Abstract
AIM: To evaluate the impacts of Schistosoma japonicum (S. japonicum) ova on the tight junction barriers in a trinitrobenzenesulfonic acid (TNBS)-induced colitis model.
METHODS: Balb/c mice were randomly divided into three groups: control group; TNBS+ova- group and TNBS+ova+ group. TNBS was used intracolonic to induce colitis and mice of the TNBS+ova+ group were pre-exposed to S. japonicum ova as a prophylactic intervention. Colon inflammation was quantified using following variables: mouse mortality, weight loss, colon extent and microscopic inflammation score. Serum expression of tumor necrosis factor-α and interferon-γ were assessed to evaluate the systemic inflammatory response. NOD2 and its mRNA were also tested. Bacterial translocations were tested by culturing blood and several tissues. ZO-1 and occludin were chosen as the representations of tight junction proteins. Both the proteins and mRNA were assessed.
RESULTS: Ova pre-treatment contributed to the relief of colitis and decreased the mortality of the models. NOD2 expression was significantly downregulated when pretreated with the ova. The TNBS injection caused a significant downregulation of ZO-1 and occludin mRNA together with their proteins in the colon; ova pre-exposure reversed these alterations. Treatment with S. japonicum ova in the colitis model caused lower intestinal bacterial translocation frequency.
CONCLUSION: S. japonicum ova can maintain epithelial barrier function through increasing tight junction proteins, thus causing less exposure of NOD2 to the luminal antigens which may activate a series of inflammatory factors and induce colitis.
Keywords: Crohn’s disease, Schistosoma japonicum ova, Tight junction protein, ZO-1, Occludin
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract. Although the etiology is multifactorial and remains incompletely understood, inflammation, immunity, genetic and environmental factors have been suggested to predispose to CD[1]. Despite the multiple mechanisms that have been investigated hitherto, proinflammatory cytokines play the most important role since they are the ultimate pathway that leads to the colitis. Therapies targeting these proinflammatory cytokines have been extensively investigated and shown to be effective both in animal experiments and clinical trials[2-3].
In addition, CD patients demonstrate increased intestinal bacterial translocation (IBT), which may be due to the increased intestinal pericellular permeability, reflecting the decreased epithelial barrier function[4]. The tight junctions (TJs), which form the pericellular barrier, are thought to be the primary determinant of mucosal permeability in the presence of an intact epithelium[5]. Impaired TJs lead to an increase of exposure of intestinal bacteria to submucosal pattern pathogen recognition receptors (PPRRs), such as toll-like receptors (TLRs) and nucleotide-binding-oligomerization domains (NODs). NOD2 has been found to exert antibacterial activity limiting survival of enteric bacteria after invasion[6-7]. As one of the main responsible genes, NOD2 and its protein have been found to be upregulated in CD patients[8].
It is well established that helminthes can protect mice from experimental colitis[9-11]. We have also demonstrated in our former study that freeze-killed Schistosoma japonicum (S. japonicum) ova could relieve the colon inflammation and prevent IBT in a trinitrobenzenesulfonic acid (TNBS)-induced model by upregulating Th2-type cytokine and downregulating TLR4 mRNA expression[12]. In the present study, we further investigate the influence of S. japonicum ova on TNBS-induced colitis in mice and whether S. japonicum ova prevent IBT by upregulating tight junction proteins. Since TLR4, one of the main extracellular receptors of enteric antigens, has been found to be upregulated in TNBS-induced colitis which was blocked by the pretreatment of S. japonicum ova[12], we further discuss here whether NOD2, one of the main intracellular receptors, has the same ability.
MATERIALS AND METHODS
Animals
Ninety Balb/c mice (Animal Center of Shanghai Laboratory, Chinese Academy of Science) were randomly divided into 3 groups. Twenty mice from the control group received an intra-colonic injection of 0.5 mL saline on day 15. Forty mice from the TNBS+ova- group received an intra-colonic injection of 0.5 mL TNBS (Sigma) on day 15. Thirty mice from the TNBS+ova+ group received intra-peritoneal injections of 10 000 freeze-killed S. japonicum ova (Zhejiang Academy of Medical Science) on day 1 and day 11, and were challenged with TNBS on day 15. The surviving mice were sacrificed on day 22. Serum expression of tumor necrosis factor (TNF)-α and interferon (IFN)-γ were detected. Ten animals from each group were randomly selected and the full-length colon from these animals was isolated and evaluated.
