Abstract
AIM: To examine the activation of the Nalp3 inflammasome and its downstream targets following lipopolysaccharide (LPS)-induced stimulation in the liver.
METHODS: Six-to-eight-week-old C57BL/6 chow fed mice were injected intraperitoneally with 0.5 μg/g bodyweight LPS and sacrificed 2, 4, 6, 18 or 24 h later. LPS-induced liver damage was confirmed by a biochemical assay to detect alanine aminotransferase (ALT) levels. To determine if LPS stimulation in the liver led to activation of the inflammasome, real-time quantitative polymerase chain reaction was used to evaluate the mRNA expression of components of the Nalp3 inflammasome. Enzyme-linked immunosorbent assays were used to determine the protein expression levels of several downstream targets of the Nalp3 inflammasome, including caspase-1 and two cytokine targets of caspase-1, interleukin (IL)-1β and IL-18.
RESULTS: We found that LPS injection resulted in liver damage as indicated by elevated ALT levels. This was associated with a significant increase in both mRNA and protein levels of the proinflammatory cytokine tumor necrosis factor (TNF)-α in the liver, as well as increased levels of TNFs in serum. We showed that LPS stimulation led to upregulation of mRNA levels in the liver for all the receptor components of the inflammasome, including Nalp3, Nalp1, pannexin-1 and the adaptor molecule apoptosis-associated speck-like, caspase recruitment domain-domain containing protein. We also found increased levels of mRNA and protein for caspase-1, a downstream target of the inflammasome. In addition, LPS challenge led to increased levels of both mRNA and protein in the liver for two cytokine targets of caspase-1, IL-1β and IL-18. Interestingly, substantial baseline expression of pre-IL-1β and pre-IL-18 was found in the liver. Inflammasome and caspase-1 activation was indicated by the significant increase in the active forms of IL-1β and IL-18 after LPS stimulation.
CONCLUSION: Our results show that the Nalp3 inflammasome is upregulated and activated in the liver in response to LPS stimulation.
Keywords: Endotoxin, Nod-like receptor, Interleukin-1β, Interleukin-18, Caspase-1
INTRODUCTION
The endotoxin lipopolysaccharide (LPS), a component of Gram-negative bacteria, plays an important role in acute liver injury as well as chronic liver diseases including fatty liver associated with either alcohol consumption or metabolic syndrome and obesity[1,2]. LPS has also been implicated in insulin resistance as well as in steatohepatitis in non-alcoholic fatty liver disease[3-5]. Increasing evidence suggests that gut-derived LPS through the gut-liver axis affects the extent of liver damage in many different types of inflammatory liver diseases[6]. LPS is a prototypical ligand for the pattern recognition receptor (PRR), Toll-like receptor 4 (TLR4). TLR4 induces downstream signaling via the MyD88 adapter molecule and induces production of proinflammatory cytokines through activation of the regulatory factor nuclear factor (NF)-κB[6]. In the liver, TLR4 is expressed in both parenchymal and immune cells, thereby providing potential for LPS-induced activation[7].
Studies have shown that the Nalp3 inflammasome is activated by both pattern associated molecular patterns (PAMPs), including LPS and bacterial RNA, and danger-associated molecular patterns (DAMPs)[8,9]. The Nalp3 inflammasome is a caspase-1 activating multiprotein complex, which has been implicated in numerous inflammatory processes and human diseases including gout, pseudogout, contact hypersensitivity, and most recently Alzheimer’s disease[10,11]. Furthermore, mutations in the Nalp3 gene that lead to gain-of-function mutations, result in several hereditary syndromes including: Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and chronic infantile neurological cutaneous and articular syndrome[12].
In response to stimulation by either DAMPs or PAMPs, Nalp3 interacts with pro-caspase-1 through the adaptor molecule [apoptosis-associated speck-like, caspase recruitment domain (CARD)-domain containing protein (ASC)] to form the inflammasome, which leads to activation of caspase-1. Active caspase-1, previously known as interleukin (IL)-1β converting enzyme, a heterodimer of p20 and p10 subunits, is the central effector protein of the inflammasome complex and promotes the cleavage of pro-IL-1β, pro-IL-18 and pro-IL-33 to their biologically active, mature forms[13]. In addition, active caspase-1 has been shown to cleave other substances, such as caspase-7 and sterol regulatory element-binding proteins, therefore playing a pivotal role in cell death and survival[14].
