Abstract
Purpose
Glioblastoma (GBM) inevitably recurs despite surgery, radiation and chemotherapy. A subpopulation of tumor cells, GBM stem cells (GSCs), has been implicated in this recurrence. The chemotherapeutic agent, etoposide is generally reserved for treating recurrent tumors, however its effectiveness is limited due to acute and cumulative toxicities to normal tissues. We investigate a novel combinatorial approach of low dose etoposide with an oncolytic HSV to enhance anti-tumor activity and limit drug toxicity.
Experimental design
In vitro, human GBM cell lines and GSCs were treated with etoposide alone, oHSV-G47Δ alone or the combination. Cytotoxic interactions were analyzed using the Chou-Talalay method and changes in caspase-dependent apoptosis and cell cycle were determined. In vivo, the most etoposide-resistant human GSC, BT74, was implanted intracranially and treated with either treatment alone or the combination. Analysis included effects on survival, therapy-associated adverse events and histological detection of apoptosis.
Results
GSCs varied in their sensitivity to etoposide by over 50-fold in vitro, while their sensitivity to G47Δ was similar. Combining G47Δ with low dose etoposide was moderately synergistic in GSCs and GBM cell lines. This combination did not enhance virus replication, but significantly increased apoptosis. In vivo, the combination of a single cycle of low dose etoposide with G47Δ significantly extended survival of mice bearing etoposide-insensitive intracranial human GSC-derived tumors.
Conclusions
The combination of low dose etoposide with G47Δ increases survival of mice bearing intracranial human GSC-derived tumors without adverse side effects. These results establish this as a promising combination strategy to treat resistant and recurrent GBM.
Keywords: GBM, cancer stem cells, chemotherapy, oHSV, drug combination, topoisomerase inhibitors
Introduction
There has been minimal progress in the treatment of glioblastoma (GBM), due in part to tumor heterogeneity, genetic diversity and the presence of radiation and chemotherapy resistant glioblastoma stem cells (GSCs)1. It has become clear that therapeutic improvements will likely depend on effective combination therapies targeting multiple aberrant signaling mechanisms. Although temozolomide is the standard chemotherapy regimen for primary GBMs, recurrent GBMs are frequently treated with high doses of DNA topoisomerase inhibitors such as irinotecan or etoposide plus platinum analogs2, 3.
Etoposide (VP-16) is a semi-synthetic derivative of a naturally occurring antibiotic, podophyllotoxin, introduced in cancer clinical trails in 1971 and FDA-approved in 19834. It inhibits topoisomerase II re-ligation of cleaved DNA molecules, resulting in the accumulation of double-strand DNA breaks. This leads to late S and G2 cell cycle arrest 4. Previous studies have reported that etoposide is effective against glioma cell lines and is currently being widely used in the treatment of lung and ovarian cancer as well as recurrent childhood brain tumors 5, 6. Although effective against GBM at high doses, etoposide often leads to toxic side effects such as nausea, weight loss, alopecia, myelosuppression with leucopenia, and thrombocytopenia 2, 3. Therefore, to avoid high dosage toxicities, metronomic etoposide is being tested in combination with a number of drugs such as Bevacizumab and Vandetanib for recurrent GBMs (Duke-clinical trials.gov) 7.
Oncolytic herpes simplex viruses (oHSV) are genetically engineered to selectively replicate in and kill cancer cells but spare normal tissue. The safety of oHSV therapy has been demonstrated in clinical trials with recurrent gliomas, however efficacy is limited8, 9. oHSV-G47Δ, tested in this study, is currently undergoing clinical trial in patients with progressive glioblastoma 10. Previously, it was shown that oHSV can combine with drugs such as taxanes and temozolomide to synergistically kill various tumor cells 11, 12. However, this study is the first to investigate combining clinically relevant etoposide (or irinotecan) with oHSV as a strategy to improve overall efficacy for GBM 13.
We have established GSCs from human GBM specimens that provide a more representative model for testing therapies on gliomas than the traditionally used adherent GBM cell lines. Tumors generated from these GSCs retain the histopathological characteristics of GBM 14. In this study, we demonstrated that low dose etoposide combined with G47Δ is efficacious against intracerebral GBMs derived from chemotherapy resistant GSCs, without causing obvious side effects.
Materials and Methods
Cell lines and Reagents
Human GBM cell lines U87, T98 and Vero cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Human astrocytes were obtained from ScienCell (San Diego, CA). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum at 37°C and 5% CO2. GSCs GBM4, GBM8, and BT74 were isolated as previously described 14. BT74 was originally isolated by Dr. D. James as GBM6 15. Cultures of GSCs were maintained as spheres in serum-free medium containing 20ng/ml recombinant human EGF (R&D systems) and 20ng/ml recombinant human FGF2 (Peprotech). Passaging of the cultures was performed by dissociating neurospheres using NeuroCult Chemical Dissociation kit (StemCell Technologies, Vancouver, BC, Canada). Etoposide (VP-16) and SN-38, used for in vitro studies, were purchased from Sigma and dissolved in DMSO as a 51 mM stock solution. The final concentrations added to cells had less than 0.5% DMSO, which is nontoxic to cells. For in vivo studies etoposide was obtained from the MGH Pharmacy in solution at 20mg/ml.
Cell susceptibility assays and Chou-Talalay analysis
Cells were dissociated and seeded into 96-well plates (4000 cells/well). The next day, the cells were treated with either etoposide and/or G47Δ after 3–6 hours, at varying doses and incubated at 37°C. 96h after incubation, MTS assays (Promega, Madison, WI) were performed according to the manufacturer’s instructions. For U87 and T98 cells, MTT assays (Sigma) were performed. For Chou-Talalay analysis, experiments were carried out as described 16. Dose-response curves and 50% effective concentration values (EC50) were obtained and fixed ratios of drug and virus were added to cells in combination. Mutually exclusive equations were then used to determine Combination Index values (CIs). Briefly, combined dose-response curves were fitted to Chou-Talalay lines 17 which are derived from the law of mass action and described by the equation; log (fa/fu)=mlogD-mlogDm, where fa is the fraction of total cells affected (percent cell death), fu is the fraction of total cells unaffected, D is the dose, Dm is the median-effect dose, and m is the coefficient signifying the shape of the dose-response curve. CI values were calculated using the equation CI=(D1/Dx1)+(D2/Dx2)+(D1)(D2)/[(Dx1)(Dx2)], where Dx1 and Dx2 are the etoposide and G47Δ doses, respectively, that are required to achieve a particular fa, and D1 and D2 are the doses of the two agents (combined treatment) required for achieving the same fa. CI=1, >1, <1 indicate additive, antagonistic, and synergistic interactions, respectively.
