Abstract
Background
Mechanisms underlying failure of influenza vaccine-induced antibody responses in HIV-infected persons are poorly understood.
Objective
To investigate innate immune factors regulating B cell function in HIV infected persons and to correlate them with serologic responses to H1N1/09 vaccine.
Methods
We evaluated immunologic characteristics of 17 HIV-infected patients and eight healthy controls (HC) at 0, 7 and 28 days (designated T0, T1 and T2) following a single 15 mcg dose of non-adjuvanted H1N1/09 influenza vaccine using Flow cytometry, ELISPOT and ELISA assays. All HC and nine patients (53%) seroconverted with >1:40 hemagglutination inhibition antibody titer at T2.
Results
In vaccine responders (R) and HC, serum levels of BAFF (B cell activating factor) and APRIL (a proliferation-inducing ligand) increased from T0 to T2 in conjunction with increases in frequencies of memory B cells. Concurrently, receptors for these factors showed changes, with increases in expression of TACI (transmembrane activator and calcium-modulator and cyclophilin ligand interactor) and decreases in BAFF receptor in memory B cells. IL-2 secreting cells and IgG antibody secreting cells increased at T2 in R and HC in ex-vivo H1N1 antigen stimulated cultures. These immunologic responses were not evident at T1 and were deficient in vaccine non-responder patients at T2. At T0, vaccine non-responders had lower frequencies of BAFF-R and TACI expressing memory B cells than responders.
Conclusion
Impaired memory B cell responses, deficiencies in serum BAFF and APRIL and alterations in their receptors on B cells were associated with failure of H1N1/09 influenza vaccine responses among virologically controlled HIV-infected patients.
Keywords: 2009 H1N1 vaccination and HIV, B cell defect in HIV, BAFF-binding receptors and HIV, Innate immune defect and HIV, T-independent humoral immune factors
INTRODUCTION
Infection with the novel influenza A H1N1/09 virus of swine-origin resulted in estimated 57 million cases in the US from April ’09 to Jan ’10 1. People <65 yrs age were deemed more susceptible due to lack of pre-existing immunity 2, 3 as this H1N1 strain was last associated with the 1918 influenza pandemic. In June ’09 a H1N1/09 influenza pandemic was declared by the World Health Organization, and the Centers for Disease Control recommended priority H1N1 vaccination of vulnerable populations including HIV-infected people 4.
Vaccination is effective in reducing the morbidity and mortality of influenza, and humoral immunity is a good predictor of protection against influenza virus infection 5. For the H1N1/09 influenza vaccine, an antibody (Ab) titer of >1:40 hemagglutination inhibition (HAI) units or a fourfold increase from baseline is considered as being protective 6. In the general population a single 15 mcg dose of non-adjuvanted H1N1/09 vaccine resulted in a 95% seroconversion rate 7, in contrast only 60% of virologically suppressed, immunologically stable HIV infected persons on combination antiretroviral therapy (cART) were found to seroconvert 8. In this context, boosters or adjuvanted H1N1 vaccines have had variable success in improving seroconversion rates 9, 10, and the immunologic basis for the failure of the H1N1 vaccine in HIV-infected persons is not well understood 11, 12. Peripheral B cell abnormalities with excessive cellular activation, impaired survival and altered maturation subsets in association with established HIV infection have been well-documented (reviewed in13). Even after virologic control with cART and CD4+ T cell recovery, the distribution of B cell subsets does not completely revert to normal in peripheral blood and the CD27+ memory B cells remain decreased in comparison to healthy uninfected donors 14, 15.
