Abstract
Nitric oxide signaling, through eNOS (or possibly nNOS), and gap junction communication are essential for normal vascular function. While each component controls specific aspects of vascular function, there is substantial evidence for cross-talk between nitric oxide signaling and the gap junction proteins (connexins), and more recently, protein-protein association between eNOS and connexins. This review will examine the evidence for interaction between these pathways in normal and diseased arteries,highlight the questions that remain about the mechanisms of their interaction, and explore the possible interaction between nitric oxide signaling and the newly discovered pannexin channels.
Keywords: endothelial nitric oxide synthase, neuronal nitric oxide synthase, connexin, pannexin, myoendothelial junctions
1. Introduction
Two of the most important mechanisms controlling vascular function, ranging from regulation of diameter and structure to protection against atherosclerosis, are endothelial production of nitric oxide and gap junction communication between cells in the vascular wall. Nitric oxide, primarily produced by the enzyme endothelial nitric oxide synthase (eNOS), induces smooth muscle relaxation via soluble guanylate cyclase(sGC)-cGMP signaling [1, 2], and is therefore important for vasodilation, particularly in larger arteries [3]. It is also essential for angiogenesis, arteriogenesis, and opposition of atherogenic changes in the vascular wall [4]. In addition to signaling through sGC-cGMP, nitric oxide has the potential to nitrosylate nearby proteins on cysteine residues [5–7], which is a major mechanism for controlling protein function [8]. Gap junctions are essential to the spread of dilation and constriction along the length of the artery, are involved in vasomotion and myogenic tone, and are implicated in non-nitric oxide, non-prostacyclin endothelium-dependent vasodilation in many vascular beds, now usually termed endothelium-dependent hyperpolarization (EDH) [9, 10]. Evidence is accumulating that nitric oxide signaling and gap junction communication are not separate pathways, but rather interdependent functions. The key constituents of these pathways, eNOS and connexins, respectively, both associate with the caveolar protein caveolin-1 and their individual activity and expression influence each other acutely and in disease states. This review will examine the relationship between these two pathways and how their interaction may affect vascular function in health and disease. In addition, the potential relationship of the newly discovered pannexin channels with the nitric oxide pathway will be explored.
2. Nitric Oxide Alters Gap Junction Function
It has been demonstrated in both cultured endothelial cells and in intact arterioles in vivo that exogenously applied nitric oxide, particularly in high doses, can acutely alter gap junction function [11–15]. In addition, some evidence indicates that endogenously produced nitric oxide also effects gap junction function [16], particularly under conditions when nitric oxide production is high. However, it is unclear from the existing evidence which vascular connexin isoforms (Cx45, Cx43, Cx40, Cx37, Cx32) are involved and which gap junction locations [endothelial-endothelial, smooth muscle-smooth muscle, myoendothelial (MEJ)] are targeted.
2.1. Evidence in cultured cells
In cultured endothelial cells, nitric oxide donors acutely alter gap junction coupling, but the effects are variable depending on whether gap junctions are newly forming or stable. In cultured HUVECs (human umbilical endothelial cells), inhibition of NOS or superoxide had no effect on newly forming gap junction coupling, but addition of a nitric oxide donor enhanced the formation of coupling, except at very high doses, and was found to be dependent upon cGMP and cAMP-PKA [12]. The cGMP pathway has the ability to stimulate the cAMP-PKA pathway by preventing the degradation of cAMP through phosphodiesterase inhibition [17]. The same study further showed that in HeLa cells (a cancer cell line that does not endogenously express connexins, which can be transfected to express various connexins), Cx40 is responsible for the NO-dependent enhancement in coupling, as only Cx40 homomeric/homotypic or Cx40-Cx37 homomeric-heterotypic channels were responsive to NO. Trafficking of Cx40 to the membrane was enhanced in HUVECs by nitric oxide donors, in a cAMP-PKA-dependent manner. These data suggest that NO regulates Cx40 translocation and function, and the authors speculate that it may do so through phosphorylation of Cx40 on a putative PKA phosphorylation site or effects on microtubular transport [12].
In cultured endothelial cells, stable gap junctions are also effected by exogenous nitric oxide donors, which decrease dye coupling (i.e., gap junction permeability) in HUVECs [11] and decrease electrical coupling in microvascular endothelial cells [13]. The study performed with microvascular endothelial cells further found that the reduction in gap junction communication was dependent upon Cx37, but not Cx40 or Cx43, and could not be explained by superoxide or peroxynitrite elevation. Serine phosphorylation of Cx37 was not required. In both studies, the reduction in gap junction communication was independent from cGMP. These studies suggest that classic nitric oxide-induced cGMP-PKG signaling is not involved in alteration of coupling, and opens the possibility that the other mechanism of nitric oxide signaling, namely S-nitrosylation, is regulating Cx37 and endothelial cell coupling. This is a very real possibility given that Cx43 can be nitrosylated [16]. However, there is currently no evidence in any cell type as to whether Cx37 also undergoes this modification and what effect it has on function.