Viral replication assays
U87 or dissociated GSCs were plated at 3× 104 cells/500μl in 24 well plates and etoposide added at a concentration lower than EC50. 3–6 hours later cells were infected with G47Δ at a multiplicity of infection (MOI) of 1, incubated at 37°C, and harvested with supernatant at indicated time points. After three freeze-thaw cycles and sonication, the titers of infectious virus were determined by plaque assay on Vero cells.
Western blotting and Caspase 3 Glo assay
Cells (1 × 105) were treated with etoposide alone (at less than EC50), G47Δ alone (MOI~1), or the combination and harvested after 24 hours. Cell pellets were lysed in RIPA buffer with a cocktail of protease and phosphatase inhibitors (Boston Bioproducts, Worcester, MA), protein concentrations measured by Bradford assay, 40μg of protein loaded onto a 12% SDS gel, electrophoresed, protein transferred to PVDF membranes, and probed with primary antibody against cleaved caspase 3 (1:1000; Cell Signaling) or Actin (1:10,000; Sigma) overnight at 4°C. This was followed by incubation with appropriate HRP-conjugated goat anti-rabbit secondary antibodies (1:5000, Promega) for 1 hour at room temperature. Protein-antibody complexes were visualized using ECL (Amersham Bioscience). Caspase-3 and -7 (caspase-3/7) activity was also evaluated using the Caspase-Glo 3/7 Assay kit (Promega), according to the manufacturer’s instruction. Briefly, cells (5000 cells per well) were plated in 96-well plates in triplicate, treated with etoposide (less than EC50) and 5 hours later infected with G47Δ at MOI of 1 or mock. The caspase-glo solution was added 20 hours after virus infection and luminescence read after one hour.
Cell cycle and apoptotic analysis
For cell cycle, gliomas cells were seeded into 10cm dishes and treated with G47Δ (MOI~0.2), etoposide or the combination. Three to 4 days later, cells were pelleted, fixed with cold 70% ethanol and stored at −20°C. Before analysis, fixed cells were washed in PBS and then resuspended with propidium iodide (50μg/ml Sigma) solution containing 0.1% sodium citrate, 0.1% Triton-X and 2μg/ml RNase (Sigma) and immediately analyzed by flow cytometry using a BD FACSCalibur. For apoptosis TUNEL assay we used an APO-BRDU kit (BD Bioscience), performed as per manufacturer’s instructions. Briefly, cells treated with etoposide (less than EC50)alone, G47Δ alone at MOI of 0.5–1, combination of both, or mock, were fixed with 1% paraformaldehyde and 70% ethanol after 48hrs. These cells were then labeled with DNA labeling solution for 1 hour followed by FITC-labeled anti-BrdU antibody. Apoptotic cells were differentiated from non-apoptotic cells via flow cytometry using a BD FACSCalibur. Data was analyzed using FlowJo software (Ashland, OR).
Animal experiments
Nude mice were obtained from NCI and maintained under standard conditions. All mice used in these studies were between 7–9 weeks of age. Dissociated human BT74 GSCs (2 × 105 cells) were implanted stereotaxically into the right striatum (2.5-mm lateral from Bregma and 2.5-mm deep), to generate orthotopic xenografts. On day 7, mice were randomly divided into four groups (n=10/gp) and etoposide (3mg/kg) was injected intraperitoneally (i.p) for 5 consecutive days to two groups of mice. On day 9, these two groups and the other 2 untreated groups were injected with either 3μl of G47Δ (1.5 × 106 pfu) or PBS intratumorally using the same coordinates as for tumor implantation. Mice were then followed for survival. Overall health of the animals, including body weight, was recorded every 2–3 days, and animals were monitored for signs of discomfort or neurological symptoms. All in vivo procedures were approved by the Subcommittee on Research Animal Care, at Massachusetts General Hospital. For histological studies, mice (21 days after tumor implantation) were treated with PBS, etoposide (5-day treatment; days 21 to 25) and/or G47Δ (day 23). 48 hours after virus injection or the last day of etoposide treatment, animals were perfused with 4% paraformaldehyde and brains were removed, embedded in OCT and sectioned. These sections were subjected to X-gal and hematoxylin staining or immunocytochemistry with antibody against cleaved caspase-3 (Cell Signaling Technology) followed by incubation with Cy3-conjugated anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA) and DAPI.
Statistical Analysis
Comparisons of data in cell survival and viral yield assays were performed using two-tailed Student’s t test. Survival was analyzed by Kaplan Meier curves, and comparisons determined by logrank test. P values of <0.05 were considered significant. Statistical analysis was conducted using Prism (GraphPad Software, Inc, San Diego, CA).