Another 30 mice were grouped according to the former methods and were sacrificed on day 16. Blood, liver, spleen and mesenteric lymph nodes (MLN) were cultured and the identification of bacteria was completed using VITEK-32 Auto Microbiotic System (bioMérieux).
Evaluations of the colonic inflammation
Mice were weighed daily and the numbers of surviving mice were recorded. The extents of colon were assessed in the sacrificed mice. The proximal 1.0 cm of the colonic segment was used for histology. The segment was fixed in 4% formaldehyde and embedded in paraffin. Morphometric analysis was performed on haematoxylin-eosin-stained 4 μm transverse sections. The microscopic slides were reviewed by two histologists blinded to the groups. The extent of damage and colonic inflammation was assessed using a modification of the histopathological grading system of Macpherson and Pfeiffer[13].
Enzyme linked immunosorbent assay for serum IFN-γ and TNF-α
Mouse serum was assembled for enzyme linked immunosorbent assay (ELISA). ELISA was performed according to the manufacturer of the kits instructions. TNF-α ELISA kit was a product of Sigma Co. IFN-γ ELISA kits were purchased from Jingmei biotech.
Realtime-PCR analysis
Colons were removed, washed in phosphate buffered solution, and then flash frozen in liquid nitrogen. Total RNA was extracted using Trizol reagent (TAKARA) according to the manufacturer’s protocol. RNA was treated with DNase (TAKARA) and was converted to cDNA using a reverse transcription kit as described by the manufacturer (INVITROGEN). Primers for PCR (INVITROGEN) were listed in Table 1. The parameters for PCR amplification were as follows: 95 °C: 10” × 1 cycle, 95 °C: 5”→60 °C 20”→55 °C 20” × 40 cycles. The concentration of each sample was calculated from the threshold cycle (Ct). The fluorescent products were detected by LightCycle system (BIO-RAD IQ5) before the completion of each cycle.
Table 1.
Primers for polymerase chain reaction
| Primer | Sequence | Product (bp) |
| ZO-1 sense | 5’-AGCCAGTCCATTCTCAGG-3’ | 151 |
| ZO-1 antisense | 5’-TGTACTGTGAGGGCAACG -3’ | |
| Occludin sense | 5’-TACAGACCCAAGAGCAGC -3’ | 177 |
| Occludin antisense | 5’-GTGGCAATAAACACCATG -3’ | |
| NOD2 sense | 5’-CCGAGGAGTCGTGATGGTT-3’ | 150 |
| NOD2 antisense | 5’-GTGTCACCCACATGCAGTG-3’ | |
| GAPDH sense | 5’-GGTGAAGGTCGGTGTGAACG -3’ | 233 |
| GAPDH antisense | 5’-CTCGCTCCTGGAAGATGGTG-3’ |
GAPDH: Glyceraldehyde phosphate dehydrogenase.
Western blotting analysis
The colons were lysed and homogenized in ice-cold RIPA lysis buffer (Beyotime, Jiangsu, China). Proteins were loaded onto each well of SDS-polyacrylamide Ready Gels (BioRad, Hercules) for electrophoresis. Proteins were transferred onto a nitrocellulose membrane (BioRad, Hercules) by electroblotting. The membrane was washed and blocked with 5% milk in Tris-buffered saline with 0.05% Tween-20, and incubated overnight with specific primary antibody at 4 °C. Rabbit anti-mouse-Occludin-mAb (2 μg/mL, ZYMED), rabbit anti-mouse-ZO-1-mAb (3 μg/mL, Abcam) and rat anti-mouse-NOD2-mAb (eBioscience) were used as the primary antibodies. HRP-conjugated goat anti-rabbit-IgG (1:1000, Santa Cruz) was employed as the secondary antibody. Membranes were washed and the assessed proteins were detected using an enhanced chemiluminescence reagent (Amersham Biosciences). Relative intensity of the bands was quantified using Leica image analysis software (Alphaln notech).
