Abstract
Background
Thyroid papillary microcarcinoma (TPMC) is an incidentally discovered papillary carcinoma that is ≤ 1.0 cm in size. Most TPMCs are indolent, whereas some behave aggressively. The aim of the study was to evaluate whether the combination of BRAF mutation and specific histopathological features allows risk stratification of TPMC.
Methods
A group of aggressive TPMC was selected based on the presence of lymph node metastasis or tumor recurrence. A group of non-aggressive tumors included TPMCs matched for age, gender, and tumor size, but with no extrathyroidal spread. Molecular analysis was performed and histological slides were scored for multiple histopathological criteria. A separate validation cohort of 40 TPMC was evaluated.
Results
BRAF mutation was detected in 77% of aggressive TPMC and 32% of non-aggressive tumors (p=0.001). Several histopathological features showed significant difference between the groups. Using multivariate regression analysis, a molecular-pathological (MP) score was developed that included BRAF status and three histopathological features: superficial tumor location, intraglandular tumor spread/multifocality, and tumor fibrosis. By adding the histologic criteria to BRAF status, sensitivity was increased from 77% to 96% and specificity from 68% to 80%. In the independent validation cohort, the MP score stratified tumors into low, moderate, and high risk groups, with the probability of lymph node metastases or tumor recurrence of 0, 20%, and 60%, respectively.
Conclusions
BRAF status together with several histopathological features allow clinical risk stratification of TPMC. The combined molecular-pathological risk stratification model is a better predictor of extrathyroidal tumor spread than either mutational or histopathological findings alone.
Keywords: thyroid cancer, papillary microcarcinoma, BRAF, histopathologic analysis
INTRODUCTION
Thyroid papillary microcarcinoma (TPMC) is a type of thyroid cancer defined by the World Health Organization Histological Classification of Tumors by two criteria: (i) 1 cm or smaller in size and (ii) an incidental finding on histopathological examination after lobectomy or total thyroidectomy performed for a larger adenoma or nodular hyperplasia.1 TPMCs are very common. In autopsy series, they are found in 3–9% of thyroid glands in many regions of North America, Europe, and South America,2–9 and appear with even higher incidence in systematically performed thyroid gland examinations in Japan (28%) and Finland (36%).10, 11 In surgical series, TPMC is reported in 5–17% of patients who have a thyroidectomy for benign lesions.12–14 Its prevalence continues to rise. In fact, TPMC represents the fastest growing type of thyroid cancer.15 Based on SEER data, papillary carcinomas 1 cm or less constituted about 30% of all papillary carcinoma in 1988 and about 40% in 2003,16 making it the most common variant of papillary carcinoma in the United States. Similar trends have been observed in France17 and many other countries around the world (reviewed in ref.18).
Clinical management of patients with TPMC remains non-standardized. These tumors are generally considered clinically innocuous, although some have an aggressive clinical behavior. A meta-analysis encompassing over 4000 cases of papillary carcinoma ≤1 cm reported found that 28% of tumors had lymph node metastasis, 0.6% distant metastasis, 3.3% of patients experienced disease recurrence, and tumor-related mortality was 0.3%.19 This suggests that as an entity, TPMCs includes at least two biologically distinct subpopulations: indolent tumors with minimal or no potential for progression, and tumors with the propensity for aggressive behavior and dissemination. The ability to stratify those relatively few patients with aggressive TPMC from the vast majority who are low-risk is crucial to offer most appropriate clinical management.
The risk factors associated with aggressive behavior of TPMC are not well defined. One of the more consistently reported features associated with the risk of tumor recurrence or metastasis is tumor multifocality or bilaterality.20–22 However, tumor multifocality is common and is found in as many as 30–40% of all TPMC.19, 23 Multifocality therefore cannot be used by itself as an accurate marker of tumor virulence. Studies demonstrate that extensive fibrosis in TPMC is associated with more frequent lymph node or distant metastases.22, 24 In some reports, the metastatic spread of TPMC was found to be limited to tumors of larger size, such as >8 mm18 or ≥5 mm,21 although other observations have found that tumor size does not correlate with aggressive disease.25 One study found that familial TPMCs were more aggressive than sporadic tumors,26 although other reports have not confirmed this observation.18, 22 A recent meta-analysis of 17 studies revealed that recurrence of TPMC was associated with younger age (<45 years), tumor multifocality, and lymph node metastasis at presentation, whereas no association with gender, tumor size, and extrathyroidal extension was found.27
More recently, genetic markers have been explored to assess tumor behavior in papillary thyroid carcinoma, including TPMC. Many studies have demonstrated the association between BRAF V600E mutation and aggressive histopathological features of papillary carcinoma, risk of tumor recurrence, and cancer-related death (reviewed in ref. 28). Several recent studies of TPMC have described correlation of BRAF V600E with extrathyroidal extension,29–32 and advanced stage at presentation,30–32 lymph node metastasis,31, 32 tumor size >5 mm,30 and multifocality.32 However, in these studies a BRAF mutation was present in 24–63% of TPMC,29–32 and it is unlikely that such a large proportion of these tumors would have aggressive behavior. Therefore, the presence of a BRAF mutation in any given tumor is not an absolute predictor of tumor aggressiveness.
