Abstract
FKBP51 and FKBP52 are diverse regulators of steroid hormone receptor signaling including regulation of receptor maturation, hormone binding, and nuclear translocation. Although structurally similar, they are functionally divergent, which is largely attributed to differences in the FK1 domain and the proline-rich loop. FKBP51 and FKBP52 have emerged as likely contributors to a variety of hormone-dependent diseases including stress-related diseases, immune function, reproductive functions and a variety of cancers. In addition, recent studies have implicated FKBP51 and FKBP52 in Alzheimer’s disease and other protein aggregation disorders. This review summarizes our current understanding of FKBP51 and FKBP52 interactions within the receptor-chaperone complex, their contributions to health and disease, and their potential as therapeutic targets for the treatment of these diseases.
FKBP51 and FKBP52 Structure and Function
It has been 20 years since the 51 and 52-kDa FK506 binding proteins FKBP51 and FKBP52 were first identified in complex with the steroid hormone receptors [1, 2]. Since that time, much progress has been made in understanding the mechanisms by which they regulate steroid hormone receptor signaling, and the resulting roles they play in endocrine-related physiological processes. Over the years, FKBP51 and FKBP52 have emerged as potential therapeutic targets for a wide variety of endocrine-related diseases including prostate cancer, breast cancer, male and female contraception, stress-related diseases, and metabolic diseases. As a result, researchers in academia and industry are increasingly focused on the identification and development of novel drugs that target FKBP51 and FKBP52.
FKBP51 and FKBP52 are Hsp90 co-chaperones that modify steroid hormone receptor (SHR) activity. FKBP51 and FKBP52 share 70% similarity and contain an active peptidyl prolyl isomerase (PPIase) domain, bind the 90-kDa heat shock protein (Hsp90) through a C-terminal tetratricopeptide repeat (TPR) domain [3], and adopt similar conformations (Figure 1) [reviewed in 4]. FKBP52 is a positive regulator of glucocorticoid receptor (GR) [5], progesterone receptor (PR) [6], and androgen receptor (AR) [7], but not estrogen receptor (ER) or mineralocorticoid receptor (MR) [5]. FKBP51 is a negative regulator of SHR activity in most studies reported [reviewed in 8]. The FKBPs compete for binding to the SHR complex and, as a result, overexpression of FKBP51 decreases receptor regulation by FKBP52 [5] and in the case of AR, hormone binding affinity is increased five-fold in the presence of FKBP52 versus FKBP51 [9].
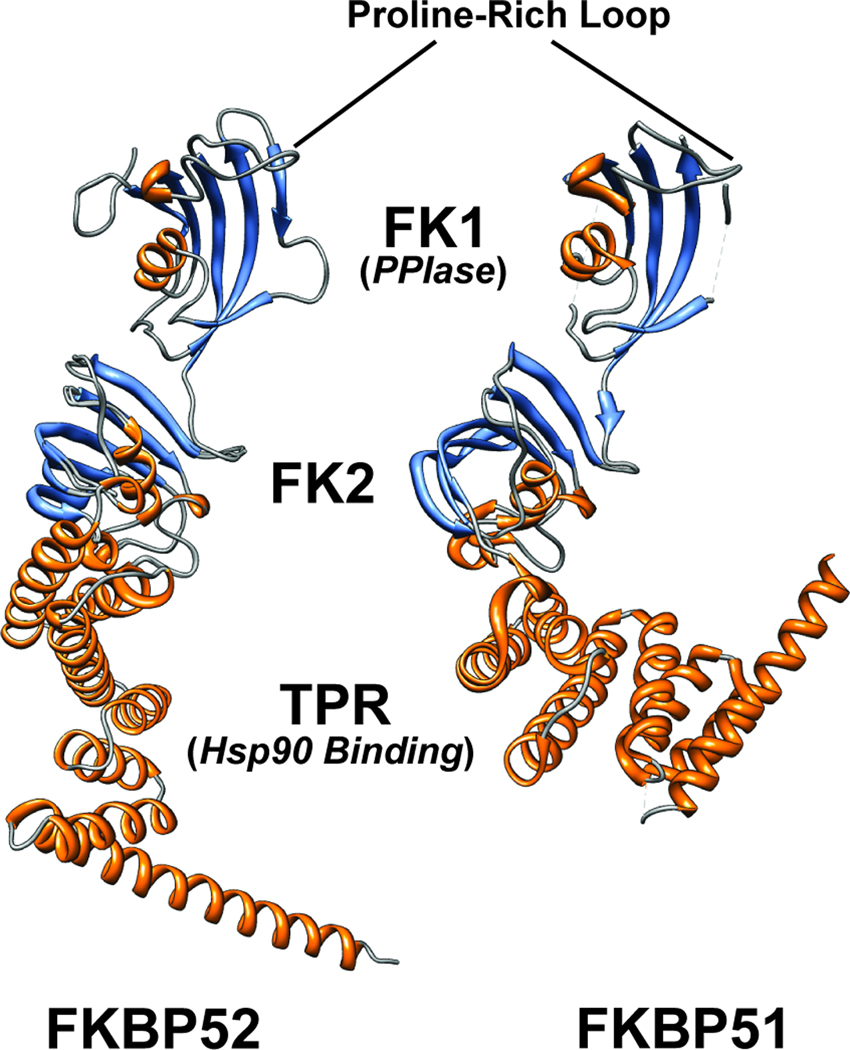
Figure 1.
FKBP51 and FKBP52 X-ray crystallographic structures. The three-dimensional structure of human FKBP51 (protein databank number 1KT0) and a composite of two partial structures for human FKBP52 (protein databank numbers 1Q1C and 1P5Q) are shown in ribbon format colored based on secondary structure. The important functional domains and regions are illustrated. The C-terminal TPR domain mediates binding to Hsp90. The FKBP12-like domains 1 and 2 are also shown. FK2 is similar to FK1 but lacks PPIase activity and does not bind the immunosuppressive ligand FK506. The FK1 domain both contains a functional PPIase active site and binds FK506. Although the PPIase activity is not required for receptor regulation, the FK1 domain is critical for FKBP function. In particular the proline-rich loop overhanging the PPIase pocket is critical for receptor regulation, is largely responsible for the divergent functions of FKBP51 and FKBP52, and may serve as a functionally important interaction surface. The figure was created, including overlaying the two partial FKBP52 structures, using UCSF Chimera version 1.5.
The major FKBP functional domains include the FKBP12-like domains 1 and 2 (FK1 and FK2) and the tetratricopeptide repeat (TPR) domain (Figure 1) [10]. The FK1 domain facilitates binding to the immunosuppressive drug FK506, confers PPIase activity [3], and is the primary regulatory domain for SHRs [5]. FKBP51 and FKBP52’s FK1 domains exhibit comparable PPIase enzymatic activity towards small peptide substrates. The FK1 domain is deemed critical for receptor potentiation by FKBP52 [5], but its enzymatic activity is not required for this potentiation. Domain integrity in and around the PPIase pocket is essential [9]. Although residues critical for PPIase activity are conserved in FKBP52 and FKBP51, residues of the proline-rich loop, suspended above the PPIase pocket, differ, thus significantly affecting protein interactions with larger peptide substrates [3, 10]. These differences are also likely responsible for the divergent functions of the FKBPs, as a randomly identified FKBP51 mutant containing two point mutations (A116V and L119P) in the FKBP51 proline-rich loop gained full FKBP52-like activity towards AR [9].