Assessment of bacterial translocation
Blood, liver, spleen and MLN were removed under sterile conditions. Blood was plated onto sheep’s blood agar and tissues were slit and cultured with sterile broth at 36 °C for 24 h. The limpid medium was then incubated for another 6 d. Each turbid medium was considered as positive regardless of its incubation time. The positive medium was plated onto sheep’s blood agar and eosin-methylene blue (EMB) agar. The blood agar and EMB agar were purchased from Shanghai Reagent Providing and Research Center for Diarrhea Disease Control. Cultures were incubated at 37 °C for 3 d. Any bacterial growth in the broth or on the agar plates was identified with standard microbiological methods on VITEK-32 Auto Microbic System.
Data analysis
All data were analyzed using SPSS 14.0 and are presented as means ± SD. Nonparametric data were analyzed by Kruskal-Wallis test with Mann-Whitney U post-hoc test. Parametric data were analyzed by 2-way ANOVA with SNK post-hoc test. The P value was considered significant if less than 0.05.
RESULTS
Mice common appearance and mortality
Exposure to schistosome eggs reproducibly ameliorated TNBS colitis in treated mice. Mice of the TNBS+ova+ group presented with inapparent bloody stools, weight loss and activity reduction. The extent of the colon was shortened in TNBS-induced models and this was reversed in the ova-treated group (Figure 1). Nothing abnormal was found in the control group. During the first week after intra-colonic injection of TNBS, the mortality of each group was as follows: control group 0/20 (0%), TNBS+ova- group 20/40 (50%), TNBS+ova+ group 6/30 (20%) (P < 0.05 between each group).
Figure 1.
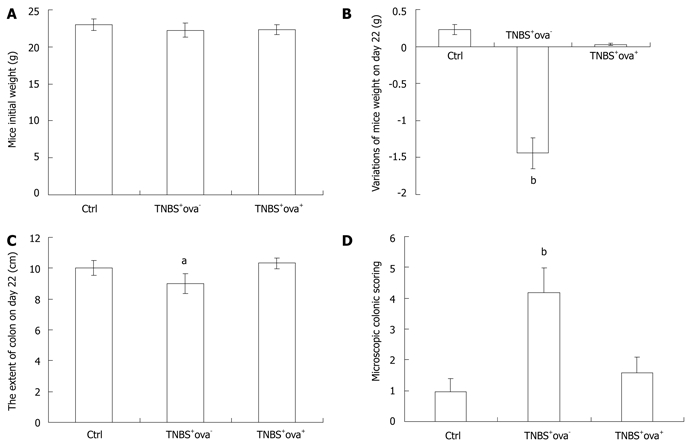
The evaluations of colitis. The initial weight of each group (A) and variations of weight (B), colon extent (C) and microscopic colonic scoring (D) on day 22. Data are presented as mean ± SD. n = 10, aP < 0.05 vs the other two groups; bP < 0.01 vs the other two groups. TNBS: Trinitrobenzenesulfonic acid.
Microscopic inflammation score
No microscopic damage was found in the colon of control animals (Figure 2A). Compared with the colon of control mice, TNBS induced a distortion of colonic mucosal architecture and an inflammatory infiltration throughout the whole colonic wall. The inflammation was characterized by hyperaemia and edema of mucosa which led to thickening of the colonic wall, ulcer formation, folliculus lymphatics hyperplasia, and transmural chronic inflammatory cell infiltration (Figure 2B). Treatment with S japonicum ova tended to relieve the inflammation with infiltration of only a few inflammatory cells (Figure 2C). The histology score also showed a significant relief of colon inflammation in TNBS+ova+ group compared with the TNBS+ova- group (Figure 1).
Figure 2.