The mRNA levels of the components the Nalp3 inflammasome are expressed in the eye, heart and lung in response to LPS stimulation[15]. Although mRNA levels of IL-1β have also been shown to increase in the liver in response to LPS stimulation, the role of the Nalp3 inflammasome in the liver in response to LPS stimulation has not been determined[16]. In the liver, the Nalp3 inflammasome is upregulated upon acetaminophen-induced toxicity[17] and the contribution of Nalp3 has been described in Propionibacterium acnes and LPS-induced acute liver injury[18]; however, a role for Nalp3 in other liver conditions has not been identified.
Although the role of the proinflammatory cytokine, tumor necrosis factor (TNF)α, has been extensively studied in both alcoholic and non-alcoholic fatty liver disease, the role of IL-1β and the inflammasome has yet to be explored. LPS plays an important role in liver disease; elevated LPS levels are detected in the portal and systemic blood of patients with alcoholic and non-alcoholic fatty liver disease[19,20]. Furthermore, there is increased sensitivity to LPS in both alcoholic liver disease and fatty liver disease[21]. Here, we examined if LPS induce the Nalp3 inflammasome in the liver. In this study, we showed that LPS stimulation led to induction of the components of the Nalp3 inflammasome at both the mRNA and protein level in the liver and this is associated with increased IL-1β production.
MATERIALS AND METHODS
Mice
Six-to-eight-week-old wild-type C57BL/6 chow fed mice received intraperitoneal injections of 0.5 μg/g LPS in PBS (Sigma, St Louis, MO, United States) (three mice per group) for 2, 4, 6, 18 and 24 h. Serum was separated from whole blood and stored at -80 °C. Liver samples were snap frozen in liquid nitrogen or stored in RNAlater (Qiagen Sciences, Germantown, MD, United States) for RNA extraction. All animals received proper care in agreement with animal protocols at the University of Massachusetts Medical School Institutional Animal Use and Care Committee.
Biochemical alanine aminotransferase assay
Serum alanine aminotransferase (ALT) was determined using a kinetic method (DTEK LLC, Bensalem, PA, United States).
RNA analysis
RNA was purified from livers using the RNeasy kit (Qiagen Sciences) and on-column DNA digestion. cDNA was transcribed with the Reverse Transcription System (Promega, Madison, WI, United States). Real-time quantitative polymerase chain reaction (qPCR) was performed using iCycler (Bio-Rad Laboratories, Hercules, CA, United States) as described previously[22]. Primer sequences are shown in Table 1.
Table 1.
Real-time polymerase chain reaction primers
| Target gene | Forward primer (5' → 3') | Reverse primer (5' → 3') |
| 18S | GTA ACC CGT TGA ACC CCA TT | CCA TCC AAT CGG TAG TAG CG |
| TNF-α | GAA GTT CCC AAA TGG CCT CC | GTG AGG GTC TGG GCC ATA GA |
| IL-1β | TCT TTG AAG TTG ACG GAC CC | TGA GTG ATA CTG CCT GCC TG |
| IL-1RA | TCA GAT CTG CAC TCA ATG CC | CTG GTG TTT GAC CTG GGA GT |
| Caspase-1 | AGA TGG CAC ATT TCC AGG AC | GAT CCT CCA GCA ACT TC |
| ASC | GA GCT GCT GAC AGT GCA AC | GCC ACA GCT CCA GAC TCT TC |
| Nalp3 | AGC CTT CCA GGA TCC TCT TC | CTT GGG CAG TTT CTT TC |
| Nlrc4 | TGG TGA CAA TAG GGC TCC TC | CTG TTC CCT TTG CTC ACC TC |
| Nalp1 | TGG CAC ATC CTA GGG AAA TC | TCC TCA CGT GAC AGC AGA AC |
| Pannexin-1 | TGT GGC TGC ACA AGT TCT TC | ACA GAC TCT GCC CCA CAT TC |
TNF: Tumor necrosis factor; IL: Interleukin; ASC: Apoptosis-associated speck-like, caspase recruitment domain-domain containing protein.