Seven to nine amino acids of the FK linker connect the FK1 and FK2 domains in FKBP51 and FKBP52. In FKBP52, this linker is crowned by the Casein Kinase 2 (CK2) phosphorylation sequence TEEED. CK2 phosphorylation at T143 could decrease binding to Hsp90 [11], but this finding was not replicated in an in vitro study in which a direct comparison of Hsp90 binding was compared between wild type FKBP52 and the phosphomimetic mutant FKBP52-T143E [12]. Phosphorylation of T143 completely abrogates FKBP52 regulation of receptor function and is predicted to reorient the conformation of the FK1 domain. In FKBP51, this loop is crowned by the sequence FED, so phosphorylation of this site is not achieved. However, this difference does not account for the lack of receptor potentiation ability of FKBP51.
The FK2 domain is still enigmatic; while structurally similar to FK1, it lacks PPIase activity and does not bind the drug FK506. Deletion of three amino acids in the FKBP51 FK2 domain (D195, H196, and D197) does not disrupt Hsp90 binding, but the mutant does not integrate normally into progesterone receptor complexes. [10]. It is possible that this mutation decreases interaction with other components of the receptor complex, possibly even the receptor itself.
The TPR domain confers Hsp90 binding ability to the FKBPs through interaction with the EEVD motif in the extreme C-terminus of Hsp90. The TPR domain is characteristic of all FKBPs, which interact with SHR complexes. Isothermal titration calorimetry suggests that FKBP51’s PPIase domain binds an Hsp90 dimer with only 25 to 33% of the affinity of FKBP52’s PPIase domain [3]. However, the FKBPs’ affinities for Hsp90 do not accurately predict the abundance of FKBPs in Hsp90-mediated receptor complexes. In a reticulocyte lysate assembly system, PR complexes showed a preference for FKBP51, GR complexes preferred FKBP51 and Protein Phosphatase 5 (PP5), and ER complexes preferred Cyclophilin 40 (Cyp40) [13]. The phenomenon of client specificity augments the possibility that the FKBPs could potentiate receptors through direct association or through unique, receptor-specific adapter proteins. Indeed, Hsp90-independent interactions have been reported between GR and FKBP52’s FK1 domain [14].
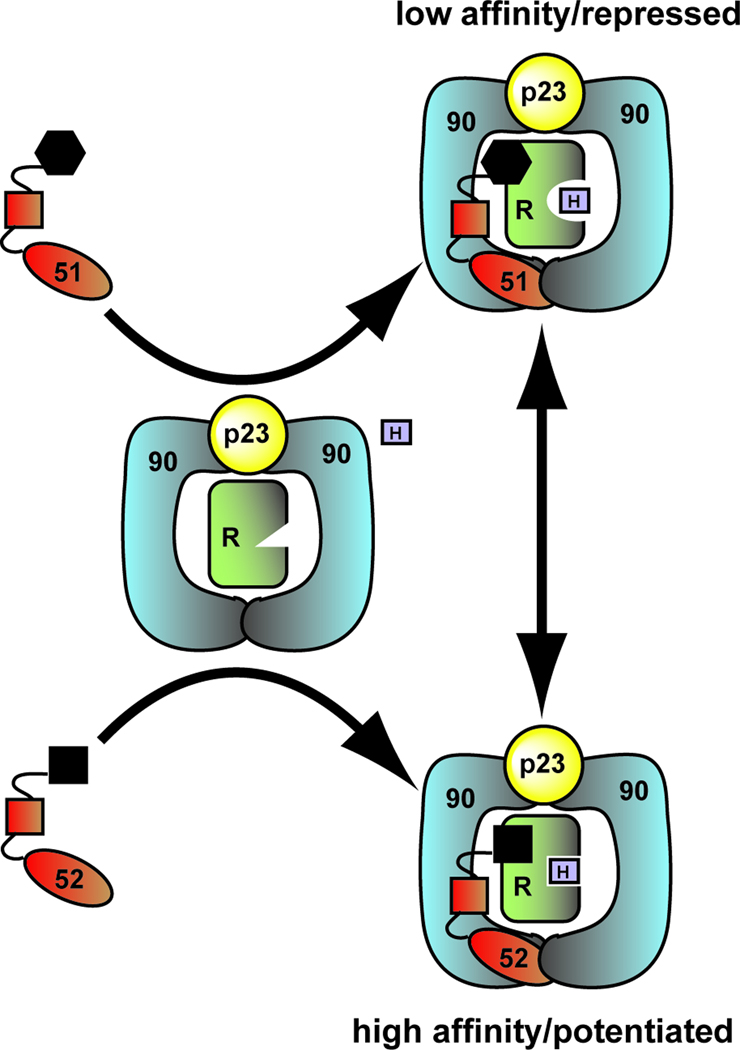
In this article we propose a model which holds that the FKBP52 FK1 domain, the proline-rich loop in particular, is not only responsible for the divergent functions of the FKBPs, but also serves as an interaction surface. The receptor specificity displayed by the FKBPs suggests that they directly contact the receptors within the receptor-chaperone complex. The fact that FKBP52 regulation has been localized to the receptor ligand binding domain (LBD) [5, 15] suggests that the FK1 interaction partner is the receptor LBD. We propose that interaction with Hsp90-receptor complex, which is stabilized by the p23 cochaperone, brings the FKBP FK1 domain into contact with the receptor LBD, directly affecting the hormone binding affinity of the receptor (Figure 2; Box 1). The FKBP interaction surface on the receptor and the molecular mechanism by which this occurs is currently under investigation (Box 3).
Figure 2.
Model for FKBP regulation of receptor maturation and hormone binding. The FK1 domain, the proline-rich loop in particular, is responsible for the divergent functions of the FKBPs and likely serves as an interaction surface. The difference in the shape of the FKBP FK1 domains shown here illustrates the structural differences in the proline-rich loop between these two proteins. The current model holds that the association of the FKBPs with the closed state of the Hsp90 dimer, which is stabilized by the p23 cochaperone, brings the FK1 domain into contact with the receptor LBD to directly influence hormone binding affinity. As a result of the differences in the FK1 domain, hormone binding is repressed in the presence of FKBP51 and potentiated in the presence of FKBP52.
Box 1. FKBP52: A diverse regulator of steroid hormone receptor signaling.