Vaccine-induced primary Ab responses are regulated by cooperative interactions of B cell intrinsic and extrinsic factors that are dependent upon a variety of accessory ligand/receptor interactions 16, 17. Under appropriate conditions of stimulation, naive B cells are activated to undergo maturation, proliferation and differentiation to generate Ab secreting cells 18. We have recently documented a role for the T cell derived cytokine IL-21 and IL-21R on B cells in the H1N1 vaccine response 15. Besides the T-B cognate interaction, there is increasing evidence that T-independent mechanisms can induce immunoglobulin (Ig) class switching and Ab production 19–21. In this context the contribution of the innate mediators belonging to the tumor necrosis family, BAFF (B-cell activating factor) and APRIL (a proliferation inducing ligand) take center stage because of their increasingly important role in B-lineage cell differentiation, class switch recombination and survival22–25. In the present study distinct changes in these innate helper factors and their receptors on B cells distinguished vaccine responders from non-responders. These studies provide insight for research directions towards delineating the molecular basis of impaired influenza vaccine responses and improvement in vaccination strategies.
METHODS
Patient characteristics and response to vaccination
Seventeen HIV-infected persons on potent cART who were given a single intramuscular dose (15 mcg; 0.5 ml) of inactivated monovalent A/California/07/2009 H1N1 vaccine (Novartis Vaccines and Diagnostics, Ltd.) as standard of care were recruited for this study during the 2009–2010 influenza season. Eight HIV uninfected persons served as controls. Summary characteristics of the study population are shown in Table 1. HAI antibody titers against H1N1 were performed at Bioqual, Inc Rockville, MD in serum samples using virus and turkey RBC 8. Immediately before vaccination (T0) H1N1 Ab titers were <1:20 in 16/17 patients and 80 in one. At 28 days post vaccination (T2), nine of 17 (52.9%) patients developed a titer of >1:40 (median, 640; range, 80–5,120) and were designated as H1N1 vaccine responders (R). In eight patients H1N1 antibody titers were <1:20 units post vaccination, and they were designated as H1N1 vaccine non-responders (NR). Of the 8 HC, pre-vaccine H1N1 Ab titers were <1:20 in seven and 1:40 in one; all developed a protective H1N1 Ab titer (median, 720; range, 80–20,480). Mean age, CD4 and CD8 T cell counts, frequencies of CD20+ B cells and plasma virus load (VL) were statistically not different between the patient groups at entry (Table 1). At T0, plasma HIV RNA was <50 copies/mL in 15 patients. In two NR, HIV RNA levels were 70 and 369 copies/mL respectively, but came down to <50 copies/mL on follow-up visits. The study was approved by the institutional review board of the University of Miami.
Table 1.
Characteristics of the study population and Ab responses to H1N1 vaccine
| Age | Gender | CD4+ T cells (cells/mm3) | CD8+ T cells (cells/mm3) | CD20+ B cells (%) | HIV RNA (copies/ml) | H1N1 Ab Titer | ||
|---|---|---|---|---|---|---|---|---|
| T0 | T2 | |||||||
| Vaccine Responders | ||||||||
| Mean | 46.9 | M=7, F=2 | 687 | 717 | 12.3 | ≤50 | <1:20 (n=8), 1:80 (n=1) | 1236.5 |
| Range | 37–57 | 126–1655 | 338–1227 | 4.2–19.1 | 80–5120 | |||
| SD | 8.6 | 463 | 280 | 4.9 | 1647.8 | |||
| Vaccine non-responders | ||||||||
| Mean | 43.7 | M=7, F=1 | 607 | 813 | 16.6 | 163 | <1:20 (n=8) | <20 |
| Range | 23–59 | 282–1177 | 327–1736 | 10.9–21.3 | <48–369 | |||
| SD | 15.1 | 309 | 424 | 4.2 | 178.7 | |||
| Healthy Controls | ||||||||
| Mean | 40.0 | M=2, F=6 | 18.7 | <1:20 (n=7), 1:40 (n=1) | 4220 | |||
| Range | 28–65 | 14.3–22.3 | 80–20480 | |||||
| SD | 13.5 | 2.8 | 7417.6 | |||||
T0, study entry; T2, day 28 post vaccination.
Processing of blood samples
Thirty mL of peripheral venous blood was collected at three time points: pre-vaccination (T0), day 7 (T1) and day 28 (T2) for isolation of peripheral blood mononuclear cells (PBMC) by density gradient isolation 26. Freshly isolated PBMC were processed for immunophenotyping and the remainder was cryopreserved in 10% DMSO and 90% fetal calf serum in liquid nitrogen for ELISPOT assays.