Cytokine enzyme-linked immunosorbent assay measurements
Whole cell lysates were extracted from liver tissue as previously described[22]. These lysates were assayed for mature IL-1β (R and D Systems, Minneapolis, MN, United States), TNF-α, total IL-18, and total IL-1β (BD Biosciences, United States). Serum was also assayed for mature IL-1β (R and D Systems).
Statistical analysis
Statistical significance was determined using Student’s t test (two-tailed distribution). Data are presented as mean ± SE and were considered significant at P < 0.05. ALT statistical significance was determined using the nonparametric Kruskal-Wallis test followed by the Mann-Whitney test.
RESULTS
LPS induces liver injury and inflammatory cytokines
Gut-derived LPS plays a role in several inflammatory liver diseases[6]. To evaluate the effects of LPS on the liver, we injected wild-type mice with LPS for 2, 4, 6, 18 or 24 h and monitored ALT levels to evaluate liver injury. We found increased ALT levels in sera from mice injected with LPS at the 4-18-h time points, reaching statistically significant levels at 4 h, indicating LPS-induced liver damage (Figure 1).
Figure 1.
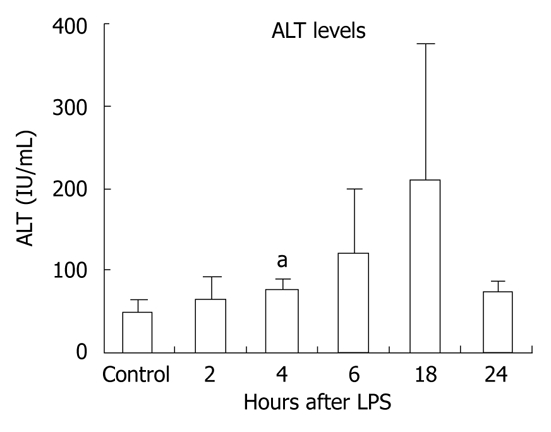
Lipopolysaccharide stimulation results in liver damage. C57BL/6 wild-type chow-fed mice (three per group) were injected intraperitoneally with lipopolysaccharide (LPS) for 2, 4, 6, 18 or 24 h. Serum was separated from whole blood and analyzed for alanine aminotransferase (ALT), which was significantly increased after 4 h of LPS stimulation. Mean ± SD are shown. aP < 0.01.
It has been shown that LPS-induced signaling through TLR4 results in production of proinflammatory cytokines, one of which, TNF-α, has been linked to liver damage[23,24]. We identified significant elevation in liver TNF-α mRNA expression at all time points after LPS stimulation compared to the unstimulated controls (Figure 2A). TNF-α protein levels were undetectable in liver tissue from unstimulated controls but were significantly increased at all time points after LPS stimulation with a maximal increase at 2 h (Figure 2B).
Figure 2.