RESULTS
Cytotoxicity of etoposide and G47Δ in human glioma cells
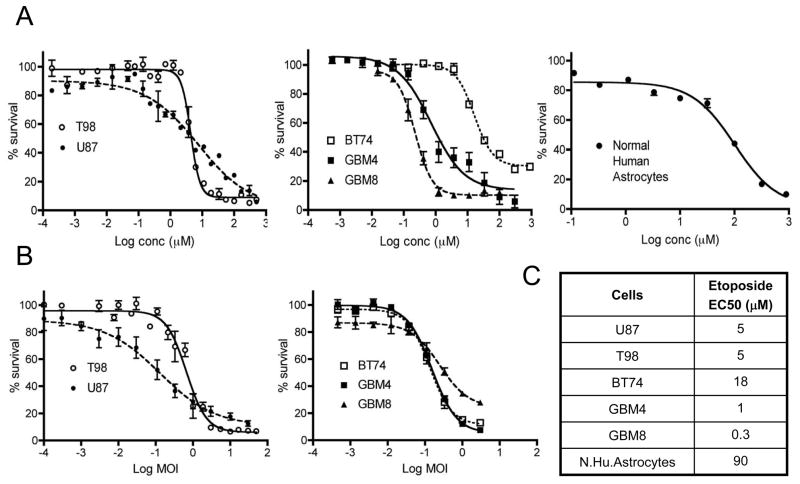
As a topoisomerase inhibitor, etoposide can cause DNA strand breaks leading to cell death. The cytotoxic effect of etoposide was initially tested in human GBM cell lines U87 and T98. Both U87 and T98 were sensitive to etoposide-induced cytotoxicity with EC50 = 5μM (Figure 1A). Others have reported similar EC50 for U87 (6.5 μM)18. Human GSCs (BT74, GBM4, GBM8) exhibited a broad range of etoposide sensitivity (50-fold range), with EC50 values of 18μM, 1μM and 0.3μM, respectively. Notably, of these GSCs, BT74 was the most resistant to etoposide. Normal human astrocytes, were even more resistant to etoposide (EC50 = 90μM; Figure 1A). Besides etoposide, we also tested the effects of SN-38, the active metabolite of the topoisomerase I inhibitor irinotecan, on U87 and T98 cell lines and BT74 GSCs. The EC50 values were 0.08μM, 0.1μM and 0.2μM, respectively (Supplementary Fig 1A). We next tested the cytotoxic activity of G47Δ oHSV. As previously reported 14, G47Δ oHSV was highly effective at killing the two GBM cell lines (EC50 of U87 at MOI 0.1; T98 at MOI 0.6) as well as all three GSCs (EC50 MOIs 0.1 to 0.3, Figure 1B), whereas normal human astrocytes are quite resistant to G47Δ replication (EC50 MOI~ 1; unpublished results).
Figure 1. Dose response curves of etoposide and G47Δ in human glioma cell lines and GSCs.
(A) Increasing concentrations of etoposide were assayed in U87 and T98 cell lines (left), three human GSCs (BT74, GBM4, GBM8) (middle) and normal human astrocytes (right). (B) Increasing MOI of G47Δ oHSV in U87 and T98 cell lines (left), and human GSCs (right). (C) Table showing EC50 values of etoposide calculated for human glioma cells and normal human astrocytes. Cells were seeded in 96 well plates and incubated with increasing concentrations of etoposide or different MOI of G47Δ, and cell viability measured 96 hours later. The percentage of viable cells relative to mock-treated controls (% survival) was plotted. Points are means of triplicate wells and three different experiments; bars, SEM.
Combination Therapy In Vitro
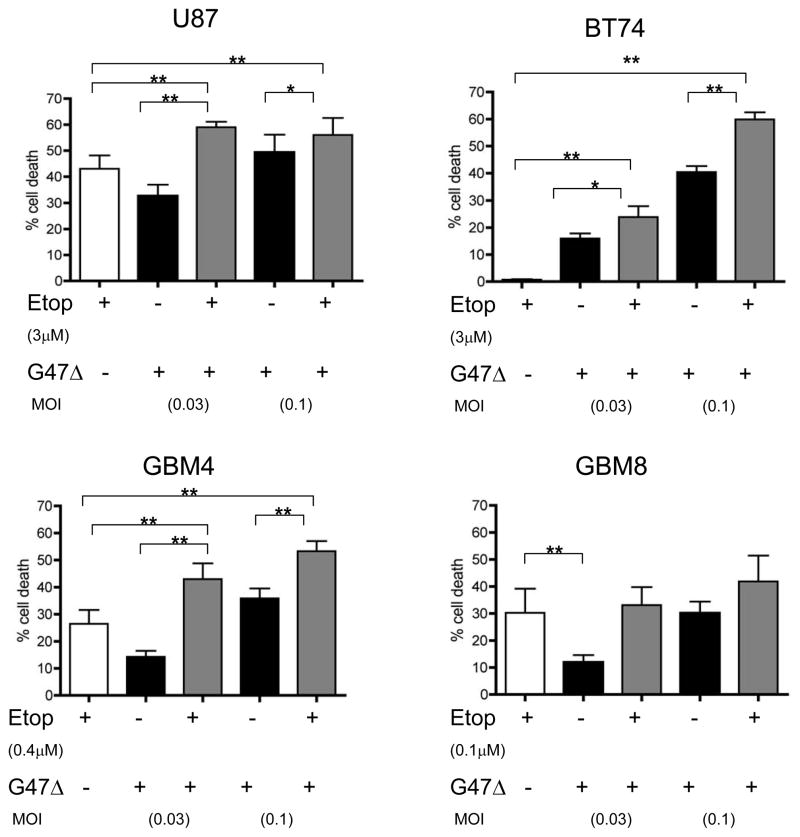
Since these two therapies (oHSV and etoposide) utilize different modes of cell killing, we evaluated the effect of combining a fixed low dose of etoposide (less than the EC50 values for each respective cell line) with G47Δ. In U87 cells, a significant increase in cell death or decrease in cell viability was observed with combination treatment when compared to either treatment alone. The combination of etoposide and G47Δ also yielded a significant increase in cell death for BT74 and GBM4 GSCs but not for GBM8 (Figure 2). In the most etoposide-resistant GSC, BT74, 3μM etoposide by itself had no effect on cell killing, however combining this with G47Δ significantly enhanced cell death over G47Δ alone at both G47Δ doses, MOIs 0.03 and 0.1.
Figure 2. Combinatorial strategy with low dose etoposide and G47Δ shows increased cytotoxicity.
Cytotoxic assays in U87, BT74, GBM4, and GBM8 cells were performed with indicated doses of etoposide (less than EC50) and/or with G47Δ at MOI 0.1 or 0.03. Cells were plated in 96 well plates and etoposide added 3–6 hours before G47Δ. Cell death was measured at 96 hours after treatment and expressed as a percentage of mock treated controls. Results are the means of triplicate wells and three different experiments; bars, SEM. * p <0.05, ** p<0.01 (by paired t-test).