The aim of this study was to evaluate the role of BRAF mutational status together with several specific histopathological tumor characteristics in identification of the subset of TPMC that demonstrate more aggressive behavior such as extrathyroidal spread. We also propose a relatively simple molecular-pathological score for risk stratification and tested its performance in an independent validation cohort of TPMC.
MATERIALS AND METHODS
Selection Criteria and Cohort Assignment
With the approval of the University of Pittsburgh Institutional Review Board (IRB), the deidentified data of 2377 patients treated at the hospitals of our university system from 1991–2004 with an inpatient or outpatient ICD-9 CM diagnosis code of 193 (thyroid malignancy) were retrieved. Among them, 888 had surgical pathology reports available electronically to confirm both tumor diagnosis and size. Of those, 403 (45%) patients had papillary cancer ≤1.0 cm without evidence of other thyroid malignancy and were used to form the original cohort for the study.
Out of 403 confirmed TPMC patients, 41 patients (10%) had cervical lymph node metastasis identified during initial pathological examination or during subsequent clinical follow-up. These patients were matched for age, gender, and tumor size to 41 TPMC patients who did not have either local metastasis or tumor recurrence. Cases from both groups that could not be further studied due to unavailability of paraffin blocks or exhaustion of tumor tissue in additional sections taken for molecular analysis were excluded, leaving 29 patients in the group with clinically aggressive TPMC, subsequently referred to as Group A of the original cohort. After similar exclusions, 30 of the original 41 cases selected as matched controls without aggressive features remained and are subsequently referred to as Group B. Groups A and B compose the original cohort used in analysis. There was no significant difference in the extent of initial surgery (total thyroidectomy vs. lobectomy) between the two groups, although completion of thyroidectomy was more common in Group A (52% vs. 17%). Since none of the tumors in either group were diagnosed preoperatively, they represented true TPMC as defined by strict WHO criteria.
In Group A, 27 out of 29 patients had lymph node metastasis detected at surgery. The follow-up for patients in this cohort ranged from 0–11.6 years, mean 5.3 years. Six patients showed recurrence in the regional lymph nodes, on average 4.3 years after initial surgery. Among these six patients, four had lymph node metastases detected during initial surgery and two had no positive lymph nodes at presentation. In Group B, patients were followed for 0–16.9 years (mean, for 4.8 years). During surveillance, none had evidence of recurrence based on serum thyroglobulin measurements, neck ultrasonography and clinical examination.
A validation cohort (Group C) was selected from cases retrieved from the year 2008 to avoid overlap with groups A and B. The validation cohort contained 40 TPMC cases, including 39 consecutive cases and one additional case of aggressive TPMC known to the authors, where tumor had thyroid bed soft tissue recurrence14 months after surgery. The follow-up in this cohort ranged from 0–2.8 years (mean, 1.5 years). Four patients had local lymph node metastasis detected at surgery, and one has been diagnosed with pulmonary metastases 2.8 years after initial surgery.
Histopathologic Evaluation
For all cases, surgical pathology reports were examined and histological slides were reviewed independently by two pathologists (L.N. and Y.E.N.) to evaluate and score the following microscopic features: anatomic location of TPMC (left lobe, right lobe or isthmus), tumor size, tumor location with respect to thyroid surface/capsule (superficial vs. intrathyroidal, as described below), status of surgical margins, presence of infiltrative tumor border (as opposed to smooth pushing border), tumor growth pattern (classic papillary, follicular, tall cell), intraglandular spread or tumor multifocality (IGS/MF), extrathyroidal extension, degree of fibrosis (as defined below), presence of psammoma bodies, and presence of significant lymphocytic thyroiditis in non-neoplastic thyroid tissue consistent with Hashimoto's thyroiditis. The distance from the edge of tumor to the closest margin of resection was measured.