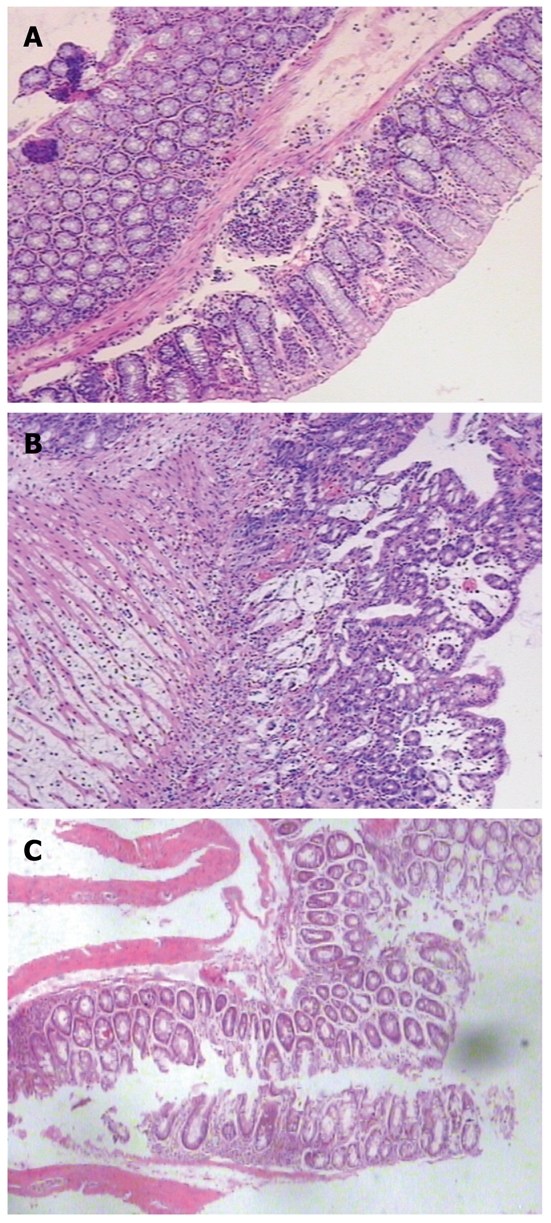
The histology of the colon in different group of mice (HE, × 10). Rare inflammation was found in controls (A). Transmural chronic inflammation was observed in TNBS-induced colitis (B). The inflammation was significantly relieved in schistosome ova treated mice (C). HE: Hematoxylin and eosin; TNBS: Trinitrobenzenesulfonic acid.
Measurement of inflammatory factors in serum
We also surveyed the expression of inflammatory factors in the serum to further evaluate the inflammatory reaction in the different groups. As shown in Figure 3, a highly increased expression of TNF-α and IFN-γ was detected in the TNBS+ova- group. In the TNBS+ova- group, the expression of TNF-α was significantly higher than that of the control group and the TNBS+ova- group. IFN-γ expression was downregulated in the TNBS+ova+ group compared with TNBS+ova- group (P < 0.05).
Figure 3.
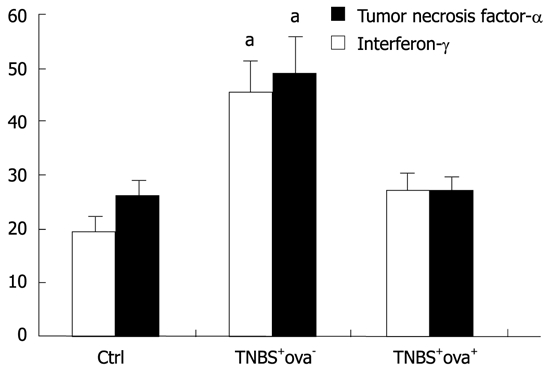
The serum levels of tumor necrosis factor-α (white bar) and interferon-γ (black bar). Data are presented as mean ± SD. There were 20 animals in the control group and the TNBS+ova- group and 24 in the TNBS+ova+ group. aP < 0.05 vs the other two groups. TNBS: Trinitrobenzenesulfonic acid.
The expression of NOD2 and its mRNA in the colon
Since NOD2 is one of the most important pattern pathogen recognition receptors which could induce a large quantity of inflammatory factors such as TNF-α and IFN-γ when inappropriately activated, we further studied the variations of NOD2 and its mRNA in the colon to verifying whether the NOD2 pathway was influenced during TNBS-induced colitis and whether it can be reversed by S. japonicum ova.
As shown in Figure 4, TNBS injection caused a distinct increase of NOD2 in the colon as compared with control mice. However, when mice were pretreated with S. japonicum ova, NOD2 expression was much lower in spite of the TNBS effect. Similar effects were also found in NOD2 mRNA expression among the three groups (Figure 5).
Figure 4.