In co-cultured endothelial and smooth muscle cells, an important in vitro model for studying gap junction communication at the myoendothelial junction (MEJ), nitric oxide has been shown to enhance MEJ communication [16]. This study found Cx43 and eNOS to be enriched at the MEJ both in co-culture and in multiple types of intact arteries, although vessels isolated from different tissue beds had unequal distribution of eNOS at the MEJ. In both co-culture and intact arteries, Cx43 was constitutively S-nitrosylated on cysteine271. This modification appears to be responsible for the open state of Cx43-containing gap junctions because denitrosylation of Cx43 specifically at the MEJ correlates with a reduction of communication, as evidenced by the restricted movement of IP3 from smooth muscle to endothelium upon phenylephrine stimulation. Moreover, enhancing Cx43 nitrosylation, through inhibition or genetic knock-down of the enzyme GSNOR, an enzyme which denitrosylates Cx43, led to greater communication through the MEJ measured directly in vitro and indirectly in intact murine thoracodorsal arteries. In this study, S-nitrosylation of Cx40, a connexin also found at the MEJ in this model [18], was not detected. This body of work provides evidence that nitric oxide regulates Cx43 gap junction permeability and heterocellular communication through the MEJ (and potentially at other sites in the intact artery) within the resistance vasculature. The unequal distribution of eNOS in different tissue beds at the MEJ may provide a unique tissue-specific control mechanism for S-nitrosylation of Cx43 composed gap junctions at the MEJ and new insight as to how various tissue beds regulate local blood flow.
Collectively, data from cell culture indicate that exogenous nitric oxide can enhance Cx40 incorporation into the endothelial cell plasma membranethrough cGMP-dependent signaling, can impair Cx37-dependent communication between endothelial cells without reliance on cGMP signaling, and endogenous nitric oxide can enhance Cx43-dependent communication at the MEJ through S-nitrosylation. Thus, three of the major vascular connexins are all affected by exogenous or endogenous nitric oxide though at least two different mechanisms. The effects on Cx37 and Cx43 were found to be rapid and reversible without changes in expression, supporting a mechanism involving post-translational modification. Notably, S-nitrosylation is a post-translational mechanism worthy of investigation in the regulation of Cx37, as other major mechanisms have been largely unsupported. It is also possible that phosphorylation of connexins or their protein binding partners may play a role in nitric oxide-mediated regulation, but there is little evidence regarding the sites of phosphorylation or the kinases involved. Even though cultured cells have substantial differences from healthy intact vascular cells [19], the effects of nitric oxide on the connexins show consistency with intact artery function, as explained in the next section.
2.2. Evidence in vivo
In vivo, gap junction function can typically be assessed by measuring conduction of vasoconstriction along the length of an artery/arteriole, conduction of vasodilation along the length of an artery/arteriole, or MEJ-mediated vasodilation. This latter function can be tested by assessing EDH-mediated vasodilation in vessels shown to utilize gap junction communication for this response, or by assessing reactive vasodilation in response to phenylephrine stimulation of smooth muscle, which requires functional MEJ [20–24]. It is not always clear, however, which connexin isoforms are responsible for each of these functions within a given vessel type or among species. It is certainly possible and likely that each gap junction site (EC-EC, SMC-SMC, MEJ) contains multiple connexins possibly in heteromeric and or heterotypic configurations. These uncertainties complicate the interpretation of in vivo data.
In vivo, both endogenous nitric oxide (as evidenced by NOS inhibitors or genetic deletion of eNOS) or exogenously added nitric oxide donors, impair the spread of vasoconstriction along mouse cremaster arterioles [14, 15]. This conducted vasoconstriction apparently relies on gap junctions between smooth muscle cells, and does not seem to involve Cx40 (as evidenced by a lack of effect in CX40 −/− mice) [14]. The inhibitory effect of nitric oxide was only partially dependent upon cGMP signaling. This means that other effects of nitric oxide, such as S-nitrosylation are possible mechanisms.
Sepsis has also been shown to impair conducted vasoconstriction in mouse cremaster arterioles, in a reversible manner that was partially nitric oxide dependent and fully tyrosine phosphorylation dependent [15]. The source of nitric oxide in septic arterioles is largely nNOS, as this enzyme’s activity is upregulated in this condition and genetic deletion of nNOS (but not eNOS or iNOS) eliminates the nitric oxide-induced impairment in conducted vasoconstriction [25]. The conducted response in these vessels was found to be dependent upon Cx37 because genetic deletion eliminated conducted vasoconstriction. However, it is possible that other connexins are also involved given that genetic deletion of one connexin usually affects expression of other connexins [26–30]. In these arterioles, the nitric oxide-induced reduction in conducted vasoconstriction was not dependent upon cGMP. So, like cultured endothelial cells, communication through Cx37-containing gap junctions is likely altered by nitric oxide through alternative mechanisms, such as S-nitrosylation.