Superficial/subcapsular tumor location was defined as location immediately adjacent to perithyroidal adipose tissue, i.e. with no benign thyroid tissue seen between the tumor and the extrathyroidal soft tissues. IGS/MF was defined as the presence of either (i) two or more separate tumors, or (ii) smaller tumor aggregates located close to the main tumor separated by a layer of normal thyroid parenchyma, or (iii) tumor cells within lymphatic channels, or (iv) isolated psammoma bodies located in thyroid stroma outside the tumor. Tumor fibrosis was scored as none, 1+, and 2+, and was defined as follows: 1+ fibrosis - mild fibrosis with the presence of few inconspicuous, delicate fibrous areas within or at the periphery of the tumor nodule, and 2+ fibrosis - moderate/extensive fibrosis that was clearly recognizable, with multiple fibrotic bands within and at the periphery of the tumor. Fibrotic tumor capsule alone, i.e. without significant fibrosis within the tumor, was not sufficient to score tumor fibrosis as 2+.
Molecular Analysis
For each tumor case, five unstained tumor slides were obtained and microdissected to collect tumor tissue. Molecular analysis was performed to test for BRAF V600E mutation and the three most common RAS mutations, including those at NRAS codon 61, HRAS codon 61, and KRAS codons 12/13. Detection of these mutations was performed using real-time PCR and fluorescence melting curve analysis (FMCA) from DNA as previously reported.33, 34 Briefly, a pair of oligonucleotide primers flanking the mutation site was designed, together with two fluorescent probes. The reaction mixture was subjected to 45 cycles of rapid PCR amplification. Post-amplification FMCA was performed by gradual heating of samples at a rate of 0.2°C/sec from 45°C to 95°C. All PCR products that showed deviation from the wild-type (placental DNA) melting peak were sequenced to verify the presence of mutation.
Statistical Analysis
Chi-squared test was used to estimate difference in accuracy between different approaches. The difference between the frequency of each feature in the two study cohorts was compared using a univariate logistic regression model for continuous variables and Fisher's exact test for categorical variables; p-values less than 0.05 were considered as statistically significant without multiple comparison correction.
Multivariate analysis and logistic regression were used to model the data, analyzing outcomes by study cohort, i.e. by defined factors of clinical aggression. Whether a tumor was assigned to the aggressive group or non-aggressive group was considered as the outcome. All evaluated factors (e.g. BRAF, tumor location) were considered as the predictors. Statistic package R was used to perform statistical computing (http://www.r-project.org/). When building the multivariate regression model, patient age, gender, and tumor size were excluded from the analysis since initially the groups were build by matching these parameters. Step-wise variable selection in the regression model was manually performed. Parameters were selected based on (i) predictability of a feature determined by its p-value in the univariate model (only variables with p-values less than 0.01 were selected as candidates for the multivariate model), (ii) overall performance of the multivariate model, and (iii) p-value of each parameter estimates in the multivariate model.
RESULTS
The histopathologic and molecular features of the 29 TMPCs noted in patients in Cohort A (tumors with aggressive features) and the 30 control patients in Cohort B (tumors without aggressive features) are summarized in Table 1.
Table 1.
Histopathologic and molecular features of TPMCs in two groups of the original cohort
| Group A (aggressive TPMCs) n= 29 | Group B (non-aggressive TPMCs) n= 30 | p value | |
|---|---|---|---|
| Anatomic location: | 0.88 | ||
| - Left lobe | 15 (52%) | 14 (46.7%) | |
| - Right lobe | 14 (48%) | 14 (47%) | |
| - Isthmus | 5 (17.2%) | 2 (7%) | |
|
| |||
| Tumor location: | <0.0001 | ||
| - Superfocial/subcapsular | 27 (93%) | 9(30%) | |
| - Intrathyroidal | 3 (10%) | 21 (70%) | |
|
| |||
| Distance to margin (cm), (mean±SD) | 0.134 ± 0.09 | 0.833 ± 1.05 | <0.0001 |
|
| |||
| Positive resection margin | 2 (7%) | 1 (3%) | 0.530 |
|
| |||
| Extrathyroidal extension | 19 (66%) | 4 (13.3%) | <0.0001 |
|
| |||
| Infiltrative border | 29 (100%) | 24 (80%) | 0.003 |
|
| |||
| Growth pattern: | |||
| - Classic papillary | 13 (45%) | 11 (37.0%) | 0.600 |
| - Follicular | 8 (28.0%) | 18 (60%) | 0.018 |
| - Tall cell | 8 (28%) | 1 (3%) | 0.012 |
|
| |||
| Tumor fibrosis: | |||
| - No | 2 (7%) | 9 (30%) | 0.042 |
| - 1+ | 3(10%) | 9 (30%) | 0.104 |
| - 2+ | 24 (83%) | 12 (40%) | 0.001 |
|
| |||
| Intraglandular spread/multifocality | 26 (90%) | 13 (43%) | <0.0001 |
|
| |||
| Psammoma bodies | 11(38%) | 7 (23%) | 0.22 |
|
| |||
| Hashimoto Thyroiditis | 10 (35%) | 8 (27%) | 0.51 |
|
| |||
| Molecular analysis: | n= 26 | n= 25 | |
| BRAF | 20 (77%) | 8 (32%) | 0.001 |
| NRAS 61 | 0 | 1 (4%) | |
| HRAS 61 | 0 | 1 (4%) | |
| KRAS 12/13 | 0 | 0 | |
Seven histopathological features showed significant difference between the groups. These features included superficial (subcapsular) tumor location, extrathyroidal extension, infiltrative border, follicular growth pattern morphology, tall cell features, tumor fibrosis, and intraglandular tumor spread/multifocality (IGS/MF). The combined category of IGS/MF was different between the two cohorts with a high degree of statistical significance, although the frequency multifocality alone did not differ between groups, which may be due to the criteria applied to distinguish between tumor multifocality and intraglandular spread.