Studies aimed at characterizing FKBP52 as a regulator of receptor maturation and hormone binding and those characterizing FKBP52 as a regulator of receptor subcellular localization, have traditionally progressed independent of each other. This review presents both ideas with equal coverage and, based on the evidence, it is likely that FKBP52 is a regulator of both receptor hormone binding and receptor localization. The only area in which the two models do not agree is the timing by which FKBP52 associates with the receptor-chaperone complex and whether or not FKBP51 has a direct role in this process. When hormone is added to in vitro PR complexes with chemically-suspended Hsp90 dissociation ability, FKBP51 quickly dissociates, yet FKBP52 remains bound [89, 90]. Secondly, in an in vivo study in which the Hsp90 complex was suspended on ice to decrease Hsp90’s dissociation rate, addition of hormone caused FKBP51 to be replaced with FKBP52 in complexes with GR [19]. Thus, evidence exists to support the hormone-induced switching of FKBP51 for FKBP52, as depicted in Figure 3. However, the fact that FKBP52 promotes increased receptor hormone binding affinity [9] suggests that FKBP52 is present in receptor-chaperone complexes prior to hormone binding and somehow primes the receptor for hormone binding. Indeed, complexes containing FKBP52 in the absence of hormone are observed. The FKBP52 FK1 domain is important for receptor regulation and recent studies suggest that the FK1 domain serves as an interaction surface that may directly contact the receptor LBD within the complex, to promote increased hormone binding, as depicted in Figure 2 [9]. Recent evidence suggests the simultaneous binding of multiple TPR proteins to Hsp90, although these studies were performed in the absence of Hsp90 client protein [91, 92]. Thus, the mutually exclusive model for TPR proteins may be more complicated than is currently thought. It is also important to point out that most studies have been performed using purified proteins, in the absence of receptor, and, more importantly, in different cell types. These differences in experimental conditions and systems could at least partially account for the differences discussed above. It is likely that FKBP52 plays an important role in the hormone binding step and continues to be critical for receptor localization, possibly even playing a role in receptor regulation in the nucleus. Further studies are needed to resolve these discrepancies and to bring these two models together.
Box 3. Open questions for future investigation.
Do the FKBPs interact directly with the receptors within the Hsp90 chaperone complex in the cytoplasm or do they influence the receptors indirectly through binding Hsp90?
What are the FKBP interaction and/or regulatory sites on the receptors? Studies suggest FKBP52 regulation is localized to the receptor LBD, but can this be narrowed to a specific surface and or surface residues within the LBD?
The exchange of FKBP51 for FKBP52 upon hormone binding is not entirely due to conformational changes in the receptor-chaperone complex, as FKBP52 can be found in association with the complex prior to hormone binding. What facilitates this exchange and what role does this exchange play in the receptor signaling pathways given that FKBP52 can still regulate receptor in experimental systems where FKBP51 is absent?
Can the FKBPs interact directly with the receptors in an Hsp90-independent manner in the nucleus and, thus, have Hsp90-independent functional roles in the receptor signaling pathways?
Why do the 52KO mice develop normal testis despite significant developmental defects in other androgen-dependent tissues?
The embryonic lethality phenotype in the double 51/52KO mice has not been characterized. At what stage do the embryos die and what causes this lethality?
What is unique within prostate cancer cells, which allows FKBP51 to act as a positive regulator of androgen receptor signaling? In line with this question, evidence suggests a more cell and/or tissue specific role for FKBP51. Within what cell and or tissue types and under what conditions can FKBP51 act in addition to prostate cancer cells?
What is the functional single nucleotide polymorphism of FKBP51 in stress-related disease and what is the mechanism by which it contributes to the disease state?
Is FKBP51 gene expression programmed (permanently altered) by stressful life experiences?
What is the role of Hsp90 in FKBP regulation of tau pathogenesis and what phosphatases and kinases are involved?
Can FKBP-specific drugs be designed that distinguish between FKBP51 and FKBP52?
Roles for FKBP51 and FKBP52 in Receptor Localization
In the absence of ligand, some SHRs reside primarily in the cytoplasm whereas others are nuclear. Regardless of their primary localization, these receptors are not confined to any particular cell compartment and shuttle continuously between cytoplasm and nucleus [16, 17]. It has always been assumed that simple diffusion is the driving force of movement for these signaling molecules. However, the observation that proteins of the dynein/dynactin complex co-immunoprecipitate with the hsp90•FKBP52 complex and also with GR [18–20] and MR [21–23], suggests that these motor proteins could power the retrograde movement of these steroid receptors. Several observations agree with this hypothesis. The steroid-dependent nuclear accumulation of primarily cytoplasmic steroid receptors is rapid (t0.5 = 4–5 min), but treatment of cells with hsp90-disrupting agents such as geldanamycin, lowers the rate of translocation by an order of magnitude (t0.5 = 40–60 min), during which the hsp90•FKBP52 complex is inactivated [18, 21]. The rapid, hsp90•FKBP-dependent movement of steroid receptors requires cytoskeletal tracts, tubulin being physically linked to the receptor•hsp90•FKBP52•motor protein complex [17, 24]. Co-immunoprecipitation of dynein and subunit components of dynactin with FKBP52 demonstrated that the motor protein complex binds the N-terminus of FKBP52 in a manner that appears to be independent of the PPIase activity of FKBP52 [23, 25]. Rather, the PPIase domain acts as a protein-protein interaction domain and the association of dynein/dynactin with FKBP52 is not affected by FK506.
Because the FKBPs and hsp90 are part of the same functional complex, it can be envisage that the disruption of the interaction between the FKBPs and the motor proteins should yield similar levels of inhibition of SHR retrotransport as that measured in the presence of hsp90 inhibitors. This was demonstrated when the receptor was “disconnected” from the transport machinery by overexpression of the PPIase peptide (interferes with dynein binding to FKBP52) [18–20], the TPR peptide (blocks FKBP52 binding to hsp90) [23] or the p50/dynactin2 subunit of dynactin [18, 20]. On the other hand, nuclear translocation of GR is delayed by FKBP51, which correlates with the poor interaction of FKBP51 with dynein [20].
In all these cases, the nuclear localization of the cargo was not fully inhibited but simply impaired, suggesting the existence of two types of transport, the rapid hsp90•FKBP52•dynein/dynactin-dependent mechanism (t0.5 = 4–5 min), and an alternative, heterocomplex-independent and less efficient mechanism (t0.5 = 40–60 min), which could be due to simple diffusion. Importantly, when the nuclear translocation rate of these receptors was impaired, they became highly sensitive to proteasomal degradation [26]. The same heterocomplex described for steroid receptors is also responsible for the cytoplasmic retrotransport of the proapototic factor p53 [27], suggesting that the hsp90-based complex may play a general role in the retrotransport of a number of hsp90-associated factors towards the nuclear surface.