The effect of nitric oxide on conducted vasodilation is less consistent. One study indicated that blockade or addition of nitric oxide had no effect on conducted vasodilation in mouse cremaster arterioles in vivo, suggesting that nitric oxide does not alter communication through endothelial gap junctions [14]. Another study performed in the same vascular bed, showed that histamine, an inflammatory factor that induces vasodilation through eNOS-dependent nitric oxide production [31], impaired conduction of vasodilation in mouse cremaster arterioles [32]. This impairment was dependent upon the presence of eNOS and the activation of the cGMP pathway. The reason for the differences in these studies is not clear, but may be related to specific effects of histamine addition in the latter study.
Examining the role of nitric oxide in modifying MEJ communication in vivo is particularly challenging. This is because EDH-mediated vasodilation is determined by blocking nitric oxide synthases (with inhibitors such as L-NAME) and cyclooxygenase, and examining the remaining dilation response. Therefore the role of endogenous nitric oxide on EDH-mediated vasodilation cannot be assessed. In addition, EDH-mediated vasodilation may depend on mechanisms other than gap junction communication, such as diffusible factors [9], which may be experimentally difficult to separate due to overlap in their signaling pathways (i.e., involvement of KCa channels)[33], the lack of specific gap junction inhibitors (although connexin-mimetic peptides may be specific)[9], and the unclear identity of the MEJ connexins. Morover, MEJ communication has been observed under some in vivo experimental conditions [34] but not others [35, 36]. Direct in vivo evidence is, therefore, lacking. Evidence in isolated vessels has indicated that exogenous nitric oxide can inhibit EDH attributed to a diffusible factor like epoxyecosotrienoic acids (EETs) [37, 38], but has no effect on EDH-mediated dilation in mouse superior mesenteric artery [39], a vessel which has functional MEJ communication (as indicated by heterocellular dye coupling) [24, 39] and the EDH response is at least partially attributed to gap junction communication in branches of this artery [24]. This very limited evidence suggests that exogenous nitric oxide does not alter this type of MEJ-mediated function.
Collectively, in vivo evidence is consistent with cell culture evidence in that elevation of nitric oxide impairs gap junction communication conducted through Cx37-containing channels, probably located at homocellular junctions. In vivo effects of nitric oxide on Cx40- and Cx43-containing channels are not clear.
3. Interrelationship of eNOS and gap junctions
3.1. Loss of eNOS affects gap junction communication
The effect of eNOS presence on connexins and gap junction communication has been studied in eNOS −/− mice. There is some evidence that loss of eNOS upregulates gap junction communication in certain arteries. With regard to conduction of vasodilation, eNOS knockout mice have normal conduction in cremaster arterioles, suggesting that endothelial cell gap junctions are not affected by the loss of eNOS [32]. Conversely, conducted vasoconstriction is enhanced in eNOS −/− mice [14], suggesting that smooth muscle cell gap junction communication is enhanced.
In male eNOS −/− mice the EDH-dependent dilation is increased in isolated skeletal muscle arterioles [40] and mesenteric arteries [41–43]. This suggests the possibility of enhanced MEJ coupling. One of these studies did find that the enhanced EDH response was blocked by the non-selective gap junction inhibitor 18alpha-glycyrrhetinic acid supporting this concept [43]. It should be noted that not all studies have found enhanced EDH-dependent vasodilation in mesenteric arteries in eNOS −/− mice. One study found no EDH-mediated responses in these arteries in either wild-type or eNOS −/− [44], and another found a decline [45].The loss of eNOS does not seem to effect connexin regulation at the transcriptional level because connexin mRNA (Cx37, Cx40, Cx43, Cx45) expression is similar in the mesenteric arterial vasculature of both wild-type and eNOS knockout mice [46]. Therefore, it is likely that post-translational mechanisms are responsible for alterations in gap junction coupling.
It is not clear whether the enhanced gap junction communication, as evidenced in most studies, with the deletion of eNOS is due to loss of the inhibitory effect of nitric oxide on gap junction communication, or due to a loss of interaction between eNOS and connexin proteins. The direct interaction between these proteins is highlighted in the next section.
3.2 Loss of connexins affects eNOS expression and function
The expression and localization of eNOS is partially dependent upon the expression of gap junction proteins. It has recently been shown that if Cx40 is absent (Cx40 −/− mice), with a coincident reduction in Cx37, then aortic eNOS expression and function are impaired [27]. This reduction in eNOS is post-transcriptional, as eNOS mRNA is comparable in Cx40−/− and wild-type aorta. Moreover, Cx40, Cx37, and eNOS may form a protein complex, as evidenced by both immunoprecipitation and in situ proximity ligation assay, indicating localization of this complex at the borders of aortic endothelial cells.