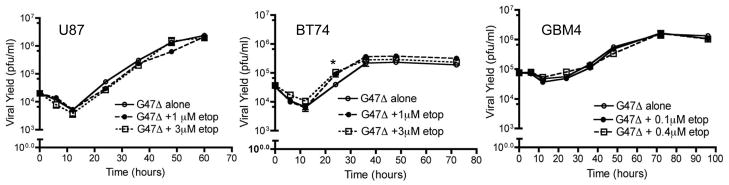
The median-effect method of Chou and Talalay was used to determine whether the combination acted synergistically. In all glioma cells tested, at a low fraction affected dose (Fa=0.2, refers to 20% cytotoxicity) showed a clearly synergistic effect of the combination of etoposide with G47Δ. At Fa =0.4, all cells except GBM8 GSC showed synergistic-additive responses (Table 1). We also investigated the combination of the irinotecan metabolite, SN-38 with G47Δ, which was antagonistic (CI value ≥1) due to inhibition of G47Δ plaque formation on Vero cells and reduced the viral yield in U87 cells at EC50 values of SN-38 (Supplementary Fig 1B, C). Previously, it was shown that certain chemotherapeutic drugs, such as mitomycin C, temozolomide and 5-Fluorouracil, increase oHSV replication, which could account for the observed synergy in cell death 13. Therefore, we tested whether the increase in cytotoxicity observed with the combination of etoposide and G47Δ was due to an increase in virus replication. However, in a single-step growth assay, there was no significant difference in virus yields at two etoposide doses, and the virus growth kinetics were similar with or without etoposide, except for a single time point in BT74 (Figure 3).
Table 1. Chou Talalay analysis (CI values) of combining etoposide with G47Δ.
The combination index values (CI), at indicated fraction affected (Fa) doses, are indicated for U87, BT74, GBM4, and GBM8. CI value =1, >1, <1 refers to additive, antagonist and synergy, respectively.
| Fa | 0.2 | 0.4 | 0.6 |
|---|---|---|---|
| Cells | |||
| U87 | 0.6 | 0.8 | 1.2 |
| BT74 | 0.6 | 0.8 | 1.1 |
| GBM4 | 0.7 | 0.7 | 0.7 |
| GBM8 | 0.7 | 1.2 | 1.6 |
Figure 3. Etoposide does not affect G47Δ replication.
U87 (left), BT74 (middle) and GBM4 (right) were infected with G47Δ at MOI=1 in the presence or absence of etoposide at the indicated concentrations (<EC50). At indicated time points, cells and medium were harvested and viral titers were determined on Vero cells. * p<0.05 versus G47Δ alone (by paired t-test)
Effect on Apoptosis and Cell Cycle
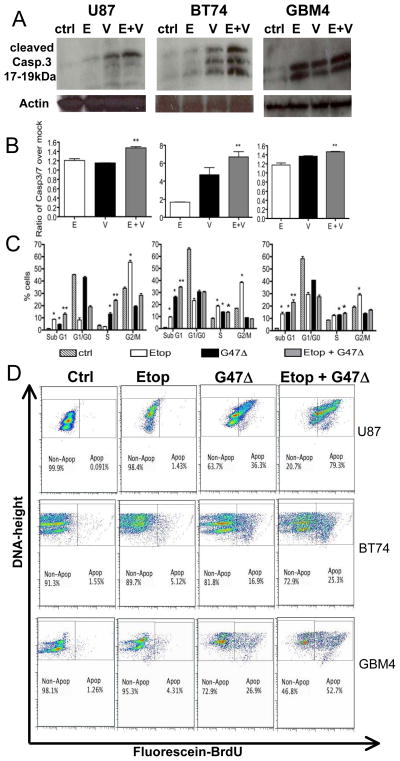
We next determined whether these moderately synergistic effects in cytotoxicity were mediated through apoptosis. Caspase 3 and 7 are common effector caspases of both the intrinsic and extrinsic pathways of programmed cell death 19. Combining etoposide with G47Δ in U87 and BT74 resulted in increased levels of cleaved caspase 3 protein compared to that with either treatment alone (Figure 4A). This was quantified using a Caspase 3/7 activity assay, where a significant increase with the combination was detected in U87, BT74, and GBM4 cells (Figure 4B). This was not observed in GBM8 GSC where the interaction was mostly antagonistic (data not shown). Although these differences may be small, it is important to point out that the differences in caspase activation were most apparent in BT74 GSC, which is extremely resistant to etoposide by itself. Thus, GSCs that are less sensitive to etoposide may respond better to the combination treatment.
Figure 4. Increased apoptosis with combination of low dose etoposide and G47.
Δ. U87 (left), BT74 (middle) and GBM4 (right) were (A) processed for western blotting with the antibody against cleaved caspase 3 (~17–19 kDa) after 24-hour treatment with mock-treated (ctrl), etoposide (E) alone (3μM in U87 and BT74 and 0.4μM in GBM4), G47Δ (V) alone (MOI=1) or the combination (E+V). Actin was used as a loading control. (B) Caspase 3/7 activity was measured by luminescence assay after 18–20 hours of the same treatments as in A. ** p<0.05 from E alone or V alone (by Student’s t-test). (C) Cell cycle analysis was performed at 3–4 days after etoposide (Etop) at the same concentrations as in A and/or G47Δ (MOI=0.2). Percentage of cells in each phase of the cell cycle was calculated. Increase in percentages *p<0.05 from ctrl, **p<0.05 from G47Δ or etoposide alone (by Student’s t-test). (D) APO-BRDU assay (right) at 48hrs after etoposide (Etop) at the same concentrations as in A and/or G47Δ (MOI=0.5–1). The graphs (Fluorescein-BrdU Vs. Total DNA- Height) show the percentages of apoptotic (Apop) and non-apoptotic (Non-Apop) cells.