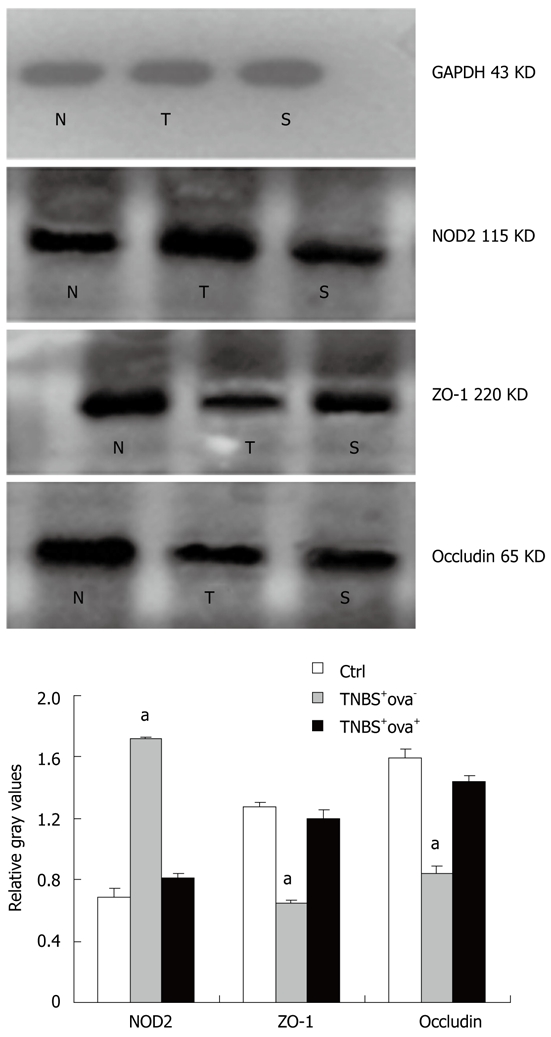
Effect of Schistosoma japonicum ova on the expression of NOD2, ZO-1 and occludin (Western blotting). White bar represents the control group (N), grey bar represents the TNBS+ova- group (T), black bar represents the TNBS+ova+ group (S). Data are presented as mean ± SE. n = 10. aP < 0.05 vs the other two groups. NOD2: Nucleotide-binding-oligomerization domain 2; TNBS: Trinitrobenzenesulfonic acid; GAPDH: Glyceraldehyde phosphate dehydrogenase.
Figure 5.
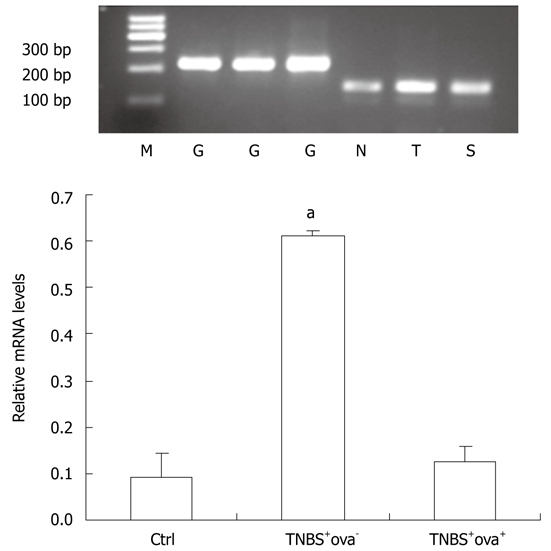
Effect of Schistosoma japonicum ova on the expression of NOD2 mRNA (Realtime-PCR). M: Marker; G: GAPDH; N: Control group; T: TNBS+ova- group; S: TNBS+ova+ group. Data are presented as mean ± SE. n = 10. aP < 0.05 vs the other two groups. GAPDH: Glyceraldehyde phosphate dehydrogenase; TNBS: Trinitrobenzenesulfonic acid.
The evaluation of the intestinal barrier function
To further understand the cause of the over-activation of NOD2, we used IBT frequency to evaluate the intestinal barrier function. Bacterial identification showed the translocated bacteria were predominantly opportunistic pathogens especially Escherichia coli, Bacillus proteus, Klebsiellas, Pseudomonas fluorescens, Pseudomonas pyocyanea and enterococci were also found in several mice. IBT was more common in the TNBS+ova- group. After exposure to S japonicum ova, the frequency of IBT to MLN, blood, liver and spleen was obviously reduced (Table 2).