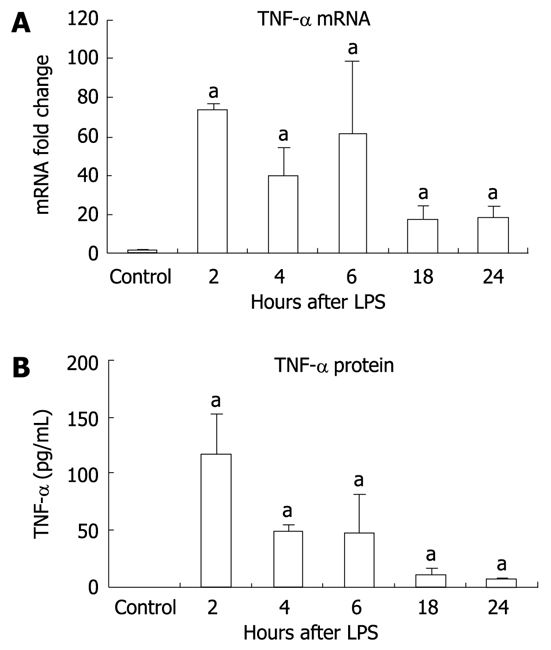
Inflammation in the liver is seen after lipopolysaccharide stimulation. A: Tumor necrosis factor (TNF)-α mRNA in liver tissue was significantly increased at all time points after lipopolysaccharide (LPS) stimulation. TNF-α mRNA was analyzed by real-time quantitative polymerase chain reaction and normalized to 18S. The values are shown as a fold change to the non-stimulated LPS control; B: Similarly, TNF-α protein was significantly elevated in liver tissue following LPS stimulation as detected by enzyme-linked immunosorbent assay. Protein levels were normalized to total protein concentration in each tissue sample. Mean ± SD are shown. n = 3 for each group (except at 4 h LPS stimulation, where n = 2 due to an outlier), aP < 0.01.
LPS upregulates mRNA expression of Nalp3 inflammasome components
For complete function, the inflammasome requires expression of its various components including Nalp3, ASC (the adapter molecule), and caspase-1[8,10]. We investigated the mRNA levels of the different types of inflammasomes, including the Nalp1 and Nlrc4 inflammasomes. Our examination revealed a significant increase in liver mRNA of Nalp3 after ≥ 4 h LPS stimulation, with a peak at 6 h (Figure 3A). Upregulation of the inflammasome was not limited to Nalp3, and there was a significant increase in Nalp1 (sevenfold) and Nlrc4 (3.5-fold) mRNA at 24 h compared to the unstimulated controls (P < 0.01, P = 0.04, respectively, data not shown). We also analyzed the mRNA of other components of the inflammasome including the pyrin and CARD-domain-containing adaptor ASC; caspase-1, an inflammatory caspase; and pannexin-1, a hemi-channel that is recruited upon activation of the P2X7 receptor and is a necessary component for inflammasome activation[25]. The mRNA levels of ASC gradually increased at each time point after LPS stimulation (Figure 3B), whereas caspase-1 levels peaked at 6 h and pannexin-1 mRNA levels peaked at 6 h (Figure 3C and D).
Figure 3.
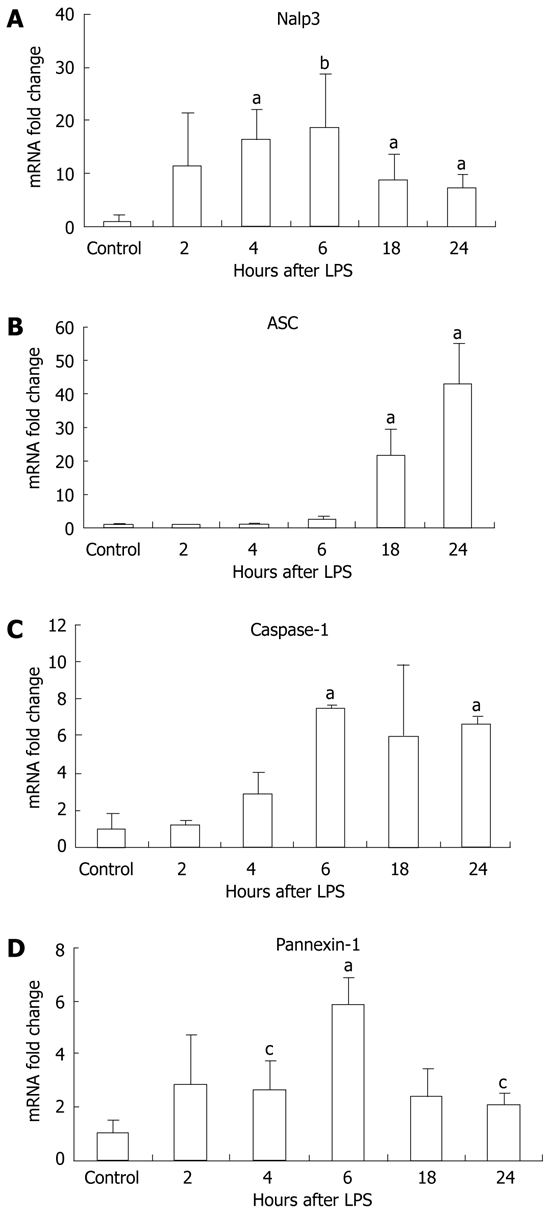
Lipopolysaccharide stimulation increased Nalp3 inflammasome mRNA expression in liver tissue. Liver RNA analysis of Nalp3 (A), apoptosis-associated speck-like, caspase recruitment domain-domain containing protein (ASC) (B), caspase-1 (C) and pannexin-1 (D) were analyzed by real-time quantitative polymerase chain reaction. The values were normalized to 18S and are shown as a fold increase to the non-lipopolysaccharide (LPS) stimulated control. Mean ± SD are shown. n = 3 per group, aP < 0.01, bP < 0.03, cP < 0.05.
