubMed] [Google Scholar]
- 18.Schrick K, Nguyen D, Karlowski WM, Mayer KF. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004;5:R41. doi: 10.1186/gb-2004-5-6-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depege-Fargeix N, Javelle M, Chambrier P, Frangne N, Gerentes D, et al. Functional characterization of the HD-ZIP IV transcription factor OCL1 from maize. J Exp Bot. 2011;62:293–305. doi: 10.1093/jxb/erq267. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee K, Burglin TR. MEKHLA, a novel domain with similarity to PAS domains, is fused to plant homeodomain-leucine zipper III proteins. Plant Physiol. 2006;140:1142–1150. doi: 10.1104/pp.105.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnani E, Barton MK. A per-ARNT-sim-like sensor domain uniquely regulates the activity of the homeodomain leucine zipper transcription factor REVOLUTA in Arabidopsis. Plant Cell. 2011;23:567–582. doi: 10.1105/tpc.110.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderman E, Mattsson J, Engstrom P. The Arabidopsis homeobox gene ATHB-7 is induced by water deficit and by abscisic acid. Plant J. 1996;10:375–381. doi: 10.1046/j.1365-313x.1996.10020375.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin Z, Hong Y, Yin M, Li C, Zhang K, et al. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008;55:301–310. doi: 10.1111/j.1365-313X.2008.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni Y, Wang X, Li D, Wu Y, Xu W, et al. Novel cotton homeobox gene and its expression profiling in root development and in response to stresses and phytohormones. Acta Biochim Biophys Sin (Shanghai) 2008;40:78–84. doi: 10.1111/j.1745-7270.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 25.Vernoud V, Laigle G, Rozier F, Meeley RB, Perez P, et al. The HD-ZIP IV transcription factor OCL4 is necessary for trichome patterning and anther development in maize. Plant J. 2009;59:883–894. doi: 10.1111/j.1365-313X.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 26.Sakakibara K, Nishiyama T, Kato M, Hasebe M. Isolation of homeodomain-leucine zipper genes from the moss Physcomitrella patens and the evolution of homeodomain-leucine zipper genes in land plants. Mol Biol Evol. 2001;18:491–502. doi: 10.1093/oxfordjournals.molbev.a003828. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Henriksson E, Soderman E, Henriksson KN, Sundberg E, et al. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev Biol. 2003;264:228–239. doi: 10.1016/j.ydbio.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Olsson AS, Engstrom P, Soderman E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol. 2004;55:663–677. doi: 10.1007/s11103-004-1581-4. [DOI] [PubMed] [Google Scholar]
- 29.Rueda EC, Dezar CA, Gonzalez DH, Chan RL. Hahb-10, a sunflower homeobox-leucine zipper gene, is regulated by light quality and quantity, and promotes early flowering when expressed in Arabidopsis. Plant Cell Physiol. 2005;46:1954–1963. doi: 10.1093/pcp/pci210. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Oh HS, Cheon CI, Hwang IT, Kim YJ, et al. Structure and expression of the Arabidopsis thaliana homeobox gene Athb-12. Biochem Biophys Res Commun. 2001;284:133–141. doi: 10.1006/bbrc.2001.4904. [DOI] [PubMed] [Google Scholar]
- 31.Soderman E, Hjellstrom M, Fahleson J, Engstrom P. The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol Biol. 1999;40:1073–1083. doi: 10.1023/a:1006267013170. [DOI] [PubMed] [Google Scholar]
- 32.Johannesson H, Wang Y, Hanson J, Engstrom P. The Arabidopsis thaliana homeobox gene ATHB5 is a potential regulator of abscisic acid responsiveness in developing seedlings. Plant Mol Biol. 2003;51:719–729. doi: 10.1023/a:1022567625228. [DOI] [PubMed] [Google Scholar]
- 33.Agalou A, Purwantomo S, Overnas E, Johannesson H, Zhu X, et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol Biol. 2008;66:87–103. doi: 10.1007/s11103-007-9255-7. [DOI] [PubMed] [Google Scholar]
- 34.Harris JC, Hrmova M, Lopato S, Langridge P. New Phytol. In press; 2011. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. [DOI] [PubMed] [Google Scholar]
- 35.Sessa G, Carabelli M, Sassi M, Ciolfi A, Possenti M, et al. A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 2005;19:2811–2815. doi: 10.1101/gad.364005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morelli G, Ruberti I. Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci. 2002;7:399–404. doi: 10.1016/s1360-1385(02)02314-2. [DOI] [PubMed] [Google Scholar]