It is likely that eNOS and Cx37 interact directly because the C-term of Cx37 contains a binding motif for eNOS [47]. The region of eNOS with a Cx37 binding motif lies in the same region that binds to caveolin-1, a negative regulator of eNOS activity [48–50] and a scaffolding protein that directs the formation of caveolae [51–55]. In cultured cells, a peptide corresponding to the eNOS binding motif reduces Cx37 channel conductance, suggesting that eNOS may be a negative regulator of Cx37 channel activity [47]. In cultured endothelial cells, not only are eNOS and Cx37 shown to associate, but knockdown of Cx37 with RNAi results in increased eNOS activity and NO production, indicating that like caveolin-1, Cx37 may be a negative regulator of eNOS [47]. The results from cultured endothelial cells must be interpreted with caution, however, because cultured endothelial cells contain 10–1000-fold less caveolae than endothelial cells within intact vessels [56], coincident with relocation of caveolin-1 from the cell membrane to the perinuclear region [47]. Because both eNOS and connexins can localize to lipid rafts and caveolae and physically associate with caveolin-1 [57–61], the loss of interaction with caveolin-1 may alter the dynamics of eNOS and its binding patterns. For example, the absence of caveolin-1 may promote binding of Cx37 to eNOS. Despite the limitations of cell culture data, the physical association of eNOS, Cx37, and Cx40 in the membrane of aortic endothelial cells in vivo [27], suggest that cell culture data is at least partly consistent with an interaction between these proteins in vivo.
3.3. Disruption of caveolae effects both nitric oxide signaling and gap junction function
In unstimulated endothelial cells, caveolin-1 binds to eNOS and suppresses its activity [48–50]. Upon stimulation, calcium-calmodulin binds to eNOS, and caveolin-1 dissociates to allow full activation of eNOS [56, 62, 63]. If caveolin-1 is absent, there is loss of normal endothelial caveolar structures and failure of proper regulation of eNOS function [52, 53, 64, 65]. Like eNOS, connexins have been found to associate with caveolin-1 [39, 57]. Cx43 is found in lipid rafts and was first discovered to associate with caveolin-1 in 293T cells transfected with both proteins [57], and later discovered to directly interact with caveolin-1 in keratinocytes [66]. Since this original discovery, both Cx43 and Cx40 have been found to co-immunoprecipitate with caveolin-1 in cultured endothelial cells [39]. If caveolin-1 expression is lost (i.e., caveolin −/− mice), expression of Cx43, Cx40, and Cx37 are all reduced in aorta and superior mesenteric artery, and this is coincident with impaired EDH-dependent vasodilation and MEJ dye transfer in superior mesenteric artery [39]. These results suggest that caveolin-1 is essential for proper vascular connexin localization to the membrane, formation of functional MEJ’s, and normal gap junction-dependent vasodilatory responses. The common association with caveolin-1 further supports the notion that eNOS and connexins colocalize in the same cellular domains and exist in the same protein complexes.
4. Potential role of nitric oxide in regulation of vascular pannexins
Pannexins are a novel protein family discovered in 2000 in the mammalian genome based on their limited sequence homology to the innexins, the gap junction proteins of invertebrates [67, 68]. Structurally, pannexins share a similar membrane topology with connexins, the constitutive protein of gap junctions in vertebrates. Similarly to connexin proteins, pannexin proteins contain four transmembrane domains and hexamerize to form a non-selective channel at the plasma membrane [69]. However, unlike connexins, there is currently no evidence of gap junctions formed by the apposition of two hexamers of pannexins, which may be due to the lower number of cysteine residues on the extracellular loop of pannexins, as compared to connexins [69, 70]. There are currently 3 known isoforms of pannexins, present on 3 different regions of the genome (Panx1, Panx2 and Panx3) [67, 68, 71]. While Panx1 is ubiquitous, Panx2 is mostly expressed in the central nervous system and Panx3 is found mainly in skin and cartilage [72]. The functional state and the cellular distribution of pannexins seem to be mainly regulated by glycosylation and interaction with other pannexins, but not by phosphorylation [69, 73, 74].
Functionally, it has been extensively shown that pannexins are paracrine channels that can release purines such as ATP, however, in theory, pannexin channels allow the passage of small molecules under 1 kDa between the cytosol and the extracellular environment [75]. The pannexin-mediated release of ATP in the extracellular milieu has been shown to serve as a key physiological signal for intercellular signaling as well as a signal to promote cell death [75]. Pannexin protein plays a key role in the inflammatory response. For example, pannexins have been recently identified as the channel mediating the “find me” signal released by apoptotic cells to recruit phagocytes in order to clear the dying cells [76], and there is growing evidence that Panx1 channels are involved in the secretion pathway of pro-inflammatory cytokines such as IL-1β [77]. In the vasculature, a recent study reported a role of Panx1 in the contraction of resistance arteries where phenylephrine activates the release of vasoactives purines, such as ATP, through Panx1 channels [78].