Activation of the inflammasome results in increased levels of total IL-18, total IL-1β, and mature IL-1β
LPS stimulation has been shown to increase levels of pro-IL-1β transcripts[26]. However, production of the biologically active IL-1β and IL-18 requires the mature caspase-1 to cleave the precursor forms into their mature forms[27]. First, we evaluated mRNA levels of IL-1β and IL-18 and found that IL-1β mRNA levels were significantly increased at all time points post-LPS stimulation peaking at 2 h (Figure 4A). Induction of IL-18 mRNA by LPS was modest but reached statistical significance at the 6-h time point (Figure 4B). There was also induction of the mRNA for the IL-1 receptor antagonist after LPS stimulation in the liver (Figure 4C).
Figure 4.
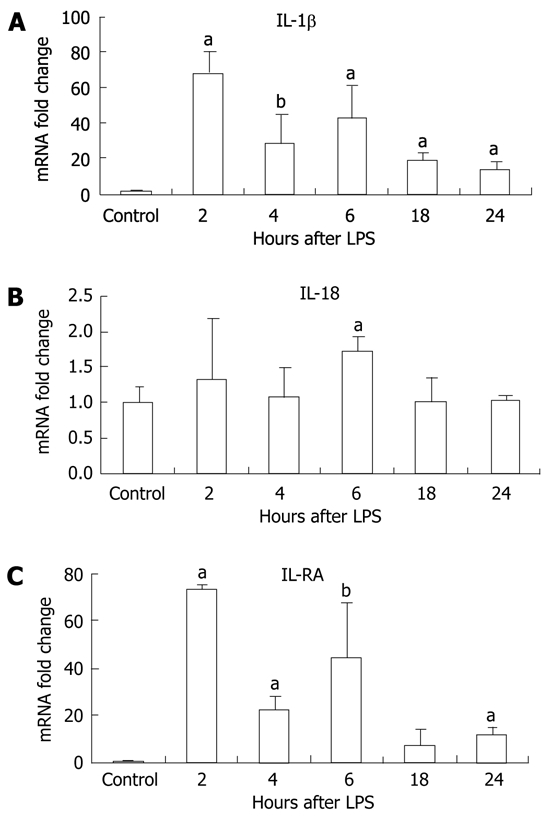
Lipopolysaccharide stimulation increased interleukin (IL)-1β and IL-18 mRNA, and IL-1RA transcription in the liver. Liver mRNA levels of IL-1β (A), IL-18 (B) and IL-1RA (C) were analyzed by real-time quantitative polymerase chain reaction and normalized to 18S. Mean ± SD are shown. n = 3 per group [except at 2 h lipopolysaccharide (LPS) stimulation, where n = 2 due to outlier], aP < 0.01, bP < 0.03.