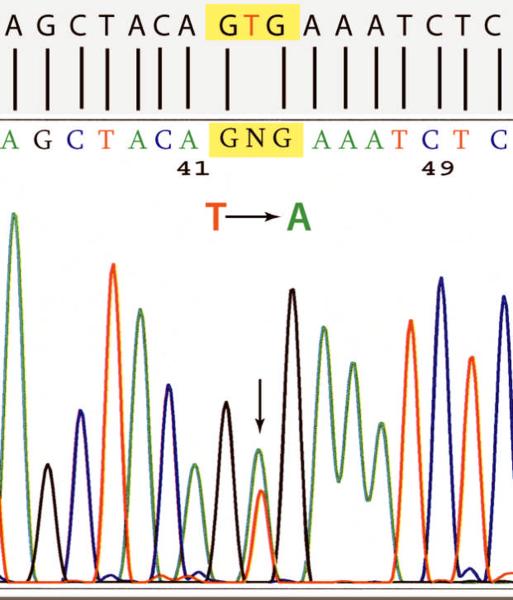
Molecular analysis was informative in 26 aggressive (group A) and 25 non-aggressive (group B) TPMC, and was uninformative for 8 tumors which were less than 3 mm in size and yielded insufficient amounts of DNA for testing. BRAF V600E mutations were detected in 20 aggressive TMPC (77%) compared to 8 (32%) non-aggressive TPMC (p=0.001) (Fig. 1). RAS mutations were present in 2 TPMCs from Group B (1 NRAS codon 61 and 1 HRAS codon 61) and were not observed in any aggressive tumors.
Figure 1.
Detection of a T→A substitution at nucleotide 1799 of the BRAF gene leading to V600E mutation in a TPMC using Sanger sequencing.
Stepwise regression analysis was used to identify the smallest set of parameters providing best separation between the two cohorts (Table 2). The set included four parameters: BRAF mutation status, tumor location (superficial), significant (2+) fibrosis, and IGS/MF (Fig. 2–4). The inclusion of additional evaluated histopathologic parameters did not improve the separation between the cohorts. As evident from Table 2, each of the four predictive parameters, when positive, contributed differently to separation of the two study cohorts. For practical use, the coefficients were rounded to the nearest integer for each included factor. The resulting weighted molecular-pathological scoring algorithm was as follows: MPW score = 4× Superficial tumor location + 3× BRAF(+) + 3× IGS/MF(+) + 2× Fibrosis (2+).
Table 2.
The multivariate regression model parameters, coefficients estimates and corresponding p-values
| Estimate | Std. Error | Z value | P value | |
|---|---|---|---|---|
| Intercept | −7.39 | 2.40 | −3.08 | 0.002* |
| BRAF mutation | 2.69 | 1.20 | 2.24 | 0.024* |
| Superficial tumor location | 3.67 | 1.26 | 2.91 | 0.004* |
| Intraglandular spread/Multifocality | 3.12 | 1.29 | 2.41 | 0.016* |
| Fibrosis (2+) | 1.69 | 1.18 | 1.43 | 0.15 |
Statistically significant on multivariate analysis
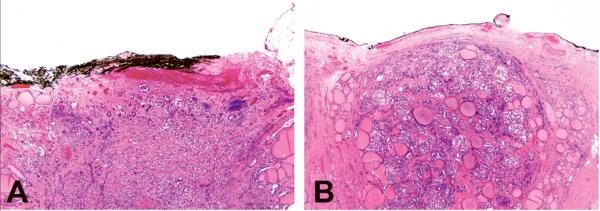
Figure 2.