As previously described in this review, the nitrosylation of cysteines have been shown to be a key element in regulating connexins, therefore, recent effort has been directed toward understanding the importance of cysteine residues in Panx1. Site-directed mutagenesis of cysteines revealed that Cys346 residue is essential in the regulation of Panx1 opening as its mutation leads to a constitutively leaky channel [79]. Furthermore, when the Cys346 residue is mutated, Panx1 is hypoglycosylated, resulting in impaired gating of the channel [73, 79, 80].
Recently, several reports demonstrated opening of Panx1 channels under oxygen and glucose deprivation in vitro, mimicking pathological conditions such as stroke [81, 82]. In these conditions, the opening of Panx1 channels led to an increase in membrane permeability, which consequently induces neuronal degeneration. Interestingly, during ischemia, NO production is enhanced, which induces changes in redox potential. Although there is no direct evidence of S-nitrosylation of cysteine residues of Panx1 during ischemic conditions, Zhang et al. described that Panx1 channel activity is enhanced in presence of NO donors [82]. Therefore, like the connexins, there is potential for nitric oxide-dependent regulation of pannexin channel function.
5. Relationship between eNOS and vascular gap junctions in disease states
5.1 eNOS and connexins in atherosclerosis
Typically eNOS production of NO in the vasculature acts in an anti-atherogenic manner [83]. However, during atherogenesis eNOS has been reported to switch to production of superoxide (instead of NO) leading to activation of reactive oxygen species (ROS) [84]. Several physiological pathways require low levels of ROS, such as superoxide anion, and it has been hypothesized that these may pass through gap junctions [85, 86] (for review, see [87]). Despite this, high levels of ROS following injury (e.g. ischemia) are associated with cellular damage, inducing lipid peroxidation of plasma membrane proteins, DNA oxidation and nitrosylation of proteins altering cellular function. In models of atherosclerosis, both up- and down-regulation of eNOS in ApoE−/− (atheroprone mice) are shown to enhance the atherosclerotic state [84]. In the double knockout eNOS−/− ApoE−/− mice, atherosclerosis is advanced, suggesting a preventative role for NO produced in atherogenesis. Yet over-expression of eNOS in ApoE−/− mice also leads to enhanced atherosclerosis. The explanation for this data is that eNOS switches function from production of NO to production of superoxide, leading to further cellular damage [84]. It is also possible that secondary pathways which sequester eNOS may play a role in these models, such as has been demonstrated for the connexins [27, 47].
Connexins and their expression are integrally linked to the progression of atherosclerosis, with associations between altered connexin expression, signaling and hemichannel function all attributed to progression of the disease state [88, 89]. Based on currently available data, the exact mechanisms for this are not yet fully understood.
In acute injury models such as ischemic re-perfusion insult, levels of activated eNOS are associated with down-regulation of Cx43 with no alterations noted in Cx37 or Cx40 [90]. Reducing eNOS in EC following application of L-NAME does not alter Cx43 expression and has not been shown to alter cellular proliferation or migration [90]. These data combined with previous data showing that NO specifically nitrosylates Cx43 [16], suggest that eNOS can induce post-translational modifications of Cx43 but that this may not be reversible following application of NO and that this pathway is not necessarily involved in phenotypic modulation of cells.
As mentioned previously, in vascular cell specific Cx40−/− mice, aortic NO release (via production of eNOS) is significantly reduced with both Cx37 and Cx40 shown to directly interact with eNOS at intercellular contact zones [27, 47]. Separate studies have shown that reductions in Cx40 in vascular cells leads to a recruitment of leukocyte adhesion indicating that it is important in atherosclerotic development [91]. Thus, the reduction in nitric oxide may be a contributing factor to the atherogenic phenotype of the Cx40 −/− mice.
Point mutations in Cx37 (C1019T mutation, encoding 319P>S) are associated with an increase in atherogenicity in humans and in mice [92]. While little is known about the physical structure of the Cx37 CT and its potential binding partners, analyses show several consensus sequences for binding partners including eNOS [47]. Using isolated single protein analyses between the wild type and mutated forms of Cx37 and native eNOS peptides, this study identified that Cx37 CT can bind with eNOS regardless of the C1019T mutation, suggesting that both native and mutated Cx37 acts in a manner to sequester eNOS and regulate vasomotor tone. One other possibility comes from reports that suggest Cx37 interacts with caveolin-1 and may be a route through which Cx37 interacts with eNOS to modulate calmodulin binding and NO production [47]. However, these previous studies do not indicate a mechanism through which Cxs 37 and 40 mediate this post-translational modification, whether through sequestration in caveloae or through direct interactions and it is not clear how loss of connexins leads to a loss of eNOS expression. Therefore it is possible that reductions in endothelial Cx37 could cause alterations in eNOS as found in atherosclerosis, but does not involve the sites associated in the known C1019T mutation.