- 37.Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, et al. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- 38.Qin YF, Li DD, Wu YJ, Liu ZH, Zhang J, et al. Three cotton homeobox genes are preferentially expressed during early seedling development and in response to phytohormone signaling. Plant Cell Rep. 2010;29:1147–1156. doi: 10.1007/s00299-010-0901-1. [DOI] [PubMed] [Google Scholar]
- 39.Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, et al. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001;126:643–655. doi: 10.1104/pp.126.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan XY, Li QJ, Shan CM, Wang S, Mao YB, et al. The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2. Physiol Plant. 2008;134:174–182. doi: 10.1111/j.1399-3054.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 41.Ito M, Sentoku N, Nishimura A, Hong SK, Sato Y, et al. Position dependent expression of GL2-type homeobox gene, Roc1: significance for protoderm differentiation and radial pattern formation in early rice embryogenesis. Plant J. 2002;29:497–507. doi: 10.1046/j.1365-313x.2002.01234.x. [DOI] [PubMed] [Google Scholar]
- 42.Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, et al. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 43.Cote CL, Boileau F, Roy V, Ouellet M, Levasseur C, et al. Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010;10:273. doi: 10.1186/1471-2229-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain M, Tyagi AK, Khurana JP. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS Journal. 2008;275:2845–2861. doi: 10.1111/j.1742-4658.2008.06424.x. [DOI] [PubMed] [Google Scholar]
- 45.Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature. 2004;428:84–88. doi: 10.1038/nature02363. [DOI] [PubMed] [Google Scholar]
- 46.Ingram GC, Magnard JL, Vergne P, Dumas C, Rogowsky PM. ZmOCL1, an HDGL2 family homeobox gene, is expressed in the outer cell layer throughout maize development. Plant Mol Biol. 1999;40:343–354. doi: 10.1023/a:1006271332400. [DOI] [PubMed] [Google Scholar]
- 47.Ingram GC, Boisnard-Lorig C, Dumas C, Rogowsky PM. Expression patterns of genes encoding HD-ZipIV homeo domain proteins define specific domains in maize embryos and meristems. Plant J. 2000;22:401–414. doi: 10.1046/j.1365-313x.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- 48.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehan MR, Freimer NB, Ophoff RA. A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. Hum Genomics. 2004;1:335–344. doi: 10.1186/1479-7364-1-5-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei F, Coe E, Nelson W, Bharti AK, Engler F, et al. Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet. 2007;3:e123. doi: 10.1371/journal.pgen.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, et al. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313x.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10:88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008;146:1333–1345. doi: 10.1104/pp.107.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, et al. Genome-wide atlas of transcription during maize development. Plant J. 2011;66:553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- 55.Moore RC, Purugganan MD. The early stages of duplicate gene evolution. Proc Natl Acad Sci U S A. 2003;100:15682–15687. doi: 10.1073/pnas.2535513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goff SA, Ricke D, Lan TH, Presting G, Wang R, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science. 2002;296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- 57.Yu J, Hu S, Wang J, Wong GK, Li S, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science. 2002;296:79–92. doi: 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
- 58.Raes J, Vandepoele K, Simillion C, Saeys Y, Van de Peer Y. Investigating ancient duplication events in the Arabidopsis genome. J Struct Funct Genomics. 2003;3:117–129. [PubMed] [Google Scholar]