Table 2.
Culture of blood, mesenteric lymph nodes, liver and spleen
| No. of mice | MLN | Blood | Liver | Spleen | |
| Control | 10 | 1/10 | 0/10 | 0/10 | 0/10 |
| TNBS+ova- | 10 | 8/10 | 10/10 | 9/10 | 5/10 |
| TNBS+ova+ | 10 | 1/10a | 3/10a | 2/10a | 1/10a |
P < 0.05 compared with the TNBS group. MLN: Mesenteric lymph nodes; TNBS: Trinitrobenzenesulfonic acid.
The expressions of ZO-1, occludin and their mRNAs in the colon
In order to gain further insight into the mechanism of intestinal barrier function alteration, we detected the tight junction proteins that constitute the main frame of the barrier. The ZO-1 and occludin expression levels were reduced in the TNBS+ova- group compared with the normal controls. Egg pre-exposure produced a significant elevation in the expression of these tight junction proteins in the experimental induced models (Figure 4). ZO-1 and occludin mRNA was examined using realtime-PCR. Similar to the study of the proteins, ZO-1 and occludin mRNA expression was obviously downregulated in the TNBS+ova- group compared with the controls. Egg pretreatment led to a dramatic upregulation of these genes (Figure 6).
Figure 6.
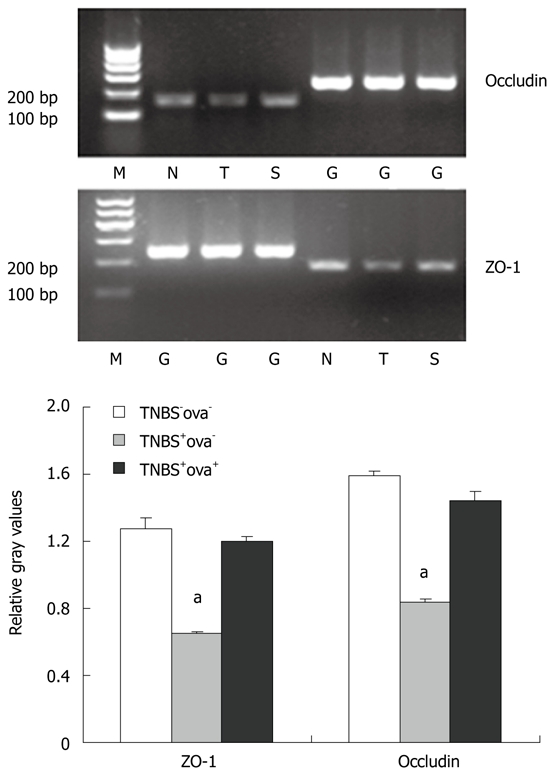
Effect of Schistosoma japonicum ova on the expression of ZO-1 and occludin mRNA (Realtime-PCR). White bar represents the control group (N), grey bar represents the TNBS+ova- group (T), black bar represents the TNBS+ova+ group (S). M: Marker; G: GAPDH. Data are presented as mean ± SE. n = 10. aP < 0.05 vs the other two groups. GAPDH: Glyceraldehyde phosphate dehydrogenase. TNBS: Trinitrobenzenesulfonic acid.
DISCUSSION
CD is a disease more prevalent in developed countries where the sanitary conditions are better. Scientists believe that intestinal hygiene might be a risk factor of CD and helminthes have been thought to be beneficial to CD[9-11]. Elliot et al[9] found that pretreating mice with Schistosoma mansoni ova could relieve TNBS-induce colitis. We have also demonstrated in our previous study that S. japonicum ova pretreatment could prevent experimental colitis in mice by a mechanism of Th2-polarizing stimuli[12]. Summers et al[9] reported a safe and effective result from their short-term study of live Trichuris suis ova therapy in 29 patients with CD. Croese et al[11] also established a potential role of Necator americanus in the remission of autoimmune disease, including CD.