In advanced disease states, stent placement may alter connexin and eNOS expression leading to endothelial dysfunction. In vitro studies in endothelial cells revealed that cells grown on stent metal demonstrated significant loss of Cx43 and eNOS expression and may be associated with endothelial cell dysfunction which could further the disease state leading to stent-restenosis [93]. Therefore endothelial dysfunction in restenosis may be mediated in part through Cx43 control of eNOS through an as yet unknown pathway.
5.2. eNOS and connexins in hypertension
Reduction in eNOS and NO production have been associated with endothelial dysfunction in hypertension [94, 95]. Although reports in humans have suggested that there is no definitive link between genetic alterations in eNOS and hypertension [96]. Reductions in the availability of NO are associated with increased pulmonary arterial hypertension (PAH; abnormally high pressure in the pulmonary artery due to narrowing of the pulmonary resistance arteries) in humans, with disruptions between the interactions of eNOS and caveolin-1 thought to reduce biologically active NO leading to PAH (for review see [97]). Conversely, increases in the expression of eNOS and production of NO have been associated with reductions in blood pressure in treated hypertensive mice [98].
As previously discussed, the vascular connexins interact with both eNOS and caveolin-1 and may play a role in hypertension. In spontaneously hypertensive rats (SHR) treated with L-NAME (inhibiting NOS species), levels of endothelial Cx37 and Cx43 (but not Cx40) are significantly reduced, with further studies showing that in aortic endothelium, Cx43 expression is reduced in pre-hypertensive mice [99, 100]. Levels of Cx37 and Cx43 in these mice can be rescued following treatments with adrenergic blocker Cardovil (and to a lesser extent atenelol) with concomitant reductions in blood pressure (in a pathway that is independent of the adrenergic blockade). These findings suggest that Cx43 and Cx37 are associated with eNOS-mediated hypertension, although the pathways have yet to be defined [99].
Multiple groups have studied how vascular endothelial cell specific deletion of Cx40 leads to hypertension in mice [101–103]. Despite the above studies, others have suggested that only reduction in Cx40 (not Cx37 and not Cx43) are associated to hypertension in SHR as compared to Wistar-Koyoto rats (WKR) [104]. While Cx40−/− mice were generally considered to be hypertensive, this is not associated with a loss of NO signaling between EC and VSMC in the resistance arterioles, rather that renin secretion in the glomerulus is significantly down-regulated [102, 103, 105]. Despite this, earlier studies have shown that the EC Cx40−/− knockout mice leads to dysregulated conduction along the arteriolar beds and that this dysfunction was independent of NO function and the renin/ angiotensin system [101]. Further studies in WKR have demonstrated that Cx37 but not Cx40 or Cx43 levels are elevated as compared to SD or SHR altered in renal arterioles. The authors conclude that this elevated Cx37 in WKR and that connexins in general are not associated with alterations in blood pressure in pre-glomerular vessels [106]. Clearly, eNOS causes alterations in hypertension although whether connexins are directly linked to this pathway is not well defined based on the current literature.
5.3. eNOS and connexins in diabetes
Enhanced production of ROS, increased degradation of NO (through NADPH oxidases) and loss of eNOS are associated with diabetes and insulin resistance in cells [107, 108]. This suggests that eNOS activity and NO production are integral in diabetes and insulin resistance pathways [98]. Studies have indicated that alterations in Cx40 and 43 in efferent arterioles may be associated with reductions in eNOS expression. Cx40 and 43 in afferent and efferent arterioles were noted to be up- and down-regulated (respectively) in diabetic mice. In eNOS −/− mice levels of Cx40 and Cx43 were found to be significantly different following diabetic induction as compared to non-treated eNOS−/− mice. These data suggest that regulation of Cx40 and Cx43 expression in diabetes requires the presence of eNOS to perform its function [109]. In diabetic-induced apoE−/−mice, levels of eNOS increase with an associated decrease in Cx37 but not other vascular connexins in small mesenteric arteries [110]. In diabetic mice with erectile dysfunction, reductions of eNOS were associated with an increase in Cx43 expression in penile tissue as compared to diabetic mice [111]. These data suggest that differential regulation of vascular connexins, i.e. upregulation of Cx40 and down regulation of Cx37 and Cx43, may act in conjunction with eNOS to regulate vascular function in diabetes.