It is well known that etoposide can arrest cells either in the G2-M or S-phase of the cell cycle 4, so we tested the effects of combination treatment on modulating the cell cycle profiles. Etoposide treatment alone caused an (1.5–2.5-fold) increase in the G2/M population of U87, BT74 and GBM4 cells, with an additional increase in S phase in BT74 (Figure 4C). G47Δ infection, as expected12, results in some growth arrest at the G1/S phase of the cell cycle, with a significant increase in sub G1 phase in BT74, GBM4 and U87 cells. The combination of G47Δ with etoposide resulted in a significantly greater proportion of cells in sub-G1 phase in all three cells, with a concomitant decrease in G1 and G2/M cells compared to etoposide alone (Figure 4C). The sub-G1 population likely consists of apoptotic cells, but could also represent mechanically damaged cells or cells with a lower DNA content. Therefore, we directly examined the induction of apoptosis using the TUNEL assay and flow cytometry after 48 hours (Figure 4D). Etoposide treatment alone in U87, BT74 and GBM4 resulted in only a small increase in DNA fragmentation (1.4%, 5.1% and 4.3% respectively). Virus treatment alone induced a greater degree of apoptosis (25-, 3-, and 6-fold greater than etoposide respectively). The combination treatment resulted in a moderately synergistic response (greater than additive), so that the majority of U87 and GBM4 cells were apoptotic (79.3% and 52.7% respectively). The apoptotic effects were also observed within 24 hours of treatment in BT74 and GBM4 cells with etoposide alone inducing apoptosis in 1.3% and 6% of cells, virus alone in 1.86% and 13.4% and the combination at 4.81% and 18.7% of cells respectively. This suggests that apoptosis is an important contributor to the combination effect in vitro.
Combination therapy with G47Δ and etoposide extends survival in an etoposide-resistant intracranial model of human GSC
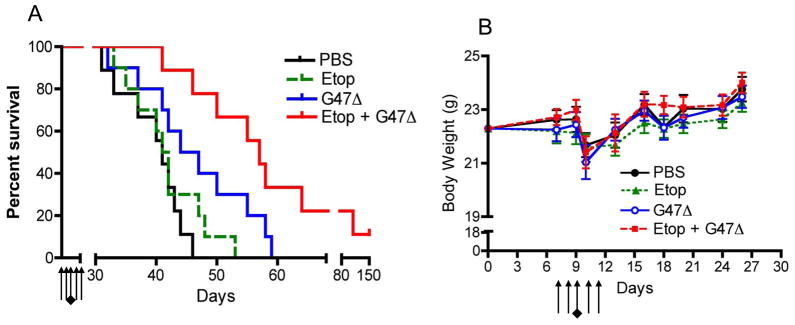
To translate the observed effects of combination treatment in vivo, we utilized BT74, which was the most etoposide insensitive GSC tested. Histopathologically BT74 forms aggressive tumors with high levels of vascularity and intratumoral hemorrhage 14, 16. The maximum tolerated dose of etoposide in mice has been published to be 40mg/kg 20. We used a single 5-day cycle of low dose etoposide at 3mg/kg/day with intratumoral G47Δ injection in the middle of the cycle (day 3 of etoposide), similar to the metronomic dose/scheme used in the clinic for oral etoposide to avoid toxicities associated with high doses7, 21. A similar low dose of etoposide was also used in the study by Bello et al., 2001 21. Control mice bearing BT74 intracerebral tumors had a median survival of 40 days and treatment with low dose etoposide alone had no effect at prolonging survival. A single intratumoral injection of G47Δ improved survival (p<0.03) to a median of 46 days similar to what was seen previously 14. However, the combination treatment of low dose etoposide with G47Δ was much more efficacious than either treatment alone, significantly extending the median survival to 57 days (p<0.05 vs. G47Δ, and p< 0.001 vs. etoposide). Two mice (out of 10) from the combination treatment group survived more than 80 days (Figure 5A). One mouse died at day 109 with a tumor, but the one alive at day 150, had a complete response with no observable tumor on histological analysis. Gross macroscopic analysis of liver/spleen as well as skin from the treated animals showed no abnormalities with any of the treatments. It is important to note that this low dose etoposide regimen (3mg/kg) with or without G47Δ did not produce any significant adverse effects as observed by measuring body weight over the period of treatment (Figure 5B). The abrupt decrease in weight on day 10 was due to anesthesia and surgery and all mice recovered similarly.
Figure 5. Treatment of intracranial BT74 tumors.
BT74 GSCs were implanted intracranially in athymic mice. Seven days later the mice were treated intraperitoneally (i.p) with etoposide (3mg/kg/day, black arrows) or PBS for five consecutive days and injected intratumorally with G47Δ (1.5 × 106 pfu, black diamond) or PBS in 3μl on day 9. (A) Kaplan-Meier survival curves with treatments. Etoposide alone is not different from PBS whereas G47Δ treatment alone is significantly different from PBS (p<0.03). Combination treatment was significantly different from G47Δ (p<0.05) as well as etoposide alone (p<0.0005). (B) Body weight of mice in gms. Arrows represent the daily etoposide injections (5 days) and a black diamond indicates the day of G47Δ intratumoral injection. The decrease in weight observed on day 10 is due to anesthesia and surgery performed the day before. No statistical differences were observed between all four groups
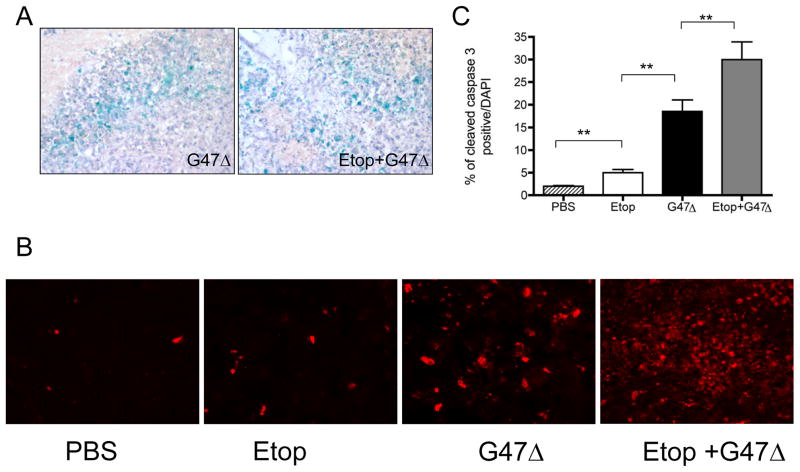
Combination therapy of G47Δ with etoposide enhances caspase 3 activation
Combining etoposide with G47Δ did not have any effect on viral infection and spread within the tumor as illustrated by X-gal staining (G47Δ-infected cells) at 48 hours after virus injection in the presence or absence of etoposide injections (Figure 6A). Since combination treatment increased apoptosis in vitro, we examined BT74 tumor sections from mice for the presence of activated caspase 3. PBS treatment induced barely detectable levels of cleaved caspase-3 within the tumor while treatment with etoposide alone induced only a small increase in cleaved caspase-3 positive cells (Figure 6BC). In contrast, G47Δ infection induced a significantly larger increase in cleaved caspase 3-positive cells, which was further increased when combined with etoposide (p<0.003 vs. G47Δ alone; p<0.0003 vs. etoposide alone) (Figure 6B,C). These high immunopositive indices for caspase 3 activation provide further support that increased apoptosis contributes to improved survival.