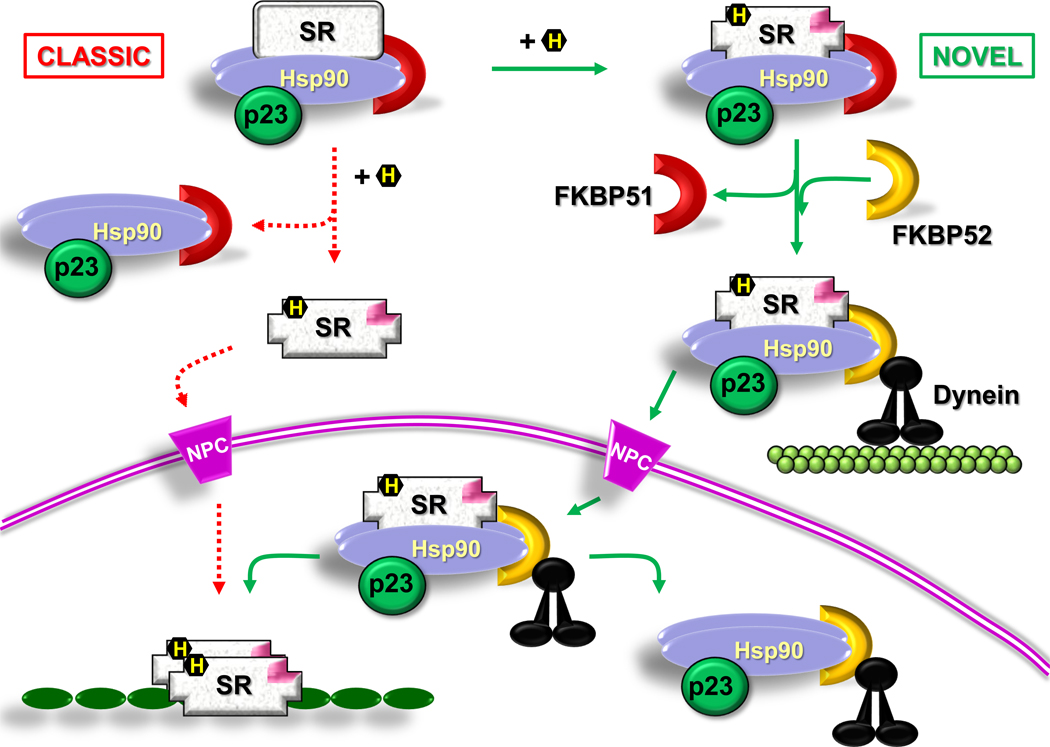
Inasmuch as the chaperone molecular bridge provides the traction chain for the nuclear factor to be transported throughout the cytoplasm via microtubule tracks, the dissociation of the hsp90-based complex from SHRs should not occur directly after ligand binding because the hsp90•FKBP52 complex is required for the normal mechanism of retrotransport [28]. This is a major modification of the model postulated decades ago for SHR activation, which sustained the principle that hsp90 “anchors” SHRs in the cytoplasmic compartment and only the cytoplasmic dissociation of the chaperone permits the nuclear translocation of the receptor [29, 30] (Figure 3; Box 1). The new model for SHR retrotransport implies that the chaperone machinery should remain associated to the receptor. It also implies that it could interact with structures of the nuclear pore complex during the nuclear translocation process. Recently, it was demonstrated that the chaperone complex binds the integral nuclear pore glycoprotein Nup62 and also β-importin [31]. This facilitates the passage of the untransformed receptor through the channel of the nuclear pore. It is possible that the chaperone complex associated to importins, nucleoporins, and the cargo itself can act as a cooperative system to prevent the aggregation of cargoes when their hydrophobic domains are exposed in the channel during the translocation step. The association of TPR proteins such as FKBP52 to Nup62 is hsp90-dependent, as shown by the dissociation of FKBP52 from Nup62 with radicicol. Nonetheless, indirect immunofluorescence assays performed in intact cells treated with radicicol still show the presence of FKBP52 in the perinuclear ring [31], suggesting that this TPR protein may also bind in an hsp90-independent manner to other perinuclear structures, for example other nucleoporins. Competition experiments by overexpression of the TPR domain showed that the perinuclear signal of FKBP52 was totally abolished, indicating that the TPR domain is required for most, if not all associations of FKBP52 with any structure of the nuclear envelope [31].
Figure 3.
Models for steroid hormone receptor translocation. According to the classic model (dashed lines) the chaperone complex dissociates in the cytoplasm from the steroid receptor (SR) upon hormone (H) binding. This transformed receptor passes through the nuclear pore complex (NPC) to reach its nuclear sites of action. The novel model is depicted with continuous lines. Upon steroid binding, the SR heterocomplex exchanges FKBP51 (brown crescent) for FKBP52 (dashed crescent), which is able to interact with dynein (black). The chaperone complex serves as a traction chain for the receptor whose retrotransport occurs on cytoskeletal tracts. The nuclear localization signal (NL1; pink) protrudes upon steroid binding and the whole SR-chaperone complex translocates through the NPC. The heterocomplex interacts with structural proteins of the pore, which are also chaperoned. Receptor transformation is nucleoplasmic and facilitates the binding of the steroid-activated receptor with promoter sites.
FKBP51 and FKBP52 in Health and Disease
Reproductive Development and Success
A role for FKBP52 in mammalian reproductive development and success emerged from the development and study of two independently derived fkbp52-deficient (52KO) mouse lines. The phenotypes observed in these mice directly correlate with observations previously obtained in biochemical and cellular studies. Male 52KO mice display phenotypes consistent with partial androgen insensitivity including dysgenic prostate and seminal vesicles, ambiguous external genitalia including hypospadias, and retention of nipples into adulthood [7, 32]. Despite the androgen insensitivity, the testis of 52KO mice develop normally. Whether some factor exists within the testis that can complement for the loss of FKBP52, or the androgen levels produced locally within the testis are enough to compensate for reduced AR activity, is currently unclear. 52KO male mice do have reduced epididymal sperm counts and the sperm display abnormal morphology. FKBP52 was also reported to be present in epididymal sperm flagella and 52KO animals display reduced sperm motility [33]. The androgen insensitivity observed in the 52KO mice does not account for reduced sperm motility, as this is an androgen-independent process. As discussed above, FKBP52 does interact with dynein motor proteins, suggesting a possible role for FKBP52 in the regulation of flagella movement. The available evidence suggests that FKBP52 may serve as an attractive target for male contraceptives; however, further studies into the role of FKBP52 in the testis and in sperm maturation and motility are needed (Box 3).
Female 52KO mice appear morphologically normal and display normal ovulation and fertilization; yet, they are infertile. The infertility is the result of embryonic implantation and decidualization failure due to progesterone insensitivity and uterine defects [6, 34, 35]. Implantation failure may be due to increased uterine oxidative stress, as 52KO animals were sensitive to paraquat-induced oxidative stress, have reduced levels of the anti-oxidant peroxiredoxin-6 (PRDX6) and addition of exogenous antioxidant rescued implantation [34, 36]. Finally, FKBP52 may promote endometriosis, as women with endometriosis show reduced FKBP52 expression, and the progesterone resistance, observed in 52KO mice, results in increased cell proliferation, inflammation, and angiogenesis, leading to endometriotic lesions [37]. Thus, evidence suggests a critical role for FKBP52 in female reproduction and uterine signaling.
The fkbp51-deficient (51KO) mice display no overt morphological phenotypes. However, the loss of both FKBP51 and FKBP52 (51/52KO) proteins results in embryonic lethality, although the cause of this phenotype has not been investigated (Box 3). Thus, FKBP51 and FKBP52 have some redundant role(s) in embryonic development. Whether or not this is an endocrine-related role is unknown and it could result from one or several of the non-endocrine related functions that have recently been characterized for the FKBP proteins. One of the more recent discoveries demonstrates a role of the FKBP proteins in microtubule assembly and tau pathogenesis [38, 39] (Box 2). This is of particular interest, as it is the first example of a PPIase-dependent function for FKBP51 and FKBP52.
Box 2. Role for the FKBPs in protein folding and aggregation disorders.
A novel role for Hsp90 and associated cochaperones in protein folding and aggregation disorders such as Alzheimer’s has emerged in recent years. Modulating Hsp90 directly may be clinically relevant for protein folding and aggregation disorders [93–96]. Hsp90 cooperates with many cochaperones to act on specific protein subclasses, such as transmembrane receptors or tau and other disordered proteins. Recent data suggest that one or more of these cochaperones could be more specific drug targets with fewer adverse consequences [39, 93]. For example, in a Caenorhabditis elegans model of tauopathy, tau levels increased and the pathological phenotype worsened when the ubiquitin ligase CHIP, which is also an Hsp90 cochaperone, and FKBP52 were silenced [97]. However, the involvement of FKBP51 in tau pathogenesis was not described in this study, since C. elegans lacks an FKBP51 gene. Recently, FKBP51 was shown to preserve tau levels, but reduce its phosphorylation, perhaps by cooperating with a set of phosphatases [39]. FKBP51 also interacts with tau in human Alzheimer’s disease brain tissue. More recently, increased levels of FKBP52 were shown to correspond with decreased tau stability [38, 98]. Thus, these two proteins may have opposing roles for tau, despite similar structural features.