6. Concluding remarks
There is clear evidence that nitric oxide affects vascular gap junction communication in vitro and in vivo, with both enhancement and inhibition of communication reported depending on the connexins involved and the cellular location within the vessel. While there’s evidence for S-nitrosylation as a mechanism by which nitric oxide affects Cx43 function, the precise mechanisms for nitric oxide regulation of Cx37 and Cx40 are not clear. Nitric oxide’s effects on Cx37 are largely independent of cGMP signaling, so S-nitrosylation is a possibility. Nitric oxide’s effects on Cx40 seem to rely on cGMP signaling, but the precise molecular alterations and interactions are unknown. Additionally, the possibility of S-nitrosylation of any of the vascular connexins other than Cx43 (Cx45, Cx40, Cx37, Cx32) or pannexin-1, has not been explored and remains a possible regulatory mechanism. It is also unknown how nitric oxide regulation of gap junctions may vary by cellular location (EC-EC, SMC-SMC, MEJ) or vascular bed.
There is new evidence that not only does nitric oxide interact with connexins, but the enzyme eNOS does as well. In endotheial cells, eNOS can exist in a complex with at least two connexins (Cx37, Cx40) both in vitro and in vivo, and this interaction effects eNOS function and expression at the membrane. It is also known that loss of eNOS affects gap junction communication in arteries, but it is unknown whether this is due to the loss of direct interaction of connexins with eNOS or the loss of nitric oxide signaling effects on connexins. It is clear that in vascular disease conditions, such as atherosclerosis, hypertension, and diabetes, both nitric oxide signaling and connexin expression and/or function are altered. A remaining question is whether there is a common mechanism for alteration in these pathways with each disease condition, or is the alteration of one of the two pathways affecting the other in sequence. Collectively, the prevailing data point to a close interrelationship between eNOS/nitric oxide signaling and connexin function, with the precise mechanisms of their interactions largely undiscovered.
Highlights.
first review of eNOS and connexins
schematic of the possible interplay between connexins/pannexins and NO in endothelium and smooth muscle
pose questions for future research in vascular function and connexins
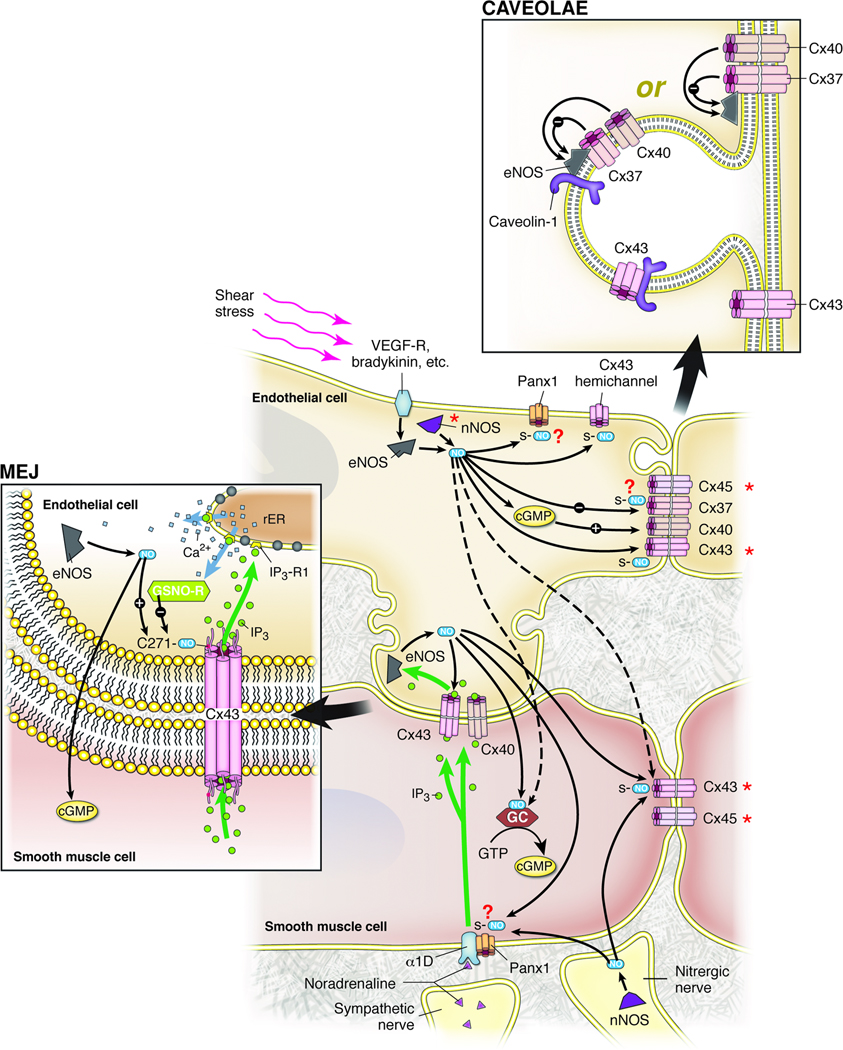
Figure 1. Model for the Interaction of the Nitric Oxide System with Vascular Connexins.