Superficial tumor location as a histological feature contributing to the molecular-pathological score included tumor location immediately at the surface of the thyroid, with extrathyroidal extension (A) or without extrathyroidal extension (B).
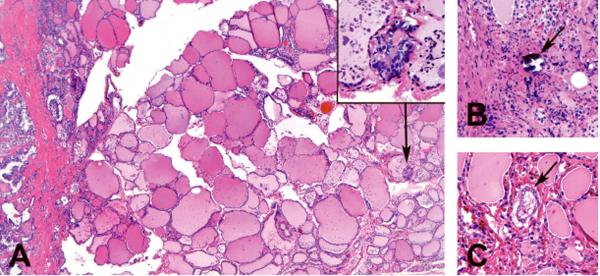
Figure 4.
Criteria for intraglandular tumor spread (IGS) included (A) small tumor focus separated from the main tumor mass by a layer of benign thyroid parenchyma (arrow and inset), (B) isolated psammoma bodies in the thyroid stroma (arrow), or (C) tumor aggregates within the lymphatic channels(arrow).
Scores from 0–7, and 8–12 correctly identified non-aggressive and aggressive TPMCs respectively with 96% sensitivity and 80% specificity. Scores of 0–7 correlated with the presence of 0–2 of the 4 features evaluated. Similarly, scores of 8–12 corresponded to the presence of 3–4 features. This allowed a simplified, unweighted version of the molecular-pathological score utilizing a sum of features: MPU score = Superficial tumor location + BRAF(+) + IGS/MF(+) + Fibrosis (2+). The simpler MPU score achieved identical sensitivity (96%) and specificity (80%) as compared to the MPW score.
The combined MP score provided a significantly better accuracy for detection of TPMCs with aggressive features than BRAF status alone (p=0.04). The sensitivity for identifying tumors with aggressive features was 77% based only on the presence of BRAF V600E but increased to 96% when the full MP model was applied. Similarly, specificity increased in the original cohort cases from 68% when BRAF only was considered to 80% when cases were evaluated with the combined MP score.
The performance of the MP score was validated in an independent set of 40 TPMCs. Among tumors in this validation cohort, 5 had aggressive features: 4 had lymph node metastasis at presentation including one patient who also developed pulmonary metastases 34 months later, and one had tumor recurrence in the thyroid bed 14 months after surgery. An MPW score of 8–12 was able to detect aggressive tumors with 100% sensitivity and 71% specificity. Identical sensitivity and specificity were again obtained when the simplified MPU score was applied. Two aggressive tumors in this set had no BRAF mutation, but both had aggressive histology and therefore were correctly identified by the MP analysis. Specificity of the MP score in the validation cohort was lower than in the original cohort (71% vs. 77%). This resulted from an increased number of cases with higher MP scores that lacked overt clinically aggressive behavior or recurrence. This is likely due to the significantly shorter follow up in the validation cohort as compared to the original cohort. It is conceivable that some of the tumors with high MP scores that did not demonstrate clinically evident disease would recur eventually with longer follow up, as one patient had already done so far.
The MP score distribution in the validation cohort of tumors suggested that scoring could be further subdivided to resolve three distinct risk groups, i.e. low, moderate and high risk. Using either version of the scoring, the 3-tiered respective observed probabilities of aggressive behavior, i.e. lymph node metastasis or tumor recurrence, was then found to rise stepwise from 0, 20%, and 60%, respectively (Table 3). The risk stratification remained quite accurate to predict the risk of tumor recurrence alone. Indeed, among 7 tumors with documented anatomic recurrence on follow-up (6 from original cohort and 1 from validation cohort), 6 tumors had the MPW score of 12 and equivalent MPU score of 4, falling into a high risk group, and 1 tumor had the MPW score of 10 and MPU score of 3, corresponding to a moderate risk tier.
Table 3.