The roles of the PPIase activity of both FKBP51 and FKBP52 could be critical for the regulation of intrinsically disordered proteins like tau, since a high percentage of proline residues is common among this family:[99]. More than 20% of the residues between I151 and Q244 of tau are proline. Most of the known functions of tau are mediated through MT binding domains distal to this proline-rich region. However many disease-associated phosphorylation events that seed tau tangle formation occur at proline-directed serine (S) and threonine (T) residues in this proline-rich region. This strongly suggests that important structural changes in the proline rich region of tau are regulating tangle formation. In particular, cis-trans isomerization around these prolines modulates protein phosphatase binding and activity at specific S/T sites. It is well established that peptidyl-prolyl isomerase 1 (Pin1) regulates tau phosphorylation in concert with protein phosphatase 2A (PP2A), specifically at T231 and T212 [100]. FKBP51 and FKBP52 likely have a similar activity; however, unlike Pin1, the FKBPs likely coordinate with Hsp90 to isomerize tau [50]. Thus, FKBP51 and FKBP52 may improve our understanding of tau biology and may be targets for drug development efforts in tauopathies.
Cell Proliferation and Cancer
Research on FKBP51 in cancer etiology and response to antineoplastic therapy has intensified recently. Initially, FKBP51 was reported as an androgen-regulated gene in prostate cancer and a modulator of androgen receptor (AR) activity [reviewed in 40]. The protein is also upregulated in prostatic hyperplasia [41]. In prostate cancer cell lines, increased levels of FKBP51 and FKBP52 were reported, as well as an inhibitory effect of FK506 on androgen-stimulated cell growth [42]. While gene knock-out strategies revealed FKBP52, but not FKBP51, as an important facilitator of physiological AR activity [7, 32], FKBP51 was also identified as a positive regulator of AR and androgen-dependent cell growth, and as a target of FK506 in prostate cancer cells [43, 44]. Potentiation of AR by FKBP51 was not found in other studies and may be cell type dependent [9, 45].
In colorectal adenocarcinoma, FKBP51 suppresses proliferation, which was ascribed to its action on GR [46]. GR action has also been invoked in myeloma cells, where dexamethasone-induced expression of FKBP51 has been interpreted as an adaptive process prior to cell death [47]. In leukemia, inhibiting FKBP51 by rapamycin abolished doxorubicin-induced activation of NF-kB and thus enhanced drug-induced apoptosis [48]. A recent study presented FKBP51 as a marker of melanocyte malignancy [49]. Irradiation caused apoptosis in cells with silenced FKBP51 and promoted autophagy in control cells. Inhibition of apoptosis in control cells involved FKBP51-dependent induction of NF-kB upon irradiation.
A major advance in mechanistic understanding was the discovery that FKBP51 negatively regulates the activity of the cell growth regulator Akt, by serving as a scaffolding protein to recruit the phosphatase PHLPP [50]. FKBP51 expression is decreased in several cancer cell lines and in pancreatic cancer tissue, and may correlate with increased Akt phosphorylation and reduced cell sensitivity to chemotherapeutic agents.
Overall, it appears that depending on cell and cancer type, promoting or reducing FKBP51 activity can produce a beneficial effect in the actions against cancer cell proliferation. Most likely this is due to the multitude of molecular functions of FKBP51, and this should be carefully considered when developing FKBP51 targeting drugs for cancer therapy [49]. Most likely, FKBP51 uses at least partially different surfaces for its divergent actions, which eventually could be exploited for specific drug development.
Given that FKBP51 expression is hormone-regulated [reviewed in 8], altered FKBP51 expression has been associated with a wide variety of cancers. In contrast, FKBP52 is ubiquitously expressed at high levels, and is slightly up-regulated under stress conditions [1]. Thus, less is known on the role of FKBP52 in cancer. Given the androgen, progesterone and glucocorticoid insensitivity phenotypes observed in the fkbp52-deficient mice [6, 7, 32, 35, 51], FKBP52 is likely to have an important role and to serve as a therapeutic target in a variety of diseases that are dependent on these hormone signaling pathways.
The prostate dysgenesis observed in 52KO mice established FKBP52 as a critically important regulator of prostate development [7], and enhanced FKBP52 levels have been observed in prostate needle biopsies from human patients [52]. In addition, a series of compounds that specifically inhibit FKBP52 regulation of androgen receptor function effectively block androgen-dependent gene expression and cell proliferation in prostate cancer cells [53]. Although FKBP52 is not a functional regulator of ER in cellular studies, FKBP52 expression is up-regulated in breast tumors, and recent studies found FKBP52 gene methylation in ER-negative, but not in ER-positive breast cancer cells [reviewed in 54]. Thus, FKBP52 may also have a role in breast cancer tumorigenesis and/or progression.
Stress-Related Diseases and Phenotypes
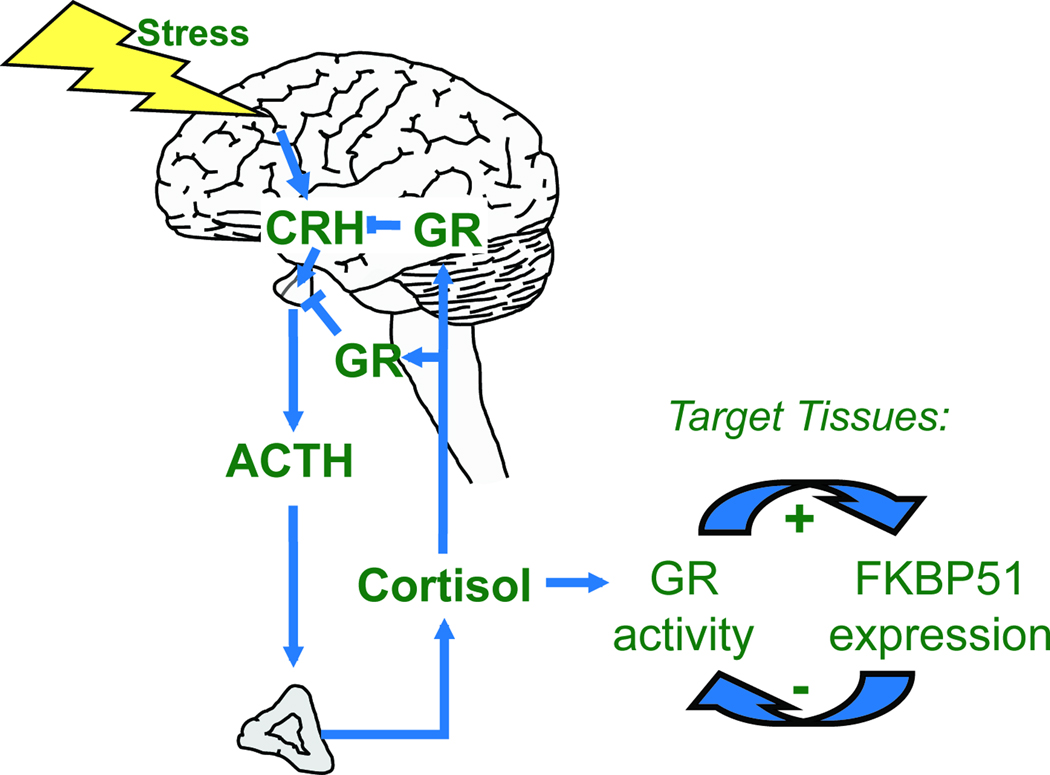
The search for molecular parameters in psychiatric disorders identified a correlation of an imbalanced stress hormone system, the hypothalamus pituitary adrenal (HPA) axis, with the risk for and course of diseases like major depression, bipolar disorder, post-traumatic stress disorder (PTSD), schizophrenia and anxiety disorders [55, 56]. The HPA axis is a hormone cascade comprising the corticotropin-releasing hormone (CRH) secreted upon stress, which triggers synthesis and release of corticotropin, resulting in the secretion of cortisol that acts on various tissues (Figure 4). A crucial characteristic of the HPA axis is the negative feedback exerted by cortisol via the glucocorticoid receptor (GR) that keeps the stress reaction in balance. The altered set-point of the HPA axis hormones observed in stress-related diseases goes along with an altered reactivity of the HPA axis, and is interpreted as the body’s inability to adequately terminate stress response, which increases the risk for disease development [55]. The corticosteroid receptor hypothesis stipulates that malfunctioning of GR is causal for the inappropriate reaction of the HPA axis to stress [56]. Thus, researchers explored the molecular mechanisms that operate to calibrate GR activity, with the aim to better understand stress-related diseases.