We sought to determine whether the increase in mRNA correlated with increased protein production for IL-1β and IL-18. We found basal expression of pro-IL-1β protein in the livers of unstimulated control mice and this was significantly upregulated at 2 h after LPS stimulation (Figure 5A). More importantly, we observed a significant increase in mature IL-1β protein in liver tissue at all time points post-LPS stimulation compared to the controls, in which no mature IL-1β was detected (Figure 5B). Consistent with inflammasome activation, we found increased serum IL-1β levels in mice after stimulation with LPS for 2 and 6 h (Figure 5C). Investigation of IL-18, which is also cleaved by caspase-1, revealed a significant increase in total IL-18 in the liver at all time points following LPS injection (Figure 5D).
Figure 5.
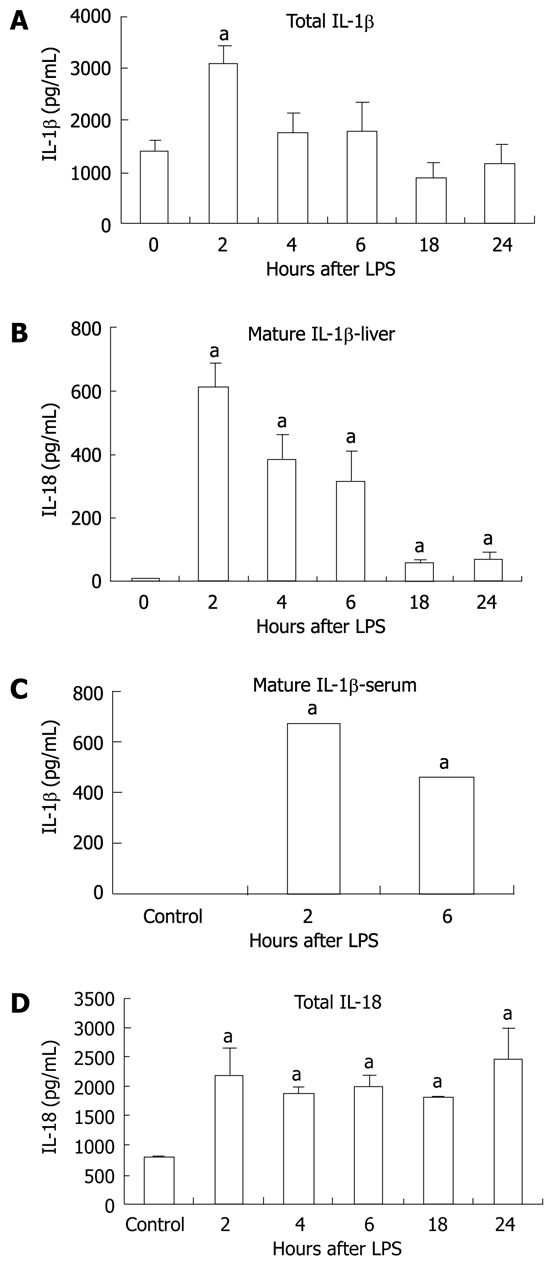
Lipopolysaccharide increased interleukin-1β and interleukin-18 protein. Protein level was detected in liver tissue by enzyme-linked immunosorbent assay for total-interleukin (IL)-1β (pro-IL-1β and cleaved IL-1β) (A), cleaved IL-1β in the liver tissue (B),cleaved IL-1β in the serum (C) and total IL-18 (pro-IL-18 and cleaved IL-18) (D) in liver tissue. The values shown are the fold change compared to non-Lipopolysaccharide (LPS) stimulated control. Protein levels were normalized to total protein concentrations in each tissue sample. Mean ± SD are shown. n = 3 per group (except at 2 h LPS stimulation, where n = 2 due to an outlier), aP < 0.01.
DISCUSSION
Although many molecules and compounds activate the Nalp3 inflammasome both in vitro and in vivo[8], the role of Nalp3 inflammasome in a healthy liver following LPS stimulation has not been determined. Our novel findings demonstrate upregulation of the Nalp3 inflammasome in the liver in response to LPS stimulation, both at the mRNA and protein levels. We also show for the first time that the components of the inflammasome pathway, including ASC and caspase-1, are upregulated in the liver after LPS challenge and this results in functional activation of the inflammasome and caspase-1, indicated by IL-1β and IL-18 secretion.