B cell characterization
Freshly isolated PBMC (1×106 cells) were stained with antibodies against surface antigens using CD3-Amcyan, CD20-allophycocyanin, CD10-PE-Cy7, CD27-Alexa700, or isotype antibodies (BD Biosciences) along with violet amine reactive live/dead cell discrimination dye. Samples were acquired on LSRII flow cytometer (BD biosciences) with collection of 200,000–500,000 events and analyzed using FlowJo software (TreeStar 8.8.6) as previously described 15. Live cells were first gated for CD3−CD20+ subset and sequentially on CD27 and CD10 to identify the CD27+CD10− memory B cells. Frequencies of memory B cells were calculated as a percentage of the total B cells, identified here as CD3−CD20+ lymphocytes. The total and memory B cell populations were further investigated for frequency and median fluorescence intensity (MFI) for BAFF-receptor, B cell maturation antigen (BCMA), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) and Ki67 22, 27 using reagents TACI-PE, BCMA-FITC (R&D systems), BAFFR-APC-Cy7 (Biolegend) and Ki67 (BD biosciences).
ELISPOT assays for H1N1 vaccine antigen stimulated IL-2, IFN-γ spot forming units (SFU) and IgG antibody secreting memory B cells (ASC) in PBMC
Cryopreserved PBMC were thawed and rested overnight and assayed by ELISPOT as previously described 28 for H1N1 antigen stimulated IL-2 and IFN-γ SFU following PBMC culture in triplicate for 24 hrs at 37°C in the presence or absence of 5 μg/ml H1N1 vaccine antigen or Staphylococcus aureus Cowan-1 strain (SAC, Sigma), 1/10,000 dilution as a positive control. Memory B cells were evaluated for IgG ASC following PBMC culture for 5 days at 37°C in the presence or absence of 5 μg/ml H1N1 vaccine antigen and SAC by ELISPOT 15 as previously described. In some experiments, IL-2, 100U/ml (R&D systems) was added to cultures together with H1N1 antigen at culture initiation. Spots were counted in an automated ELISPOT reader (Immunospot version 2.08, Cellular Technologies Ltd).
ELISA assays for serum levels of BAFF and APRIL
Briefly, serum diluted 1:1 was added in duplicate, 50 μl/well to ELISA plates for human BAFF (R&D systems) or APRIL (Bender Medsystems, Austria) followed by incubation for 2 hours at room temperature. Thereafter, plates were washed and developed as per manufacturer’s instructions. Values of BAFF were reported as pg/ml and APRIL as ng/ml. Sensitivity of the assays were 3.38 pg/ml for BAFF and 0.4 ng/ml for APRIL.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (version 4.01). Differences between pre- and post-vaccination values were assessed, within a group (paired 2-tailed) and between different groups (unpaired 2-tailed) using Student’s t test. Data for each group are presented as individual data points or box plots with median and 25th and 75th percentile borders and error bars at 10th and 90th percentiles. Correlation between variables was determined either by Pearson correlation or by linear regression analysis. P values < 0.05 were considered significant.
RESULTS
H1N1/09 vaccine responders show expansions of memory B cells post vaccination
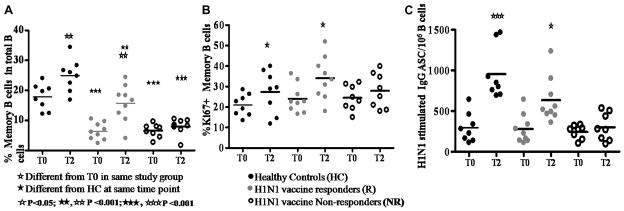
In agreement with previous studies by Moir et al 14, 29 and as reported previously 15, distribution of B cell maturation subsets, including memory B cells at T0 revealed differences between HC and HIV-infected patients, but the R and NR were not different from each other. At T2 however expansions in memory B cells occurred only in R patients and HC (Fig 1A). Concurrently, at T2, R patients and HC exhibited higher Ki67 expressing memory B cell subsets than NR (Fig 1B). The functional memory B cell response to ex-vivo stimulation of PBMC with H1N1 antigen for 5 days increased IgG ASC from T0 to T2 in R patients and in HC (Fig 1C) but not in NR patients. Thus NR patients did not show alterations in phenotypic or functional memory B cell from T0 to T2 following vaccination. B cell characteristics at T1 were not different from T0 in any group, and are not shown. The R and NR HIV+ patients were similar in SAC stimulated IgG secreting cell response in ELISPOT (3374±572.8 and 3161±549.5) respectively which were significantly less (p= <0.01) than response of HC (5181±896); these responses did not change at T1 or T2 in any group (not shown).