In the present study, we also found an obvious inflammatory reaction both in the colon and the blood. The innate immune system plays an important role in the production of these inflammatory factors. Among varieties of the PPRRs, TLRs and NODs are the most important in antigen recognition. In our former study we demonstrated that TLR4 expression was elevated after the mice were injected with TNBS and was downregulated in the ova group[12]. NOD2/CARD15, famous as the first predisposing gene of CD, codes a cytosolic protein which provides a second line of innate immune defense within IECs and adjacent immune cells, expanding immune surveillance beyond membrane-associated TLRs[14]. Thus we assume that the ova might also decrease the production of the inflammatory cytokines through downregulating NOD2 expression in a direct or indirect pattern. We detected the expression of NOD2 mRNA using realtime-PCR. The results showed that in the TNBS+ova- group, the NOD2 mRNA was increased and the ova pre-exposure significantly downregulated NOD2 mRNA expression. We suppose that the ova relieved colitis and suppressed the inflammatory reaction partially because of the downregulation of PPRRs which might be inappropriately activated and thus produced large quantities of inflammatory cytokines.
The intestinal epithelial barrier also plays an important role in the onset and progression of CD. Increased IBT to the MLN, liver, blood and spleen was found in the present study. We supposed it might be due to the increased intestinal pericellular permeability, which was usually caused by impaired intestinal barrier function. The intestinal barrier function was maintained by a series of epithelial TJs, protecting the body from pathogens and other toxic luminal substances. Transmembrane proteins (such as occludin, tricellulin, claudins and junctional adhesion molecule) interact with cytoplasmic peripheral membrane proteins (such as ZO-1, ZO-2, ZO-3 and cingulin), and thus constitute the frames of TJs[15].
An obvious downregulation of ZO-1 and occludin was found in the present study using Western blotting, which could be prevented by pre-exposure to the S. japonicum ova. We further tested the expression of ZO-1 and occludin mRNA and the outcome was consistent with the protein study.
Inflammatory mediators such as cytokines, toxins and reactive oxygen species contributed to the impairment of TJs[16-20]. The best-studied cytokine that causes barrier dysfunction due to epithelial tight junction regulation is TNF-α. Poritz et al[19] incubated MDCK cells with different concentrations of TNF-α and found the fragmentation staining of ZO-1, suggesting a disruption of the TJs. They also found that claudin-1 expression was decreased along with an increasing concentration of TNF-α. It was demonstrated that the TNF-α-induced TJs impairment was mediated by NF-κB activation in Caco-2 cells[19]. Treatment of T84 cells with IFN-γ also caused a dose- and time-dependent increase in monolayer permeability. Examination of specific proteins associated with TJs by immunoblotting and confocal microscopy revealed changes in the localization and expression levels of the tight junction proteins after exposing the cells to IFN-γ. Specifically, they found that IFN-γ treatment resulted in an almost total loss of ZO-1[20].
Impaired barrier function leads to the exposure of submucosal immunocytes to luminal antigens and initiates the innate immune reaction. TLRs and NODs are PPRRs that are expressed by intestinal epithelial cells and submucosal immunocytes. The inappropriate activation of TLRs and NODs may cause the over-reaction of the immune system and overproduction of cytokines (such as IFN-γ and TNF-α), leading to the formation of CD. We assume that treating mice with S. japonicum ova might cut down the impairment of the intestinal epithelial barrier through protecting the TJs, thus preventing the activation of PPRRs and downregulating inflammatory mediators like TNF-α and IFN-γ, leading to the remission of colitis.
Despite various therapeutic measures against CD, even the most experienced clinician might have problems with treatment of many cases, especially those with complications. Helminthes might be beneficial in CD. The clarification of this mechanism contributes to finding out the key effective component, which may be a novel potential way of treating CD.
COMMENTS
Background
Crohn’s disease (CD) is more prevalent in developed countries where sanitary conditions are better. Scientists have found that helminthes can protect mice from experimental colitis. The intestinal epithelial barrier plays an important role in the onset and progress of CD. Tight junctions (TJs), which form the pericellular barrier, are thought to be the primary determinant of mucosal permeability in the presence of an intact epithelium. The impaired TJs lead to an increase of exposure of intestine bacteria to submucosal proteins, like nucleotide-binding-oligomerization domains (NODs). Nucleotide-binding-oligomerization domain 2 (NOD2) has been found to exert antibacterial activity limiting survival of enteric bacteria after invasion. As one of the main responsible genes, NOD2 and its protein have been found to be upregulated in CD patients.