Figure 4.
FKBP51 is linked to the HPA axis. Upon perception of stress, CRH is released from the hypothlamus, which promotes synthesis and release of ACTH from the pituitary. ACTH in turn increases release of cortisol from the adrenal glands. The inhibitory action of cortisol-activated GR on CRH and ACTH terminates the hormonal stress response and keeps the HPA axis in balance. FKBP51 expression is increased by GR, and acts back on GR in an inhibitory manner. This ultrashort feedback loop provides a mechanism by which FKBP51 regulates HPA axis activity, affects the impact of cortisol in target tissues, and links stress to its other molecular and physiological functions.
The discovery of divergent actions of FKBP51 and FKBP52 on GR [5, 20] along with the correlation of FKBP51 with altered set-points of the HPA axis in squirrel monkeys spurred their inclusion as candidates in an association study in major depression [57]. This study was the first to identify a correlation of FKBP51 gene variants with response to antidepressant treatment, and also with the reactivity of the HPA axis in the dexamethasone (DEX)-CRH test. FKBP51 genetic polymorphisms and their relationship to antidepressant responses were confirmed in several large and small patient samples [58–61]. An association of FKBP51 genotype with depression disease status was also discovered, and in one study, it revealed a gender-specific effect of this association [60, 62–64]. In addition, FKBP51 polymorphisms were linked to suicide in several samples [63, 65–67] and a gender-specific FKBP51 genotype association with the personality traits of harm avoidance and cooperativeness has also been reported [68]. Moreover, polymorphisms of FKBP51 influenced recovery from psychosocial stress in healthy individuals [69].
FKBP51 gene variants are also associated with peritraumatic dissociation [70], an established risk factor for the development of posttraumatic stress disorder (PTSD) [71]. Several studies link FKBP51 to PTSD and FKBP51 was found to be less expressed in PTSD, consistent with the observation of enhanced GR responsiveness [72]. Intriguingly, recent studies suggested that FKBP51 polymorphisms modify the effects of early life trauma in PTSD [73–75] and major depression [76].
The reports on FKBP51 and PTSD in particular suggest a function of FKBP51 as a modulator of the response to stressful life events and as a mediator of gene-environment interactions. Thus, the risk of disease development critically depends on the FKBP51 gene status. Even though it is very likely that FKBP51 operates in stress physiology through its action in GR and the HPA axis function, the exact mechanism of the gene environment interaction remains to be elucidated, and no associating polymorphism has been reported so far. However, a recent study reported decreased DNA methylation and increased expression of FKBP51 in mice after chronic exposure to corticosterone [77]. Thus, it is possible that FKBP51 gene expression is programmed by stressful life events in a genotype-dependent fashion, and results of research on this are eagerly awaited.
Immune Function
Several studies report a role of FKBP51 in immune-related diseases and inflammation. Expression of FKBP51 was enhanced in bone marrow cells from patients suffering from rheumatoid arthritis [78]. Evidence has also been provided for a modulation of NF-kB-dependent gene expression by FKBP51, with possible implications for various pathways [79, 80]. This possibly links FKBP51 to NF-kB and its regulation in cell proliferation and survival, inflammation, immune regulation, metabolic diseases and hematopoiesis [81, 82]. Another study found FKBP51 and NF-kB up-regulated in bone marrow-derived mononuclear cells from patients with rheumatoid arthritis [83]. Treatment of chronic obstructive pulmonary disease goes along with increased expression of FKBP51 [84]. FKBP51 has also been reported to mediate the effect of the immunosuppressant FK506 in inhibiting endogenous MHC class II-restricted antigen presentation [85]. Since GR is an established modulator of immune function, FKBP51 mediated regulation of GR activity per se could provide the basis for a role of FKBP51 in immune processes. Additional FKBP51 effects, such as its impact on NF-kB, are also operative. Mechanistically, evidence from glioma cells suggests that FKBP51 impacts on the stability of IkB and on phosphorylation of NF-kB, and enhances DNA binding of NF-kB [79].
FKBP51 is also able to mediate the inhibition of the phosphatase calcineurin, which activates Nuclear Factor of Activated T cells, by FK506 [86, 87]. This function, however, is not unique to FKBP51, as the smaller FKBPs also mediate this effect, some of them even more efficiently [87]. Moreover, FKBP51 has been reported, by some, to interact with calcineurin in the absence of FK506 [88], but not by others [87].
Concluding Remarks
It is becoming increasingly clear that FKBP52 associates with SHR-chaperone complexes to regulate hormone binding and continues to play a critical role in receptor translocation to the nucleus, possibly even regulating the receptors in the nucleus. Although, FKBP51 is generally considered as a negative regulator of receptor function, evidence suggests that it displays tissue and/or cell type specific effects on receptor signaling. As a result, both FKBP51 and FKBP52 are implicated in a variety of diseases, and could serve as therapeutic targets for the treatment of these diseases. Efforts to therapeutically target the FKBP proteins are currently underway and these efforts would be enhanced by studies aimed at improving our understanding of FKBP interactions within the receptor-chaperone complex both in the cytoplasm and nucleus.
Acknowledgements
The authors are in part supported by the Grant Number 5G12RR008124 (to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. We thank the Border Biomedical Research Center’s Biomolecule Analysis Core Facility (BACF), Tissue Culture Core Facility (TCF), and the DNA Analysis Core Facility (DACF) for the use of the instruments. M.B.C. is also supported in part by American Recovery and Reinvestment Act (ARRA) funds through grant number SC1GM084863 from the National Institute of General Medical Sciences, NIH. M.D.G. is supported by grants ANPCyT (PICT2011-1170) and UBACyT (X085). C.A.D. is supported by grants R01NS073899 and R00AG031291 from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging respectively.