Figure 1. H1N1/09 vaccine responders show expansion of memory B cells.
Frequencies of (A), Frequencies of memory B cells (CD20+CD27+CD10−) in total (CD20+) B cells. (B), KI67+ memory B cells and (C), IgG producing ASC following ex-vivo stimulation with H1N1 antigen.
H1N1/09 vaccine responders show increase in levels of serum BAFF and APRIL post vaccination
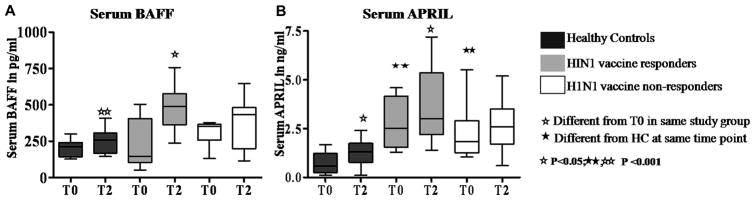
To examine mechanisms for the observed differences in memory B cells in the R and NR patient groups, we turned our attention to the receptor/ligand associations for the TNF ligand superfamily members BAFF and APRIL (Fig 2), which are known to be important for promoting B cell activation and immunoglobulin production. Serum BAFF levels were equivalent in the study groups at T0 and increased at T2 in HC and R patients, and not in NR patients (Fig 2A). Serum APRIL levels in patients, although higher than HC at T0 in both R and NR groups, increased significantly at T2 only in HC and R patients and not in NR patients (Fig 2B).
Figure 2. Serum BAFF and APRIL are increased in H1N1/09 vaccine responder patients.
Serum levels of BAFF and APRIL were measured by ELISA. (A), Serum levels of BAFF. (B), Serum levels of APRIL.
BAFF binding receptors (BBR) on B cells are differentially influenced by H1N1/09 vaccination
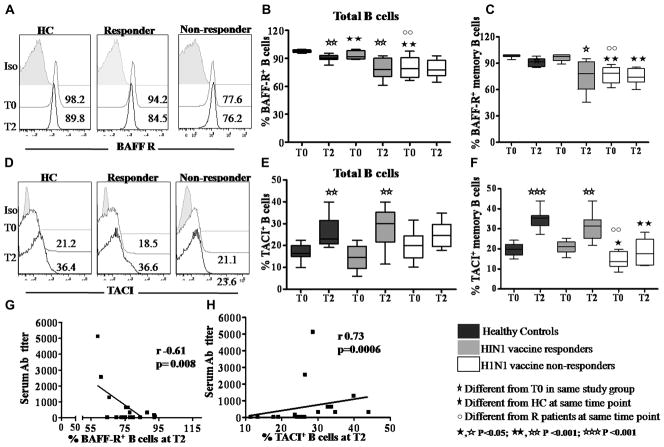
Emerging data in humans indicates that the expression of BBR is coordinately regulated during B cell differentiation. BAFF binds exclusively with high affinity to BAFF-R, and both BAFF and APRIL bind to TACI and BCMA25, 30. Frequencies and histograms of BAFF-R and TACI expressing B cells at T0 and T2 are shown in Figs 3A–F. In HC BBR were in the range of 95.7–99.7% for BAFF R, 9.9 – 22.4% for TACI and 0.6–2.9% for BCMA (not shown) and were similar in total B and memory B cells. At T0, compared to HC, R patients and NR patients had similar levels of TACI expression but significantly fewer BAFF-R expressing B cells. Following vaccination, at T2, frequencies of BAFF-R+ B cells decreased and of TACI+ B cells increased in HC and R patients but were unchanged in NR patients. In HIV infected patients, serum H1N1 Ab titer correlated inversely with BAFF-R+ total B cells (Fig 3G) and memory B cells (r= −0.75, p=0.0002; not shown) and directly correlated with TACI+ total B cells (Fig 3H) and memory B cells (r=0.61, p=0.005, not shown). Similar pattern of correlations were observed in HC; serum H1N1 antibody titer correlated inversely with BAFF-R+ total B cells (r=−0.46, p=0.003, not shown) and BAFF-R+ memory B cells (r= −0.55, p=0.002; not shown) and directly with TACI+ total B cells (r=0.63, p=0.007) and TACI+ memory B cells (r=0.66, p=0.002). Expression of BCMA did not change significantly between T0 and T2 (not shown).