- 59.Paterson AH, Bowers JE, Chapman BA. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci U S A. 2004;101:9903–9908. doi: 10.1073/pnas.0307901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swigonova Z, Lai J, Ma J, Ramakrishna W, Llaca V, et al. Close split of sorghum and maize genome progenitors. Genome Res. 2004;14:1916–1923. doi: 10.1101/gr.2332504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang C, Gu X, Peterson T. Identification of conserved gene structures and carboxy-terminal motifs in the Myb gene family of Arabidopsis and Oryza sativa L. ssp. indica. Genome Biol. 2004;5:R46. doi: 10.1186/gb-2004-5-7-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salse J, Bolot S, Throude M, Jouffe V, Piegu B, et al. Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell. 2008;20:11–24. doi: 10.1105/tpc.107.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murat F, Xu JH, Tannier E, Abrouk M, Guilhot N, et al. Ancestral grass karyotype reconstruction unravels new mechanisms of genome shuffling as a source of plant evolution. Genome Res. 2010;20:1545–1557. doi: 10.1101/gr.109744.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maere S, De Bodt S, Raes J, Casneuf T, Van Montagu M, et al. Modeling gene and genome duplications in eukaryotes. Proc Natl Acad Sci U S A. 2005;102:5454–5459. doi: 10.1073/pnas.0501102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanc G, Hokamp K, Wolfe KH. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res. 2003;13:137–144. doi: 10.1101/gr.751803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dezar CA, Gago GM, Gonzalez DH, Chan RL. Hahb-4, a sunflower homeobox-leucine zipper gene, is a developmental regulator and confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res. 2005;14:429–440. doi: 10.1007/s11248-005-5076-0. [DOI] [PubMed] [Google Scholar]
- 67.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 68.Finn RD, Mistry J, Schuster-Bockler B, Griffiths-Jones S, Hollich V, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letunic I, Doerks T, Bork P. SMART 6: recent updates and new developments. Nucleic Acids Res. 2009;37:D229–232. doi: 10.1093/nar/gkn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 72.Bailey TL, Elkan C. The value of prior knowledge in discovering motifs with MEME. Proc Int Conf Intell Syst Mol Biol. 1995;3:21–29. [PubMed] [Google Scholar]
- 73.Guo AY, Zhu QH, Chen X, Luo JC. [GSDS: a gene structure display server]. Yi Chuan. 2007;29:1023–1026. [PubMed] [Google Scholar]
- 74.Zhang X, Feng Y, Cheng H, Tian D, Yang S, et al. Relative evolutionary rates of NBS-encoding genes revealed by soybean segmental duplication. Mol Genet Genomics. 2011;285:79–90. doi: 10.1007/s00438-010-0587-7. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Deng D, Bian Y, Lv Y, Xie Q. Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays. L.). Mol Biol Rep. 2010;37:3991–4001. doi: 10.1007/s11033-010-0058-6. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Guo K, Li Y, Tu Y, Hu H, et al. Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC Plant Biol. 2010;10:282. doi: 10.1186/1471-2229-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MP phylogeny of maize, rice and Arabidopsis HD-Zip proteins. The MP tree was generated with MEGA5.0 program using the full-length amino acid sequences of the maize, Arabidopsis and rice HD-Zip proteins. The bootstrap analysis was performed using 1,000 replicates with the pairwise deletion option. The tree was divided into four classes, and was largely consistent with the results of the NJ tree in figure 2.
(TIF)
ML phylogeny of maize, rice and Arabidopsis HD-Zip proteins. The ML tree was generated with MEGA5.0 program using the full-length amino acid sequences of the maize, Arabidopsis and rice HD-Zip proteins. The bootstrap analysis was performed using 1,000 replicates with the pairwise deletion option. The tree was classified into four classes, and was largely consistent with the results of the NJ tree in figure 2.
(TIF)
Multiple sequence alignment of 55 maize HD-Zip proteins. The complete amino acid sequences of the 55 Zmhdz proteins were aligned using ClustalW and revised manually. The HD, Zip, START and MEKHLA domains are indicated by white box, grey box, straight line and dashed line, respectively. Due to the diverse structures of class III and IV proteins, the conserved domains were cycled by black rectangles.
(TIF)
Analysis of the expression patterns of known drought-responsive and housekeeping genes by qRT-PCR. (A) Expression levels of two drought-responsive genes in the maize B73 seedlings at the five-leaf stage were analyzed under drought stress. (B) Expression levels of the Actin 1 gene and one putative housekeeping gene were compared under the same drought treatment.
(TIF)
Sequence characteristics of 55 HD-Zip genes identified in maize.
(DOC)
Specific primers used for qRT-PCR in this study. Primer sequences used for qRT-PCR to validate the expression patterns of Zmhdz I genes under drought stress.
(XLS)
Amino acid sequences of maize, rice and Arabidopsis HD-Zip proteins used for phylogenetic analysis.
(TXT)