Glossary of Terms
- Akt
Akt is a serine/threonine protein kinase that regulates apoptosis, cell migration, transcription, proliferation, and glucose metabolism.
- Antineoplastic therapy
Chemotherapy that targets all actively dividing cells.
- β importin
During nuclear import, β importin tethers incoming proteins to the nuclear pore complex.
- FKBP51
51kDa FK506 binding protein that binds the immunosuppressive drug FK506 without initiating immunosuppression.
- FKBP52
52kDa FK506 binding protein that binds the immunosppressive drug FK506 without initiating immunosuppression.
- FK1 Domain
FKBP12-like domain 1 of FKBP51 and FKPB52, responsible for binding to FK506, for PPIase activity, and for steroid hormone receptor regulation.
- FK2 Domain
FKBP12-like domain 2, present in FKBP51 and FKBP52, which differs slightly from the FK1 and lacks PPIase and FK506 binding ability.
- FK506
Also called tacrolimus, is a macrolide drug that complexes with FKBP12 and inhibits calcineurin phosphatase activity. This leads to immunosuppression by blocking T cell signal transduction cascades and Interleukin-2 transcription.
- Geldanamycin
This benzoquinone ansamycin antibiotic directly binds Hsp90 and inhibits its function.
- Hsp90
The 90 kDa heat shock protein is a molecular chaperone involved in protein folding, tumor repression, and cell signaling. Steroid hormone receptors require association with Hsp90 to fold to a conformation capable of binding ligand.
- Hypothalamus pituitary adrenal (HPA) Axis
Interactions between the hypothalamus, the pituitary gland, and the adrenal glands are critical parts of the neuroendocrine system. This pathway is responsible for the regulation of stress reactions, energy storage and output, emotion and affect, sexuality, digestion, and the immune system.
- MHC II
Major Histocompatibility Complex Class II molecules are located on subclasses of antigen presenting cells and display extracellular protein fragments to CD4+ helper T-cells to determine the immune response.
- NFκB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells is a transcription factor complex found in most animal cells that regulates cellular responses to infections and stress.
- Nup62
Nucleoporin 62 is a complex of proteins which associates with the importin αβ complex of the nuclear pore to assist in the import of proteins with nuclear localization signals.
- PPIase activity
Peptidyl Prolyl Isomerase activity catalyzes cis-trans isomeration reactions of peptide bonds involving the amino acid proline. PPIase activity is required for the proper folding of several, but not all, proteins.
- Protein aggregation disorders
The agglomeration of proteins occurs in diseases such as Alzheimer's, Mad Cow, and Huntington's diseases.
- Radicicol
Also known as monorden, radicicol is a macrolactone antibiotic that binds Hsp90 and alters its function. It also inhibits tyrosine kinase and is anti-angiogenic.
- Reticulosyte lysate assembly system
This cell free assembly system contains all necessary eukaryotic cofactors for steroid hormone receptor folding and allows for the reconstitution of the receptors with chaperone complexes in vitro.
- Tau
Tau proteins are found primarily in neurons of the Central Nervous System and normally help in microtubule stabilization. Defective folding of tau and resultant agglomeration is associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s Diseases.
- TPR Domain
The tetratricopeptide repeat is a structural motif utilized in protein-protein interactions. The TPR domains on FKBP51 and FKBP52 bind specifically to the extreme C-terminus of Hsp90.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265:22067–22070. [PubMed] [Google Scholar]
- 2.Smith DF, et al. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990;265:3996–4003. [PubMed] [Google Scholar]
- 3.Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. 2001;308:795–806. doi: 10.1006/jmbi.2001.4595. [DOI] [PubMed] [Google Scholar]
- 4.Cheung-Flynn J, Place, Sean P, Cox, Marc B, Prapapanich V, Smith, David F. 12 FKBP Co-Chaperone in Steroid Receptor Complexes. In: Calderwood SK, editor. Cell Stress Proteins. Springer: 2007. pp. 275–306. [Google Scholar]
- 5.Riggs DL, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tranguch S, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung-Flynn J, et al. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 8.Cox MB, Smith DF. Functions of the Hsp90-Bindign FKBP Immunophilins. In: Blatch GL, editor. The Networking of Chaperones by Cochaperones. Eurekah.com; 2006. [Google Scholar]
- 9.Riggs DL, et al. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinars CR, et al. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 2003;100:868–873. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata Y, et al. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci U S A. 1997;94:14500–14505. doi: 10.1073/pnas.94.26.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox MB, et al. FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol Endocrinol. 2007;21:2956–2967. doi: 10.1210/me.2006-0547. [DOI] [PubMed] [Google Scholar]
- 13.Barent RL, et al. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein AM, et al. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A, et al. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–10480. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witchel SF, DeFranco DB. Mechanisms of disease: regulation of glucocorticoid and receptor levels--impact on the metabolic syndrome. Nat Clin Pract Endocrinol Metab. 2006;2:621–631. doi: 10.1038/ncpendmet0323. [DOI] [PubMed] [Google Scholar]
- 17.Galigniana MD, et al. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus. 2010;1:299–308. doi: 10.4161/nucl.1.4.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galigniana MD, et al. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200. [DOI] [PubMed] [Google Scholar]
- 19.Davies TH, et al. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 20.Wochnik GM, et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 21.Piwien Pilipuk G, et al. Evidence for NL1-independent nuclear translocation of the mineralocorticoid receptor. Biochemistry. 2007;46:1389–1397. doi: 10.1021/bi0621819. [DOI] [PubMed] [Google Scholar]
- 22.Gallo LI, et al. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry. 2007;46:14044–14057. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- 23.Galigniana MD, et al. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol. 2010;30:1285–1298. doi: 10.1128/MCB.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell JM, et al. Evidence for glucocorticoid receptor transport on microtubules by dynein. J Biol Chem. 2004;279:54647–54654. doi: 10.1074/jbc.M406863200. [DOI] [PubMed] [Google Scholar]
- 25.Galigniana MD, et al. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z. [DOI] [PubMed] [Google Scholar]
- 26.Galigniana MD, et al. Retrograde transport of the glucocorticoid receptor in neurites requires dynamic assembly of complexes with the protein chaperone hsp90 and is linked to the CHIP component of the machinery for proteasomal degradation. Brain Res Mol Brain Res. 2004;123:27–36. doi: 10.1016/j.molbrainres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Galigniana MD, et al. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J Biol Chem. 2004;279:22483–22489. doi: 10.1074/jbc.M402223200. [DOI] [PubMed] [Google Scholar]
- 28.Galigniana MD, et al. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus. 2010;1:299–308. doi: 10.4161/nucl.1.4.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt WB. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987;35:51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- 30.Schaaf MJ, Cidlowski JA. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol Cell Biol. 2003;23:1922–1934. doi: 10.1128/MCB.23.6.1922-1934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echeverria PC, et al. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol Cell Biol. 2009;29:4788–4797. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong W, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong J, et al. Deficiency of co-chaperone immunophilin FKBP52 compromises sperm fertilizing capacity. Reproduction. 2007;133:395–403. doi: 10.1530/REP-06-0180. [DOI] [PubMed] [Google Scholar]