Figure 3. Differential expression of BAFF-R and TACI in vaccine responder and non-responder patients.
Representative histograms showing frequency of (A) BAFF-R, (D) TACI in total B cells at T0 (grey) and T2 (black) compared to isotype controls (filled). Expression of BAFF-R+ in (B), total B cells; (C), memory B cells. TACI expression in (E), total B cells; (F), memory B cells. Correlation between the H1N1 Ab titer with (G), BAFF-R+ B cells and (H), TACI+ B cells.
IL-2 secreting cells are selectively induced ex-vivo in vaccine responders
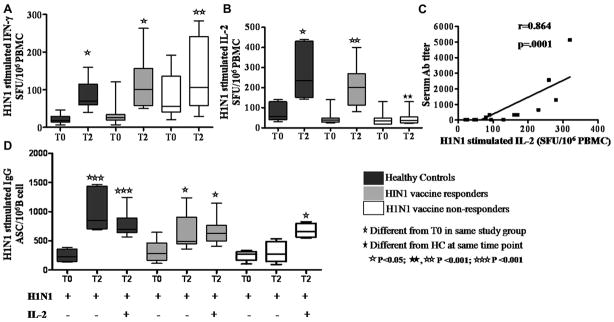
To evaluate H1N1-specific cell mediated adaptive T cell response, we performed IL-2 and IFN-γ ELISPOT assays following stimulation of PBMC with H1N1 vaccine antigen at T0 and T2 (Fig 4). An increase in IFN-γ SFU from T0 to T2 was noted in both patient groups and HC (Fig 4A) but increase in IL-2 SFU was observed only in HC and R patients and not in NR patients (Fig 4B). Moreover, H1N1 induced IL-2 SFU response at T2 in HC was significantly higher than in NR patients. Serum H1N1 antibody titers correlated with H1N1 stimulated IL-2 SFU at T2 in patients (Fig 4C) and in HC (r−0.73, p=0.005, not shown). We investigated if exogenous IL-2 supplementation at culture initiation would influence the H1N1-stimulated IgG ASC responses, particularly in NR patients (Fig 4D). Supplementation of cultures with exogenous IL-2 resulted in augmented H1N1 antigen stimulated IgG secreting cells at T2 in all 3 study groups.
Figure 4. H1N1 antigen-stimulated IL-2, IFNγ and IgG responses in healthy controls and HIV-infected patients pre- and post- H1N1 vaccination.
Frequencies of H1N1-stimulated (A), IFN-γ and (B), IL-2 producing SFU in PBMC at T0 and T2. (C), Correlation between the H1N1 Ab titer and IL-2 SFU in PBMC ELISPOT assay. (D), Mean H1N1-stimulated IgG ASC with and without exogenous IL-2 at T0 and T2.