LPS is recognized by TLR4, which induces an intracellular signaling cascade leading to activation of NF-κB, as well as the production of pro-IL-1β and pro-IL-18[28]. Previous studies have suggested that signaling through the TLRs alone is insufficient to generate the production of mature IL-1β and IL-18, and that a second signal is needed that leads to ionic perturbation[29]. These perturbations may include ATP signaling through the P2X7 channel, leading to changes in intracellular potassium concentrations and membrane perturbations. The physiological importance of ATP in IL-1β secretion is unclear and the second signal that leads to cleavage of pro-IL-1β in the liver is still under investigation[30]. In vitro studies have shown that a second signal is needed in addition to LPS to obtain cleavage of pro-IL-1β[31]. It is possible that a DAMP released by injured cells could act as the second signal. In vivo studies, however, have shown that LPS alone is sufficient to increase cleavage of IL-1β and IL-18[31]. Our novel data demonstrate that, after in vivo LPS challenge, the inflammasome is activated in the liver and this results in increased inflammasome function and IL-1β secretion. We found that LPS stimulation not only increased IL-1β mRNA levels and the expression of pro-IL-1β protein, but it significantly increased the levels of the 18-kDa form of mature IL-1β in the liver. Secretion of mature IL-1β also resulted in elevated IL-1β in the serum. Interestingly, we found significant levels of pro-IL-1β protein expressed in the liver without exogenous stimulation. This suggests that the exogenous LPS probably provided the second signal for IL-1β production. The mechanisms for the presence of pro-IL-1β protein in the liver remain to be determined, however, one possibility is stimulation via PAMPs, such as LPS, which are being constantly supplied by the gut through the portal vein to the liver[6].
Cleavage of pro-IL-1β is mediated by activated caspase-1; consistent with this, we found increased levels of IL-1β protein in the serum after LPS challenge. The other target of the caspase-1 complex and inflammasome activation is IL-18. Consistent with LPS-induced inflammasome and caspase-1 activation, we found an increase in IL-18 mRNA and total IL-18 levels in the liver. Due to limitations of the detection assay, we could not distinguish between the mature form of IL-18 and pro-IL-18, but the increase in total IL-18 after LPS stimulation was most likely due to an increase in both.
We found elevated basal levels of pro-IL-1β in the liver, and pro-IL-1β was further increased by LPS with the highest levels at 2 h following stimulation. It is likely that LPS-induced inflammasome activation can cleave the pre-existing pro-IL-1β to the mature form by caspase-1. In addition to activation of the Nalp3 inflammasome, our data suggests that other types of caspase-1-activating inflammasomes, Nalp1 and Nlrc4, may also play a role in caspase-1 activation and subsequent IL-1β cleavage in the liver. Our data showed that both Nalp1 and Nlrc4 mRNA levels were upregulated in the liver following LPS stimulation. This suggests that other inflammasome-activating signals lead to full activation in the liver.
The contribution of the Nalp3 inflammasome to inflammation in the liver in response to LPS stimulation may have implications for several different liver diseases. Increased gut-derived LPS has been shown to contribute to liver inflammation in alcoholic and non-alcoholic fatty liver diseases, as well as other forms of liver damage such as hepatitis C virus infection[32]. In healthy subjects, the liver plays a central role in elimination of gut-derived endotoxins and other pathogens to maintain immune homeostasis[33]. Thus, it is tempting to speculate that gut-derived ligands of TLRs constantly entering the liver could contribute to the high basal expression of pro-IL-1β found in the liver in our experiments. The high basal expression of pro-IL-1β represents the first step of inflammasome activation, and this appears to provide a pre-activated state where even single LPS stimulation can result in inflammasome activation, as seen in the liver after in vivo LPS challenge in our experiments. The specific role of Nalp3 and the inflammasome remains to be evaluated in different types of liver diseases where inflammation is related to LPS and other pathogen-derived or endogenous danger signals.
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1 DK075635. The authors thank Jan Petrasek, Karen Kodys, Donna Catalano, Angela Dolganiuc, Shashi Bhala and Miguel Marcos-Martin for their support in the Szabo lab.