Figure 6. Staining of brain sections from treated tumor-bearing mice.
(A) Lac Z staining of brain sections from mice treated with injection of intratumoral G47Δ. Mice receiving G47Δ alone or combined with etoposide were sacrificed 48 hrs after G47Δ injection. Hematoxylin was used for counterstain (40x magnification) (B) Representative images of brain tumor sections immunostained with an antibody to cleaved caspase-3 (red) (30x magnification) (C) Quantification of cleaved caspase-3 immunopositivity. Pictures were taken from 3–4 random fields/section and positive staining quantified in 2 mice/group (** p< 0.05).
DISCUSSION
We report here the first study to demonstrate that low dose etoposide can be combined with oHSV to effectively target GSCs. This combinatorial strategy holds great promise for the treatment of both primary and recurrent GBMs as it could enhance efficacy while minimizing toxicity 22.
In vitro, etoposide was just as effective at targeting human GSCs as the human gliomas cell lines. These results may seem counterintuitive, however, recent evidence has shown that cancer stem cells are genetically diverse, can differ in drug efflux resistance mechanisms, and that certain chemotherapeutics may preferentially target GSCs23, 24. BT74 was the most resistant GSC to etoposide when compared to the other GSCs and glioma cell lines tested (Figure 1). In contrast, GBM8 was very sensitive to etoposide alone (EC50 0.3 μM). Reasons for the differences in etoposide sensitivity observed in GSCs include differences in proliferation rate, cell-cycle distribution, DNA repair, and drug resistant efflux mechanisms 25. Some studies also suggest that cells with wild-type p53 and Akt-myr-transduced Ink4a/Arf−/− are more sensitive to cell death by etoposide 26, 27. This is in line with our in vitro etoposide susceptibility data with GBM8, expressing wild type p53, the most sensitive to etoposide, followed by GBM4 that is heterozygous for p53 (unpublished data) and BT74 with mutated p53, being the least sensitive15, 28. Further genetic analysis of the GSCs may identify signaling pathways responsible for the apoptotic response to DNA damage by etoposide in GSCs with mutant p53.
We show that etoposide and oHSV when combined cooperatively kill GBM cells including GSCs much more effectively than either agent alone. Some drugs can predispose cancer cells towards increased viral replication by increasing the expression of ribonucelotide reductase (RR) or GADD34 29, 30, which would amplify the progeny and spread of oncolytic virus to adjacent cells to enhance cytotoxicity as with temozolomide11. Although there appears a significant but minimal increase in the kinetics of viral replication after etoposide treatment in BT74 cells, over time the viral yield plateaus off and is similar to control (Figure 3). Negligible differences in G47Δ titers with or without etoposide treatment were seen regardless of the cell line or GSC tested, and, thus increased viral replication is not the cause of the additive to moderately synergistic cytotoxic effects observed with combination treatment (Figure 2, Table 1). Such effects on cell killing with no increase in viral replication have been observed previously with other chemotherapeutics such as paclitaxel, docetaxel and cisplatin12, 13. Importantly, we observed that unlike etoposide, SN-38, the active metabolite of irinotecan, inhibits G47Δ replication in U87 glioma cells even at low doses (Suppl. Figure 1). Accordingly the additive to synergistic cytotoxic responses observed with the G47Δ-plus-etoposide combination, were not observed with irinotecan. This difference of action between the two types of topoisomerase inhibitors may be related to the finding that topoisomerase II levels are found to be higher during the S phase and in proliferating cells, whereas topoisomerase I levels are almost constant during the cell cycle 31. The ability of etoposide to induce cells to enter the S or G2-M phases may make cells more sensitive to oncolysis by G47Δ. Such mechanisms have been proposed for treatment with adenovirus delta 24 and S-phase dependent treatments 12, 32.
We performed cell cycle analysis since both etoposide and oHSV have been reported to block the cell cycle at different phases. Etoposide alone induced predominantly a G2/M arrest, as reported by others4, and a decrease in G0/G1 cells. oHSV has been reported to cause a G1/S phase arrest33, but the most predominant effect of G47Δ in GSCs and U87 was the increase in Sub-G1. Treatment with the combination of etoposide and G47Δ resulted in a significant increase in the Sub-G1 population, and a decrease in the G2/M phase. This knowledge of cell cycle phases both in terms of the drug’s cytostatic effect and its ability to induce apoptosis may provide helpful information for designing future combination treatments34.
Although cells in sub-G1 may be undergoing apoptosis35, to directly measure apoptosis, we labeled DNA fragmentation, a classic irreversible apoptosis marker, using the TUNEL assay (Figure 4D). The combination of G47Δ with a low concentration of etoposide, greatly increased apoptosis over either treatment alone, in U87, BT74 and GBM4 cells. However, it is worth noting that other cell death pathways, such as autophagy, necrosis or pyroptosis (caspase-1-dependent inflammation), could also contribute to the enhanced efficacy of the combination in vivo. Etoposide has been reported to induce autophagy in certain cells36 and it is known that caspase activation can also be induced in glioma cells undergoing autophagy37. The differences in the extent of apoptosis amongst the cells may potentially be due to p53 status or activation of Cdk1 checkpoint but further studies may be required to understand this38.