DISCUSSION
Among HIV-infected patients on cART, a high proportion (47%) failed to seroconvert in response to the H1N1/09 non-adjuvanted inactive H1N1 vaccine and failed to expand phenotypic and functional memory B cells. Here we identified a novel association of vaccine responses in this population with increase in innate immune factors BAFF and APRIL in serum and changes of BBR in total and memory B cells. Importantly, frequencies of BAFF-R and TACI in memory B cells pre-vaccination were higher in R than in NR patients, while other B cell phenotypic characteristics were not distinguishable between the two groups. These findings underscore the importance of innate immunity in vaccine responses and uncover evidence of residual immune deficiency despite control of virus replication in a subset of HIV-infected patients.
Expansions of memory B cells in response to vaccination are an important benchmark of immune competence. The activation, proliferation and differentiation of naïve B cells in response to immunization generates plasmablasts in the germinal centers in draining lymph nodes and memory B cells that are and are readily identifiable in peripheral blood. Memory B cells expand upon re-exposure to the same antigen in vivo 31, 32 and develop into antibody secreting cells upon ex-vivo antigen stimulation15. The long lived CD10−CD27+ memory B cells increased at T2 and ex-vivo H1N1-stimulated memory ASC responses correlated with the H1N1 Ab titer in serum in vaccine responders but not in vaccine non-responders.
Mechanisms underlying vaccine-induced generation of memory B cells are multifactorial, comprised of B cell intrinsic and extrinsic factors 16, 17. To probe into the underlying mechanisms contributing to failure of H1N1 vaccine-induced B cell responses, we examined the TNF family of molecules BAFF and APRIL, the innate immune mediators secreted predominantly by accessory cells including monocytes, macrophages and dendritic cells. These factors are important sources of T-independent help for B cells and play definitive roles in regulating antibody responses 19, 33, 34. BAFF is known to deliver cell survival signals to B cells at different stages of development and differentiation 35. The enhancement of Ig isotype switching in naïve B cells by activated DC is also BAFF dependent, and is augmented by interaction of DC with activated T cells 19, 36. In respiratory syncitial virus (RSV) bronchiolitis in infants, BAFF and APRIL are locally expressed in respiratory epithelium and correlate with specific and total immunoglobulin levels in secretions in surviving infants 37. APRIL is important for survival of differentiated B cells and is also involved in Ig class switch to IgA 21, 24, 38. In our studies, BAFF and APRIL were both upregulated in serum at T2 following H1N1/09 vaccination in R patients and HC but not in NR patients. We did not examine these factors at T1 and it is possible that the increases in BAFF/TACI preceded the expansions in memory B cells.
Expression of BAFF binding receptors is coordinately regulated during B cell differentiation 22, 25, 30, 39. Of the three BAFF binding receptors, only BAFF-R is expressed in all differentiation stages of B cells prior to development of plasma cells, in immature, transitional, naïve and activated memory B cells.. Memory B cells express both BAFF-R and TACI and plasmablasts downregulate BAFF-R, retain TACI and acquire BCMA. The observed decrease in BAFF-R expression in memory B cells of vaccine responders post vaccination implies that the cells were undergoing differentiation following vaccination-induced activation for generating an effective Ab response 30, 39, 40. In contrast to BAFF-R, TACI was upregulated in responders post vaccination. TACI upregulation is known to occur rapidly after B cell activation and enables them to become responsive to BAFF and APRIL. TACI ligation also activates class switch recombination (CSR) in differentiating B cells 19, 30, promotes Ig isotype switch to IgA and facilitates plasma cell generation. In data not shown we observed that ex-vivo H1N1 antigen stimulation for 24 hours on day 180 post vaccination resulted in significant upregulation of TACI on B cells of vaccine responders but not in non-responders. BCMA, expressed primarily on plasmablasts, is believed to play a role in their differentiation into plasma cells 40–42 was present in low frequency in peripheral blood and was unaffected by H1N1 vaccination. Dysfunction of the cytokines BAFF/APRIL and BBR have been implicated in B cell mediated immunopathology resulting from excessive or insufficient signaling by these molecules. TACI mutations are associated with common variable immunodeficiency with Ig deficiency 43, 44. Chronically elevated serum BAFF and APRIL have been reported in progressive HIV infection 45, 46 In autoimmunity and hematopoietic malignancies, these factors are implicated in sustaining undesirable autoreactive or malignant cells through inhibition of apoptosis 47. To our knowledge this is the first description of the role of these TNF superfamily receptor/ligand molecules in facilitating influenza vaccine-induced B cell activation.