Research frontiers
TJs could be affected by a large amount of factors, such as cytokines, toxins and reactive oxygens. Many proteins and signal transduction systems participate in the regulation of TJs such as Rho/Ras-GTPases. Recently, it was reported that Notch-1 plays an important role in maintaining epithelial barrier function and promoting tight junction protein expression.
Innovations and breakthroughs
Till now various studies have proved that helminthes and their eggs do favor the relief of colitis both in experimental models and in some small scale clinical trials, but the rationale of how this works remains unknown. It was usually thought that helminthes might relieve colonic inflammation by a T cell immunity balance adjustment. Zhang and his co-workers investigated the impact of S. japonicum ova on trinitrobenzenesulfonic acid (TNBS)-induced colitis from a new point of view. They found that helminthes and their eggs might protect the intestine from inflammation by increasing the TJs and protecting the intestinal barrier function.
Applications
Despite various therapeutic measures against CD, even the most experienced clinician might have problems with treatment of many cases, especially those with complications. Helminthes and their eggs might be beneficial to treatment of CD. The clarification of this mechanism contributes to finding out the key effective component, which may be a novel potential method for treating CD.
Terminology
Intestinal bacterial translocation: The bacteria in the intestine migrate to the peripheral circulation and other organs (liver, spleen, etc.) through the portal vein due to the increased intestinal permeability; NOD2: One of the pattern pathogen recognition receptors which exists in the cytoplasm and recognize muramyl dipeptide of the bacteria. NOD2 is also the first predisposing gene of CD; Tight junctions: tight junctions form a network of close contacts between membranes of adjacent cells. They control the pericellular transport of ions, water, and solutes, and in addition they constitute a fence separating apical and basolateral membrane proteins.
Peer review
The paper is an intriguing and well designed paper which addresses a relevant issue.
Footnotes
Peer reviewer: Giuseppe Chiarioni, Dr., Gastroenterological Rehabilitation Division of the University of Verona, Valeggio sul Mincio Hospital, Azienda Ospedale di Valeggio s/M, Valeggio s/M 37067, Italy
S- Editor Sun H L- Editor O’Neill M E- Editor Zhang DN
References
- 1.MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 2.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 4.Takesue Y, Ohge H, Uemura K, Imamura Y, Murakami Y, Yokoyama T, Kakehashi M, Sueda T. Bacterial translocation in patients with Crohn’s disease undergoing surgery. Dis Colon Rectum. 2002;45:1665–1671. doi: 10.1007/s10350-004-7256-z. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis. 2009;27:443–449. doi: 10.1159/000233282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mühlbauer M, Cheely AW, Yenugu S, Jobin C. Regulation and functional impact of lipopolysaccharide induced Nod2 gene expression in the murine epididymal epithelial cell line PC1. Immunology. 2008;124:256–264. doi: 10.1111/j.1365-2567.2007.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 8.Okumura S, Yuki K, Kobayashi R, Okamura S, Ohmori K, Saito H, Ra C, Okayama Y. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin Immunol. 2009;130:175–185. doi: 10.1016/j.clim.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 11.Croese J, O’neil J, Masson J, Cooke S, Melrose W, Pritchard D, Speare R. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136–137. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Zhang S, Jiang L, Jiang J, Liu H. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid-induced colitis and bacterial translocation in mice. J Gastroenterol Hepatol. 2009;24:1775–1780. doi: 10.1111/j.1440-1746.2009.05986.x. [DOI] [PubMed] [Google Scholar]
- 13.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 14.Mueller T, Podolsky DK. Nucleotide-binding-oligomerization domain proteins and toll-like receptors: sensors of the inflammatory bowel diseases’ microbial environment. Curr Opin Gastroenterol. 2005;21:419–425. [PubMed] [Google Scholar]
- 15.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 17.Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293:G308–G318. doi: 10.1152/ajpgi.00582.2006. [DOI] [PubMed] [Google Scholar]
- 18.Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol Med. 2002;8:353–366. [PMC free article] [PubMed] [Google Scholar]
- 19.Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res. 2004;116:14–18. doi: 10.1016/s0022-4804(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 20.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]