Most chemotherapy regimens are inherently toxic when administered at high doses, and their therapeutic benefits are based upon the balance between maximizing antitumor effects and minimizing non-specific toxicities on normal cells. Multiple studies have shown that the anti-tumor activity of etoposide is schedule dependent, as smaller doses over several days or small daily doses result in higher response rates than a single large dose 39, 40. Our in vivo study was devised to utilize one injection of G47Δ, a clinically relevant oHSV, sandwiched within one cycle (5 day treatment) of low dose metronomic etoposide. This low dose etoposide treatment was very well tolerated by the animals as demonstrated by the absence of any weight loss or adverse effects. There has been evidence that multi-drug resistant tumors can be effectively targeted by low doses of cytotoxic drugs given at close regular intervals or metronomic doses with minimal toxic side effects 41. Recent observations also suggest that chemotherapeutic agents may have additional properties of therapeutic relevance such as anti-angiogenic effects through targeting of endothelial cells and beneficial effects on the regulatory arms of the immune system42–45. These may in part account for our observation of increased cleaved caspase 3 expression with etoposide treatment alone in vivo, despite the etoposide resistance of BT74, however this needs to be further investigated. It is important to note that although this dose of etoposide did not produce an improvement in survival on its own, the combination with G47Δ was effective at enhancing survival. Furthermore, the combination treatment of etoposide and G47Δ resulted in a significant increase in activated caspase 3 in vivo over either agent alone. Our findings have important implications for the design of protocols with combination treatment and can influence the construction of more rational oncolytic therapeutics16, 46. One such example is HSV-2 mutant (Delta-PK) in which the relatively limited virus replication is associated with a robust tumor cell killing via a bystander effect through functionally distinct proteases47.
A combination therapy regimen to combat a treatment-refractory cancer like GBM is likely to have a higher probability of success than treatments with single agents, given the need to counter a wide variety of possible resistance mechanisms. Indeed, the use of oral etoposide combinations such as with angiogenesis inhibitors, chemotherapy and/or radiation has demonstrated activity in a number of mouse tumor models and in patients 48, 49. As an FDA approved drug, etoposide is ideally suited for use in combination with G47Δ to treat primary or recurrent GBM. In summary, our data highlights the importance of exploring combinations of low dose chemotherapeutic agents with oHSV to treat drug resistant human GBMs without inducing toxic side effects. Additional studies will be needed to evaluate the optimal dosing and scheduling of combination oHSV with metronomic etoposide in human patients, along with genetic analysis to provide insights for future stratification of patients based on chemotherapy resistant mechanisms. However, metronomic chemotherapy can be cost effective and well-tolerated, and thus these data can reposition an existing drug into a new clinically-relevant role 50.
Supplementary Material
Translational Relevance.
Despite aggressive multi-modal therapies (including chemotherapy with etoposide), the outcome for patients with glioblastoma (GBM) has not improved much over the last three decades. Patients frequently encounter side effects of current chemotherapy regimens and more effective and less toxic strategies are needed to eradicate GBM including the glioblastoma stem cells (GSCs). This study shows for the first time that the topoisomerase inhibitors, etoposide and irinotecan, are effective at killing GSCs. More importantly, topoisomerase II inhibitor etoposide, but not topoisomerase I inhibitor irinotecan, can synergistically to additively cooperate with the oncolytic herpes simplex virus (oHSV-G47Δ), to induce apoptosis of GSCs and improve survival in mice with intracranial human GSC tumors without inducing toxic side effects. Since both etoposide and G47Δ have already been used independently in clinical trials, these findings provide support for a clinical trial utilizing the combination of low dose etoposide with G47Δ for the treatment of GBM.
Acknowledgments
Acknowledgements and Grant support
We would like to thank Melissa Marinelli for laboratory assistance and Katz Folz-Donahue and Laura Prickett-Rice from the Harvard Stem Cell Institute Flow Cytometry Core facility for assistance with cell cycle and Apo-BrdU analysis. These studies were supported by an NIH Grant NS-032677 (R.L.M) and an American Brain Tumor Association training fellowship (T.A.C).
References
- 1.Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S. Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting. Protein Cell. 2010;1:638–55. doi: 10.1007/s13238-010-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner JC, Brown LD, Cascino TL, Gerstner JB, Krook JE, Westberg MW, et al. Phase II evaluation of infusional etoposide and cisplatin in patients with recurrent astrocytoma. J Neurooncol. 1990;9:249–54. [PubMed] [Google Scholar]
- 3.Jeremic B, Grujicic D, Jevremovic S, Stanisavljevic B, Milojevic L, Djuric L, et al. Carboplatin and etoposide chemotherapy regimen for recurrent malignant glioma: a phase II study. J Clin Oncol. 1992;10:1074–7. doi: 10.1200/JCO.1992.10.7.1074. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents. 2005;5:363–72. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- 5.Ashley DM, Meier L, Kerby T, Zalduondo FM, Friedman HS, Gajjar A, et al. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14:1922–7. doi: 10.1200/JCO.1996.14.6.1922. [DOI] [PubMed] [Google Scholar]
- 6.Kakolyris S, Samonis G, Koukourakis M, Vlachonicolis I, Chalkiadakis G, Kalbakis K, et al. Treatment of non-small-cell lung cancer with prolonged oral etoposide. Am J Clin Oncol. 1998;21:505–8. doi: 10.1097/00000421-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101:1986–94. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 9.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ino Y. A clinical study of a replication-competent, recombinant herpes simplex virus type 1 (G47delta) in patients with progressive glioblastoma. 2009 [cited; Available from: http://apps.who.int/trialsearch/trial.aspx?trialid=JPRN-UMIN000002661.