COMMENTS
Background
The endotoxin lipopolysaccharide (LPS) is a component of Gram-negative bacteria, and plays an important role in both acute liver injury as well as chronic liver diseases, including fatty liver associated with either alcohol consumption or metabolic syndrome and obesity. Several pathogen-associated molecular patterns, including LPS, induce inflammasome activation, an intracellular multiprotein complex leading to release of inflammatory cytokines and cell death.
Research frontiers
Inflammasomes are required for effective clearance of several viral and bacterial pathogens. In addition, inflammasomes have been reported to play a major role in several diseases with sterile inflammation such as gout, Alzheimer’s disease, atherosclerosis and type 2 diabetes. Here, the authors show the contribution of the Nalp3 inflammasome to LPS-induced inflammation in the liver.
Innovations and breakthroughs
Recently, the contribution of the Nalp3 inflammasome to Propionibacterium acnes plus LPS-induced acute liver injury has been described. Here, authors show the increased expression and role of the inflammasome in healthy liver following LPS stimulation.
Applications
The contribution of the Nalp3 inflammasome to inflammation in the liver in response to LPS stimulation may have implications for several liver diseases, including alcoholic and non-alcoholic fatty liver disease, as well as hepatitis C virus infection where the role of gut-derived endotoxin has been shown previously. A better understanding of the pathological mechanism may help future attempts to develop effective therapy.
Terminology
Nalp3, also called cryporin is a nucleotide oligomerization domain-like receptor that forms one of the most characterized inflammasome complexes.
Peer review
This is a well done study that examines the role of the inflammasome in the liver following LPS exposure.
Footnotes
Supported by NIH grant RO1 DK075635
Peer reviewer: Eddie Wisse, Professor, Cell Biology and Histology of the Faculty of Medicine and Pharmacy, Free University of Brussels, Laarbeeklaan 103, B 1090 Brussels-Jette, Belgium
S- Editor Yang XC L- Editor Kerr C E- Editor Li JY
References
- 1.Nath B, Szabo G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin Liver Dis. 2009;29:166–177. doi: 10.1055/s-0029-1214372. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–1483. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo G, Velayudham A, Romics L, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29:140S–145S. doi: 10.1097/01.alc.0000189287.83544.33. [DOI] [PubMed] [Google Scholar]
- 5.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F. Detection of immune danger signals by NALP3. J Leukoc Biol. 2008;83:507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 9.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- 11.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 14.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 15.González-Benítez JF, Juárez-Verdayes MA, Rodríguez-Martínez S, Cancino-Diaz ME, García-Vázquez F, Cancino-Diaz JC. The NALP3/Cryopyrin-inflammasome complex is expressed in LPS-induced ocular inflammation. Mediators Inflamm. 2008;2008:614345. doi: 10.1155/2008/614345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19:538–546. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- 17.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamura M, Tsutsui H, Yasuda K, Uchiyama R, Yumikura-Futatsugi S, Mitani K, Hayashi S, Akira S, Taniguchi S, Van Rooijen N, et al. Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol. 2009;51:333–341. doi: 10.1016/j.jhep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 20.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romics L, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, Szabo G. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. Hepatology. 2004;40:376–385. doi: 10.1002/hep.20304. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 28.Matsushima K, Taguchi M, Kovacs EJ, Young HA, Oppenheim JJ. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986;136:2883–2891. [PubMed] [Google Scholar]
- 29.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 30.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 31.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 32.Caradonna L, Mastronardi ML, Magrone T, Cozzolongo R, Cuppone R, Manghisi OG, Caccavo D, Pellegrino NM, Amoroso A, Jirillo E, et al. Biological and clinical significance of endotoxemia in the course of hepatitis C virus infection. Curr Pharm Des. 2002;8:995–1005. doi: 10.2174/1381612024606983. [DOI] [PubMed] [Google Scholar]
- 33.Iakovlev MIu. Elements of endotoxin theory in human physiology and pathology. Fiziol Cheloveka. 2003;29:98–109. [PubMed] [Google Scholar]