MP scores in the validation cohort of TPMCs and risk of more aggressive tumor behavior
| MPU score | 0–2 | 3 | 4 |
|
| |||
| MPW score | 0–7 | 8–10 | 12 |
|
| |||
| TPMCs with aggressive behavior (n=5) | 0 | 2 | 3 |
|
| |||
| TPMCs with no evidence of aggressive behavior (n=35) | 25 | 8 | 2 |
|
| |||
| Risk category (Probability of extrathyroidal spread or recurrence) | LOW (0) | INTERMEDIATE (20%) | HIGH (60%) |
The validation cohort allowed an estimation of the proportion of TPMCs that fall into each risk group based on the MP score. Based on this cohort, 64% of tumors would be placed in low risk category (MPU score 0–2), 26% of tumors in intermediate risk category (MPU score 3), and 10% in high risk category (MPU score 4).
DISCUSSION
In this study, we identify a number of molecular and histopathological features that correlate with more aggressive behavior of TPMC, and offer a practical and simple scoring system to assess the clinical behavior of this common type of thyroid cancer. The scoring system relies on BRAF mutation status and three histopathological features to assign tumors to one of three risk categories. Neither mutational status for BRAF V600E nor histologic features alone were adequate to provide accurate risk stratification.
The BRAF V600E mutation has been found in many studies to correlate with more aggressive behavior of thyroid papillary carcinoma.28 Although the reported data on TPMCs are more limited, this mutation has been found to correlate with a number of more aggressive histopathological characteristics of these tumors in several recent studies.29–32 In this study, we observed that BRAF V600E mutation was highly prevalent in TPMC with metastatic spread to lymph nodes or tumor recurrence as compared to a matched control cohort of non-recurrent TPMC without metastatic spread. The difference between the two cohorts was significant on univariate and multivariate analyses. As a result, BRAF mutation status was included as an important component of the combined molecular-pathological scoring algorithm. However, BRAF mutation status alone was not sufficient to reliably identify all TPMCs with aggressive characteristics. Specifically, among aggressive tumors in the original and validation cohorts, 8 had no BRAF mutation, but all had aggressive histology, and would not have been identified if molecular analysis only was performed. Conversely, the histopathological features alone were not adequate to identify all tumors demonstrating aggressive clinical features. In the original cohort, among tumors with aggressive clinical features, 20 tumors were BRAF positive. However, only 13 of these tumors also demonstrated all three aggressive histologic features that would be sufficient to render an aggressive MP score. The remaining 7 had only two aggressive histologic features and would have been missed without molecular assessment for BRAF mutation.
Tumor extrathyroidal extension, multifocality, and significant tumor fibrosis have been associated with aggressive behavior of TPMCs in previous studies.20–22, 24 Our series confirm these findings. However, we observed that not only was extrathyroidal extension associated with aggressive features of TPMCs, but the simple presence of tumor located superficially at the surface of the thyroid gland also showed strong correlation with the risk of tumor spread to regional lymph nodes and/or recurrence. Tumor location at the thyroid surface obviously predisposes to extrathyroidal extension of the tumor, which was seen in 75% of these cases. It is likely that even in the absence of detectable extrathyroidal extension, superficial tumor location may facilitate tumor spread into lymphatic channels and regional lymph nodes, explaining the increased risk associated with this parameter.
Significant tumor fibrosis was also a predictor of risk in this study. We have previously observed the association between significant, sclerotic type fibrosis in TPMC and lymph node or distant metastasis.24 This association has also been observed by others,22 and was confirmed in this study. The biological basis for this finding requires further investigation. However, tumor desmoplasia is a well-known feature of invasive neoplastic growth and it is plausible that increased aggressiveness of these TPMCs may be related to their propensity for matrix formation and angiogenesis.
We treated intraglandular tumor spread and tumor multifocality as a combined feature (IGS/MF) due to the inherently subjective manner in which in some cases two tumors are designated as synchronous primary or as one tumor with intraglandular spread. The combined parameter of IGS/MF retained statistical significant on multivariate analysis, and allowed its use as a reproducible predictor of risk in this study.
Tall cell morphology has also been reported as a risk factor in TPMC but was not part of the final molecular-pathological scoring algorithm, since it did not improve its performance further. In the original cohort, 9 tumors showed tall cell features, and 8 of those from the aggressive tumor cohort. All 8 of these tumors were positive for BRAF mutation and were therefore indirectly included in our score.
The MP score can be calculated using the same four parameters that are weighted differently (MPW score) or in a simplified, unweighted way (MPU score). It is conceivable that in a very large data set, the MPW score may provide additional prognostic information. For example, the intermediate risk category that corresponds to MPU score 3, encompasses tumors with the MPW score in the range of 8–10, and whether or not incrementally higher score within this category would confer a progressively higher risk remains unknown.