- 11.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 12.Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–60. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai R, Wakimoto H, Cheema T, Rabkin SD. Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol. 2010;6:619–34. doi: 10.2217/fon.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 16.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A Novel Oncolytic Herpes Simplex Virus that Synergizes with Phosphatidylinositol 3-Kinase/Akt Pathway Inhibitors to Target Glioblastoma Stem Cells. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 18.Das CM, Aguilera D, Vasquez H, Prasad P, Zhang M, Wolff JE, et al. Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol. 2007;85:159–70. doi: 10.1007/s11060-007-9402-7. [DOI] [PubMed] [Google Scholar]
- 19.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 20.Linden CJ. Toxicity of intraperitoneally administered antitumour drugs in athymic rats. In Vivo. 1989;3:259–62. [PubMed] [Google Scholar]
- 21.Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–6. [PubMed] [Google Scholar]
- 22.Kroeger KM, Muhammad AK, Baker GJ, Assi H, Wibowo MK, Xiong W, et al. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discov Med. 2010;10:293–304. [PMC free article] [PubMed] [Google Scholar]
- 23.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–15. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 24.Frosina G. Frontiers in targeting glioma stem cells. Eur J Cancer. 2011;47:496–507. doi: 10.1016/j.ejca.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Nakai E, Park K, Yawata T, Chihara T, Kumazawa A, Nakabayashi H, et al. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Invest. 2009;27:901–8. doi: 10.3109/07357900801946679. [DOI] [PubMed] [Google Scholar]
- 26.Oh SY, Sohn YW, Park JW, Park HJ, Jeon HM, Kim TK, et al. Selective cell death of oncogenic Akt-transduced brain cancer cells by etoposide through reactive oxygen species mediated damage. Mol Cancer Ther. 2007;6:2178–87. doi: 10.1158/1535-7163.MCT-07-0111. [DOI] [PubMed] [Google Scholar]
- 27.Skladanowski A, Larsen AK. Expression of wild-type p53 increases etoposide cytotoxicity in M1 myeloid leukemia cells by facilitated G2 to M transition: implications for gene therapy. Cancer Res. 1997;57:818–23. [PubMed] [Google Scholar]
- 28.Meley D, Spiller DG, White MR, McDowell H, Pizer B, See V. p53-mediated delayed NF-kappaB activity enhances etoposide-induced cell death in medulloblastoma. Cell Death Dis. 2010;1:e41. doi: 10.1038/cddis.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett JJ, Adusumilli P, Petrowsky H, Burt BM, Roberts G, Delman KA, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–3. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 30.Passer BJ, Cheema T, Zhou B, Wakimoto H, Zaupa C, Razmjoo M, et al. Identification of the ENT1 antagonists dipyridamole and dilazep as amplifiers of oncolytic herpes simplex virus-1 replication. Cancer Res. 2010;70:3890–5. doi: 10.1158/0008-5472.CAN-10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holm C, Covey JM, Kerrigan D, Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989;49:6365–8. [PubMed] [Google Scholar]
- 32.Gomez-Manzano C, Alonso MM, Yung WK, McCormick F, Curiel DT, Lang FF, et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin Cancer Res. 2006;12:556–62. doi: 10.1158/1078-0432.CCR-05-1892. [DOI] [PubMed] [Google Scholar]
- 33.Ehmann GL, McLean TI, Bachenheimer SL. Herpes simplex virus type 1 infection imposes a G(1)/S block in asynchronously growing cells and prevents G(1) entry in quiescent cells. Virology. 2000;267:335–49. doi: 10.1006/viro.1999.0147. [DOI] [PubMed] [Google Scholar]
- 34.Evan GI, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr Opin Cell Biol. 1995;7:825–34. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 35.Hirose Y, Berger MS, Pieper RO. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61:1957–63. [PubMed] [Google Scholar]
- 36.Xie BS, Zhao HC, Yao SK, Zhuo DX, Jin B, Lv DC, et al. Autophagy inhibition enhances etoposide-induced cell death in human hepatoma G2 cells. Int J Mol Med. 2011;27:599–606. doi: 10.3892/ijmm.2011.607. [DOI] [PubMed] [Google Scholar]
- 37.Emdad L, Qadeer ZA, Bederson LB, Kothari HP, Uzzaman M, Germano IM. Is there a common upstream link for autophagic and apoptotic cell death in human high-grade gliomas? Neuro Oncol. 2011;13:725–35. doi: 10.1093/neuonc/nor053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastan MB, Canman CE, Leonard CJ. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995;14:3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- 39.Martin M, Lluch A, Casado A, Santabarbara P, Adrover E, Valverde JJ, et al. Clinical activity of chronic oral etoposide in previously treated metastatic breast cancer. J Clin Oncol. 1994;12:986–91. doi: 10.1200/JCO.1994.12.5.986. [DOI] [PubMed] [Google Scholar]
- 40.Needle MN, Molloy PT, Geyer JR, Herman-Liu A, Belasco JB, Goldwein JW, et al. Phase II study of daily oral etoposide in children with recurrent brain tumors and other solid tumors. Med Pediatr Oncol. 1997;29:28–32. doi: 10.1002/(sici)1096-911x(199707)29:1<28::aid-mpo5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haggerty TJ, Dunn IS, Rose LB, Newton EE, Martin S, Riley JL, et al. Topoisomerase inhibitors modulate expression of melanocytic antigens and enhance T cell recognition of tumor cells. Cancer Immunol Immunother. 2010;60:133–44. doi: 10.1007/s00262-010-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 44.Panigrahy D, Kaipainen A, Butterfield CE, Chaponis DM, Laforme AM, Folkman J, et al. Inhibition of tumor angiogeneisis by oral etoposide. Experimental and Therapeutic Medicine. 2010;1:739–46. doi: 10.3892/etm.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: changing the paradigm that more is better. Curr Oncol. 2009;16:7–15. doi: 10.3747/co.v16i2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S, Gao L, Yeagy B, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–9. [PubMed] [Google Scholar]
- 47.Colunga AG, Laing JM, Aurelian L. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 2010;17:315–27. doi: 10.1038/gt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khafif A, Canfield VA, Syzek EJ, Medina JE. Results of phase I-II trial of concomitant hyperfractionated radiation and oral etoposide (VP-16) in patients with unresectable squamous cell carcinoma of the head and neck. Am J Otolaryngol. 2003;24:1–5. doi: 10.1053/ajot.2003.7. [DOI] [PubMed] [Google Scholar]
- 49.Vaishampayan U, Fontana J, Du W, Hussain M. Phase II trial of estramustine and etoposide in androgen-sensitive metastatic prostate carcinoma. Am J Clin Oncol. 2004;27:550–4. doi: 10.1097/01.coc.0000135922.12198.e4. [DOI] [PubMed] [Google Scholar]
- 50.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–83. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.