The innate immune factors describe herein do not minimize the importance of adaptive T cell responses in vaccine induced immunity. Quantitative loss of CD4 T cell correlates with impaired B cell responses to seasonal influenza vaccines in HIV infected patients 48. All patients were on cART, most with reconstitution of CD4 T cells. Qualitatively, the T cells were deficient in IL-2 responses in ELISPOT following ex-vivo H1N1 Ag stimulation, while IFN-γ responses were normal. These assays were performed in PBMC, and the main contributor of IFN-γ was most likely cytotoxic CD8 T cells, whereas CD4 T cells are presumed to be the main producers of IL-2 49. The addition of IL-2 to ELISPOT cultures resulted in low but significant enhancement of H1N1-stimulated ASC responses in all three study groups. IL-2 is well known for imparting T cell help to B cells in the presence of appropriate B cell activation signals 50. We speculate that IL-2 either acted directly on B cells or indirectly provided bystander help via stimulating secretion of other cytokines, and was able to facilitate activation and proliferation of Ag-specific B cells ex-vivo in each of the study groups. We have also observed lower levels of serum IL-21 post-HIN1 vaccination in vaccine non-responder HIV infected patients 15. Collectively, our findings concur with the concept that integrated signals from a wide spectrum of sources regulate the ability of B cells to differentiate into pathways resulting in generation of memory cells and protective antibody response17, 51. Investigation of the molecular basis for the failure of B cell responses, including transcriptional regulation of their survival, proliferation, differentiation, class switch and somatic mutation merit future investigation.
Despite the small sample size the data were striking and the proportion of patients who failed to seroconvert closely matched the published data 8, 10. Distinct and significant differences in immune responses were identified that set the NR apart from R patients and HC. HIV-infected patients who could successfully mount Ab responses to a single dose of non-adjuvanted H1N1 vaccine had demonstrable expression of innate T-independent B cell helper factors APRIL and BAFF as well as T-dependent cytokine IL-2, with expansion of memory B cells, upregulation of TACI and downmodulation of BAFF-R. These immunologic responses were deficient in vaccine non-responder patients and add to the known HIV associated B cell hyperactivation, dysfunction and residual abnormalities in chronically infected patients on cART 14. Our studies point to the need for continuing investigations to examine the cumulative signaling elements that control B cell differentiation 52 for insight into novel adjuvant strategies for local and systemic vaccines, in which T cell-independent and T cell-dependent stimuli synergize to optimally stimulate B lymphocytes. A focus on T-independent factors that can shape the primary antiviral antibody response of B cells following vaccination may be critically important in an immune deficient setting such as HIV/AIDS where cognate T cell help may be suboptimal.
Acknowledgments
The Laboratory Sciences Core of the Developmental Center for AIDS Research facilitated conduct of the flow cytometry studies and Dr. Kristopher L. Arheart of the Clinical Sciences Core provided statistical input. We thank Drs Wasif Khan, Bonnie Blomberg and Daniela Frasca of the Department of Microbiology and Immunology for helpful discussions and the patients for their participation in this study.
Source of funding: This work was supported by NIH grant A1077501 to SP and a specialty laboratory grant from International Maternal, Pediatric and Adolescent AIDS Clinical Trials Group (IMPAACT). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632].
Abbreviations
- cART
combination antiretroviral therapy
- R
Vaccine responder patients
- NR
vaccine non-responder patients
- ASC
antibody secreting cells
- BAFF
B-cell activating factor
- APRIL
a proliferation inducing ligand
- TACI
transmembrane activator and calcium-modulator and cyclophilin ligand interactor
- HAI
hemagglutination inhibition
- BCMA
B cell maturation antigen
Footnotes
Potential conflict of interest: none
Clinical Implications: The investigations reported indicate that some HIV infected people on antiretroviral therapy did not respond to the monovalent HINI/09 vaccine during the 2009 epidemic because of underlying immune defects.