The proposed scoring system assigns 65% of all TPMCs to the low risk category, i.e. tumors with virtually no risk of aggressive behavior, 25% to the intermediate risk category, and only 10% to the high risk category. Our model is therefore consistent with the expected biological behavior of TPMC, i.e. the majority are indolent, non-aggressive tumors, and also allows clinically relevant risk stratification that can further inform treatment decision-making. The revised American Thyroid Association management guidelines for patients with thyroid nodules released in 2009 recommend against completion of thyroidectomy and subsequent radioiodine ablation for tumors <1 cm when tumors are unifocal, intrathyroidal, node-negative, and lack other high-risk features such as certain histologic subtypes (tall cell variant, poorly differentiated carcinoma).35 In our validation cohort of 39 consecutive TPMC, 19 (48%) were either multifocal or showed extrathyroidal extension or tall cell morphology. As a result, almost 50% of TPMCs would have been candidates for more aggressive treatment based on the current ATA guidelines. Based on our analysis, only 10% of these TMPCs were at notably high risk for recurrence, and this figure is increased to only 35% if the TMPCs of indeterminate risk are included. Additionally, our model provides a means of assigning proportional risk based on the number of positive features present.
How exactly the proposed scoring system should be applied to the management of patients with these tumors requires further investigation. It seems reasonable to suggest that patients with low risk tumors should be followed conservatively, and patients with high risk tumors would probably benefit from total thyroidectomy, possibly with central compartment or lateral neck nodal sampling. It is likely that patients with moderate and high risk TPMC would also benefit from more intensive post-operative follow-up surveillance. The role of radioactive iodine ablation in treatment of these patients, and whether those at highest risk might benefit from this intervention remains unclear.
This study has several limitations that must be considered. The aggressiveness of TPMC in both tumor sets was defined primarily based on lymph node metastasis found at presentation. Therefore, the presence of malignant adenopathy remained the primary outcome measure that defined aggressive tumor behavior in this study. Few cases demonstrated tumor recurrence either in cervical lymph nodes or soft tissues of thyroid bed. Distant metastasis and tumor-related death are exceedingly rare in TPMC and were not observed in any of the cases in this series. However, it is important to note that lymph node involvement at presentation has strong correlation with recurrence of TPMC27 and therefore we believe that it can be reliably used to consider a given tumor as being more aggressive. Additional validation will be required to determine how well our model can predict the discrete outcomes of tumor recurrence and mortality associated with TPMC.
In summary, in this study we describe a number of clinical and histopathologic characteristics that correlate with the risk of extrathyroidal spread and recurrence of TPMC and offer a simple molecular-pathological score that can be used to assess tumor aggressiveness. The combined MP score allows for a more robust risk stratification than either molecular or histopathologic evaluation alone, and may be helpful in clinical management of patients with this common type of thyroid tumors.
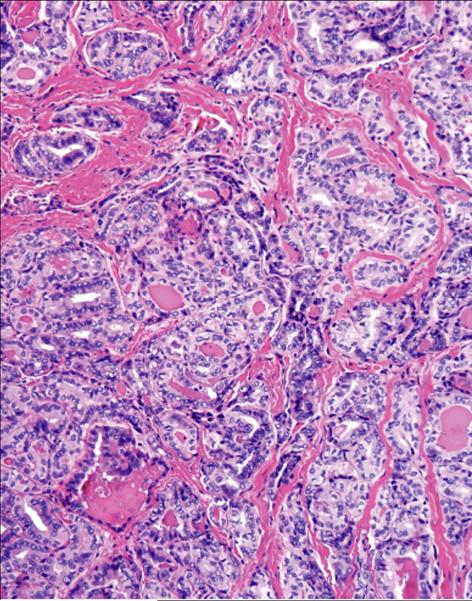
Figure 3.
Significant (2+) sclerotic-type tumor fibrosis that contributed to the molecular-pathological score.
Acknowledgements
This study was supported in part by the NIH grant R01 CA88041 and the Carrie L. Hughes Endocrine Genetics Research Fund of the University of Pittsburgh School of Medicine.