- 34.Tranguch S, et al. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirota Y, et al. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirota Y, et al. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambraud B, et al. A role for FKBP52 in Tau protein function. Proc Natl Acad Sci U S A. 2010;107:2658–2663. doi: 10.1073/pnas.0914957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinwal UK, et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J.Neurosci. 2010;30:591–599. doi: 10.1523/JNEUROSCI.4815-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;10:10. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Malley KJ, et al. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69:1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Periyasamy S, et al. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology. 2007;148:4716–4726. doi: 10.1210/en.2007-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni L, et al. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Periyasamy S, et al. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene. 2010;29:1691–1701. doi: 10.1038/onc.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SchÅlke JP, et al. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS.One. 2010;5:e11717. doi: 10.1371/journal.pone.0011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukaide H, et al. FKBP51 expressed by both normal epithelial cells and adenocarcinoma of colon suppresses proliferation of colorectal adenocarcinoma. Cancer Invest. 2008;26:385–390. doi: 10.1080/07357900701799228. [DOI] [PubMed] [Google Scholar]
- 47.Rees-Unwin KS, et al. Proteomic evaluation of pathways associated with dexamethasone-mediated apoptosis and resistance in multiple myeloma. Br.J.Haematol. 2007;139:559–567. doi: 10.1111/j.1365-2141.2007.06837.x. [DOI] [PubMed] [Google Scholar]
- 48.Avellino R, et al. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400–1406. doi: 10.1182/blood-2005-03-0929. [DOI] [PubMed] [Google Scholar]
- 49.Romano S, et al. FK506 binding proteins as targets in anticancer therapy. Anticancer Agents Med.Chem. 2010;10:651–656. doi: 10.2174/187152010794479816. [DOI] [PubMed] [Google Scholar]
- 50.Pei H, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warrier M, et al. Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology. 2010;151:3225–3236. doi: 10.1210/en.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JF, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–2605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- 53.De Leon JT, et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A. 2011;108:11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivils JC, et al. Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52) Curr Opin Pharmacol. 2011;19:19. doi: 10.1016/j.coph.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Kloet ER, et al. Stress and the brain: from adaptation to disease. Nat.Rev.Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 56.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 57.Binder EB, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat.Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 58.Kirchheiner J, et al. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. doi: 10.2217/14622416.9.7.841. [DOI] [PubMed] [Google Scholar]
- 59.Laje G, et al. Pharmacogenetics studies in STAR*D: strengths, limitations, and results. Psychiatr.Serv. 2009;60:1446–1457. doi: 10.1176/appi.ps.60.11.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lekman M, et al. The FKBP5-gene in depression and treatment response--an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol.Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou YF, et al. Meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neurosci.Lett. 2010;484:56–61. doi: 10.1016/j.neulet.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Velders FP, et al. Genetics of cortisol secretion and depressive symptoms: A candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willour VL, et al. Family-based association of FKBP5 in bipolar disorder. Mol.Psychiatry. 2008;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavebratt C, et al. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J.Affect.Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- 65.Brent D, et al. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am.J.Psychiatry. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy A, et al. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Supriyanto I, et al. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2011;35:252–256. doi: 10.1016/j.pnpbp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 68.Shibuya N, et al. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neurosci.Lett. 2010;485:194–197. doi: 10.1016/j.neulet.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Ising M, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur.J.Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 70.Koenen KC, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol.Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 71.Ozer EJ, et al. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol.Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 72.Yehuda R, et al. Gene Expression Patterns Associated with Posttraumatic Stress Disorder Following Exposure to the World Trade Center Attacks. Biol.Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 73.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koenen KC, Uddin M. FKBP5 polymorphisms modify the effects of childhood trauma. Neuropsychopharmacology. 2010;35:1623–1624. doi: 10.1038/npp.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie P, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann P, et al. Interaction of variants in the FKBP5 gene and adverse life events in predicting the first depression onset: results from a ten-year prospective community study. American Journal of Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.10111577. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee RS, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsushita R, et al. Enhanced expression of mRNA for FK506-binding protein 5 in bone marrow CD34 positive cells in patients with rheumatoid arthritis. Clin.Exp.Rheumatol. 2010;28:87–90. [PubMed] [Google Scholar]
- 79.Jiang W, et al. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway. Neoplasia. 2008;10:235–243. doi: 10.1593/neo.07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park J, et al. Glucocorticoids modulate NF-kappaB-dependent gene expression by up-regulating FKBP51 expression in Newcastle disease virus-infected chickens. Mol.Cell Endocrinol. 2007;278:7–17. doi: 10.1016/j.mce.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Baker RG, et al. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakamura N, et al. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res. 2006;13:169–183. doi: 10.1093/dnares/dsl006. [DOI] [PubMed] [Google Scholar]
- 84.Holownia A, et al. Increased FKBP51 in induced sputum cells of chronic obstructive pulmonary disease patients after therapy. Eur.J.Med.Res. 2009;14 Suppl 4:108–111. doi: 10.1186/2047-783X-14-S4-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imai A, et al. Inhibition of endogenous MHC class II-restricted antigen presentation by tacrolimus (FK506) via FKBP51. Eur.J.Immunol. 2007;37:1730–1738. doi: 10.1002/eji.200636392. [DOI] [PubMed] [Google Scholar]
- 86.Baughman G, et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15:4395–4402. doi: 10.1128/mcb.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weiwad M, et al. Comparative analysis of calcineurin inhibition by complexes of immunosuppressive drugs with human FK506 binding proteins. Biochemistry. 2006;45:15776–15784. doi: 10.1021/bi061616p. [DOI] [PubMed] [Google Scholar]
- 88.Li TK, et al. Calcium- and FK506-independent interaction between the immunophilin FKBP51 and calcineurin 1692. J.Cell Biochem. 2002;84:460–471. doi: 10.1002/jcb.10026. [DOI] [PubMed] [Google Scholar]
- 89.Smith DF, et al. Reconstitution of progesterone receptor with heat shock proteins. Mol Endocrinol. 1990;4:1704–1711. doi: 10.1210/mend-4-11-1704. [DOI] [PubMed] [Google Scholar]
- 90.Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 91.Li J, et al. Mixed Hsp90-cochaperone complexes are important for the progression of the reaction cycle. Nat Struct Mol Biol. 2011;18:61–66. doi: 10.1038/nsmb.1965. [DOI] [PubMed] [Google Scholar]
- 92.Hildenbrand ZL, et al. Hsp90 can Accommodate the Simultaneous Binding of the FKBP52 and HOP Proteins. Oncotarget. 2011;2:45–58. doi: 10.18632/oncotarget.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waza M, et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- 95.Sittler A, et al. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum Mol Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 96.Auluck PK, et al. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 97.Kraemer BC, et al. Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Hum.Mol.Genet. 2006;15:1483–1496. doi: 10.1093/hmg/ddl067. [DOI] [PubMed] [Google Scholar]
- 98.Chambraud B, et al. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. FASEB J. 2007;21:2787–2797. doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- 99.Elbi C, et al. A novel in situ assay for the identification and characterization of soluble nuclear mobility factors. Sci STKE. 2004;2004:pl10. doi: 10.1126/stke.2382004pl10. [DOI] [PubMed] [Google Scholar]
- 100.Galas MC, et al. The peptidylprolyl cis/trans-isomerase Pin1 modulates stress-induced dephosphorylation of Tau in neurons. Implication in a pathological mechanism related to Alzheimer disease. J Biol Chem. 2006;281:19296–19304. doi: 10.1074/jbc.M601849200. [DOI] [PubMed] [Google Scholar]