References
- 1.Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–8. [PubMed] [Google Scholar]
- 2.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106:20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 4.Klein MB, Lu Y, DelBalso L, Cote S, Boivin G. Influenzavirus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis. 2007;45:234–40. doi: 10.1086/518986. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot HK, Keitel W, Cate TR, Treanor J, Campbell J, Brady RC, et al. Immunogenicity, safety and consistency of new trivalent inactivated influenza vaccine. Vaccine. 2008;26:4057–61. doi: 10.1016/j.vaccine.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 8.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–92. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 9.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, Stephan C, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–5. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 10.Ho J, Moir S, Wang W, Posada JG, Gu W, Rehman MT, et al. Enhancing effects of adjuvanted 2009 pandemic H1N1 influenza A vaccine on memory B-cell responses in HIV-infected individuals. AIDS. 2011;25:295–302. doi: 10.1097/QAD.0b013e328342328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–80. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O’Shea MA, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–9. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 15.Pallikkuth S, Kanthikeel SP, Silva YS, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 Receptor on B cells and IL-21 secretion distinguishes Novel 2009 H1N1 vaccine responders from non-responders among HIV infected persons on combination antiretroviral therapy. J Immunol. 2011;186:6173–81. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–50. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 18.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–25. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 19.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 21.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 24.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 26.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 27.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–82. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 28.Buseyne F, Catteau A, Scott-Algara D, Corre B, Porrot F, Rouzioux C, et al. A vaccinia-based elispot assay for detection of CD8+ T cells from HIV-1 infected children. J Immunol Methods. 2005;298:105–18. doi: 10.1016/j.jim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–7. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 31.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 33.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–8. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 35.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–17. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol Cell Biol. 2005;83:554–62. doi: 10.1111/j.1440-1711.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 37.Reed JL, Welliver TP, Sims GP, McKinney L, Velozo L, Avendano L, et al. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis. 2009;199:1128–38. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 38.Geha RS. Role of APRIL in the mucosal Iga antibody response. J Pediatr Gastroenterol Nutr. 2005;40 (Suppl 1):S27. doi: 10.1097/00005176-200504001-00015. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–88. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 40.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–54. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi J, Liu C, Aghamohammadi A, Bergbreiter A, Du L, Lu J, et al. Novel mutations in TACI (TNFRSF13B) causing common variable immunodeficiency. J Clin Immunol. 2009;29:777–85. doi: 10.1007/s10875-009-9317-5. [DOI] [PubMed] [Google Scholar]
- 44.Poodt AE, Driessen GJ, de Klein A, van Dongen JJ, van der Burg M, de Vries E. TACI mutations and disease susceptibility in patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156:35–9. doi: 10.1111/j.1365-2249.2008.03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS. 2003;17:1983–5. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine J, Chagnon-Choquet J, Valcke HS, Poudrier J, Roger M, Montreal Primary HIVI, et al. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood. 2011;117:145–55. doi: 10.1182/blood-2010-08-301887. [DOI] [PubMed] [Google Scholar]
- 47.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–47. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 48.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–50. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 49.North ME, Ivory K, Funauchi M, Webster AD, Lane AC, Farrant J. Intracellular cytokine production by human CD4+ and CD8+ T cells from normal and immunodeficient donors using directly conjugated anti-cytokine antibodies and three-colour flow cytometry. Clin Exp Immunol. 1996;105:517–22. doi: 10.1046/j.1365-2249.1996.d01-795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingari MC, Gerosa F, Carra G, Accolla RS, Moretta A, Zubler RH, et al. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984;312:641–3. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- 51.Kurosaki T, Aiba Y, Kometani K, Moriyama S, Takahashi Y. Unique properties of memory B cells of different isotypes. Immunol Rev. 2010;237:104–16. doi: 10.1111/j.1600-065X.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 52.Gauld SB, Dal Porto JM, Cambier JC. B cell antigen receptor signaling: roles in cell development and disease. Science. 2002;296:1641–2. doi: 10.1126/science.1071546. [DOI] [PubMed] [Google Scholar]