REFERENCES
- 1.DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press; Lyon: 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 2.Bisi H, Fernandes VS, de Camargo RY, Koch L, Abdo AH, de Brito T. The prevalence of unsuspected thyroid pathology in 300 sequential autopsies, with special reference to the incidental carcinoma. Cancer. 1989;64:1888–1893. doi: 10.1002/1097-0142(19891101)64:9<1888::aid-cncr2820640922>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Bondeson L, Ljungberg O. Occult papillary thyroid carcinoma in the young and the aged. Cancer. 1984;53:1790–1792. doi: 10.1002/1097-0142(19840415)53:8<1790::aid-cncr2820530831>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Neuhold N, Kaiser H, Kaserer K. Latent carcinoma of the thyroid in Austria: a systematic autopsy study. Endocr Pathol. 2001;12:23–31. doi: 10.1385/ep:12:1:23. [DOI] [PubMed] [Google Scholar]
- 5.Sampson RJ, Woolner LB, Bahn RC, Kurland LT. Occult thyroid carcinoma in Olmsted County, Minnesota: prevalence at autopsy compared with that in Hiroshima and Nagasaki, Japan. Cancer. 1974;34:2072–2076. doi: 10.1002/1097-0142(197412)34:6<2072::aid-cncr2820340629>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Sobrinho-Simoes MA, Sambade MC, Goncalves V. Latent thyroid carcinoma at autopsy: a study from Oporto, Portugal. Cancer. 1979;43:1702–1706. doi: 10.1002/1097-0142(197905)43:5<1702::aid-cncr2820430521>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Bondeson L, Ljungberg O. Occult thyroid carcinoma at autopsy in Malmo, Sweden. Cancer. 1981;47:319–323. doi: 10.1002/1097-0142(19810115)47:2<319::aid-cncr2820470218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Komorowski RA, Hanson GA. Occult thyroid pathology in the young adult: an autopsy study of 138 patients without clinical thyroid disease. Hum Pathol. 1988;19:689–696. doi: 10.1016/s0046-8177(88)80175-8. [DOI] [PubMed] [Google Scholar]
- 9.Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol. 1988;90:72–76. doi: 10.1093/ajcp/90.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga FH, Yatani R. Geographic pathology of occult thyroid carcinomas. Cancer. 1975;36:1095–1099. doi: 10.1002/1097-0142(197509)36:3<1095::aid-cncr2820360338>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Fink A, Tomlinson G, Freeman JL, Rosen IB, Asa SL. Occult micropapillary carcinoma associated with benign follicular thyroid disease and unrelated thyroid neoplasms. Mod Pathol. 1996;9:816–820. [PubMed] [Google Scholar]
- 13.Pelizzo MR, Piotto A, Rubello D, Casara D, Fassina A, Busnardo B. High prevalence of occult papillary thyroid carcinoma in a surgical series for benign thyroid disease. Tumori. 1990;76:255–257. doi: 10.1177/030089169007600309. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita H, Nakayama I, Noguchi S, et al. Thyroid carcinoma in benign thyroid diseases. An analysis from minute carcinoma. Acta Pathol Jpn. 1985;35:781–788. doi: 10.1111/j.1440-1827.1985.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 21:231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007. [Google Scholar]
- 17.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 18.Roti E, Rossi R, Trasforini G, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–2178. doi: 10.1210/jc.2005-2372. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13:498–512. doi: 10.4158/EP.13.5.498. [DOI] [PubMed] [Google Scholar]
- 20.Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998;83:553–559. doi: 10.1002/(sici)1097-0142(19980801)83:3<553::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98:31–40. doi: 10.1002/cncr.11442. [DOI] [PubMed] [Google Scholar]
- 22.Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004;89:3713–3720. doi: 10.1210/jc.2003-031982. [DOI] [PubMed] [Google Scholar]
- 23.Rassael H, Thompson LD, Heffess CS. A rationale for conservative management of microscopic papillary carcinoma of the thyroid gland: a clinicopathologic correlation of 90 cases. Eur Arch Otorhinolaryngol. 1998;255:462–467. doi: 10.1007/s004050050100. [DOI] [PubMed] [Google Scholar]
- 24.Kumar SM, Mehta N, Steward DL, Nikiforov YE. Correlation between clinicopathologic features of papillary thyroid microcarcinomas and tumor behavior. Mod Pathol. 2006;19:95A. [Google Scholar]
- 25.Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407. doi: 10.1097/01.SLA.0000055273.58908.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lupoli G, Vitale G, Caraglia M, et al. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet. 1999;353:637–639. doi: 10.1016/S0140-6736(98)08004-0. [DOI] [PubMed] [Google Scholar]
- 27.Roti E, degli Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159:659–673. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 28.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 29.Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 30.Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253:854–860. doi: 10.1148/radiol.2533090471. [DOI] [PubMed] [Google Scholar]
- 31.Lee X, Gao M, Ji Y, et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16:240–245. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 32.Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. The BRAF Mutation Is Predictive of Aggressive Clinicopathological Characteristics in Papillary Thyroid Microcarcinoma. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1129-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 34.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]