Nitric oxide production can be stimulated by several mechanisms, including, but not limited to: 1) activation of endothelial cells by shear stress or agonists such as bradykinin leading to activation of eNOS and possibly nNOS, 2) activation of endothelial eNOS through smooth muscle α1-adrenergic stimulation followed by movement of IP3 through myoendothelial gap junctions (MEJs) and release of Ca++ from the endothelial sarcoplasmic reticulum (illustrated in MEJ panel), and 3) activation of nNOS in nitrergic nerves.
Nitric oxide action includes S-nitrosylation of Cx43 (and possibly other connexins and Panx1), which increases the open state of Cx43-containing gap junctions in the MEJ, and possibly at other sites in the vascular cells. Nitric oxide also inhibits Cx37 channel conductance in a generally non-cGMP-dependent manner, while it increases de novo formation of Cx40-containing gap junctions in a cGMP-dependent manner.
eNOS, Cx37, and Cx40 can exist in a complex, but it is not clear whether this complex is contained within the caveolae (illustrated in CAVEOLAE panel). Cx37 interaction decreases eNOS activity, while Cx40 expression is essential for proper expression of eNOS at the membrane. Both eNOS and Cx43 have been found to bind directly to caveolin-1.
Although connexins are shown in homomeric/homotypic gap junction channels, it is quite possible that more than one connexin isoform bind to form heteromeric and/or heterotypic gap junction channels. * indicates tissue variability in expression.
Acknowledgements
We thank Anita Impagliazzo for the illustration. This work was supported by NIH HL088554 (BEI), AHA SDG (BEI), an AHA post-doctoral fellowship (MB; SRJ), an NRSA post-doctoral fellowship (ACS), R15 HL102742-01 (RLW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friebe A, Koesling D. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 2.Murad F. J Clin Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. Proc Natl Acad Sci U S A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel T, Vanhoutte PM. Pflugers Arch. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Proc Natl Acad Sci U S A. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 7.Qian J, Zhang Q, Church JE, Stepp DW, Rudic RD, Fulton DJ. Am J Physiol Heart Circ Physiol. 2010;298:H112–H118. doi: 10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima B, Forrester MT, Hess DT, Stamler JS. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit C, Griffith TM. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 10.Garland CJ, Hiley CR, Dora KA. Br J Pharmacol. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. J Cell Physiol. 2005;203:233–242. doi: 10.1002/jcp.20218. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Gloe T, Pohl U, Zahler S. Cardiovasc Res. 2003;60:421–430. doi: 10.1016/j.cardiores.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 13.McKinnon RL, Bolon ML, Wang HX, Swarbreck S, Kidder GM, Simon AM, Tyml K. Am J Physiol Heart Circ Physiol. 2009;297:H93–H101. doi: 10.1152/ajpheart.01148.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodenwaldt B, Pohl U, de Wit C. Am J Physiol Heart Circ Physiol. 2007;292:H2341–H2348. doi: 10.1152/ajpheart.01061.2006. [DOI] [PubMed] [Google Scholar]
- 15.Lidington D, Ouellette Y, Li F, Tyml K. J Vasc Res. 2003;40:149–158. doi: 10.1159/000070712. [DOI] [PubMed] [Google Scholar]
- 16.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Arterioscler Thromb Vasc Biol. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis SH, Busch JL, Corbin JD, Sibley D. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakson BE, Duling BR. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 19.Sandow SL, Grayson TH. Am J Physiol Heart Circ Physiol. 2009;297:H1–H7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- 20.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 21.Dora KA, Doyle MP, Duling BR. Proc Natl Acad Sci U S A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yashiro Y, Duling BR. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- 23.Isakson BE, Ramos SI, Duling BR. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 24.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 25.McKinnon RL, Lidington D, Bolon M, Ouellette Y, Kidder GM, Tyml K. Cardiovasc Res. 2006;69:236–244. doi: 10.1016/j.cardiores.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Day KH, Damon DN, Duling BR. Proc Natl Acad Sci U S A. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso F, Boittin FX, Beny JL, Haefliger JA. Am J Physiol Heart Circ Physiol. 2010;299:H1365–H1373. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 28.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 29.Kruger O, Beny JL, Chabaud F, Traub O, Theis M, Brix K, Kirchhoff S, Willecke K. J Vasc Res. 2002;39:160–172. doi: 10.1159/000057764. [DOI] [PubMed] [Google Scholar]
- 30.Simon AM, McWhorter AR. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 31.Payne GW, Madri JA, Sessa WC, Segal SS. Am J Physiol Heart Circ Physiol. 2003;285:H493–H498. doi: 10.1152/ajpheart.00071.2003. [DOI] [PubMed] [Google Scholar]
- 32.Payne GW, Madri JA, Sessa WC, Segal SS. Faseb J. 2004;18:280–286. doi: 10.1096/fj.03-0752com. [DOI] [PubMed] [Google Scholar]
- 33.Feletou M. Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, Hill CE. J Physiol. 589:2607–2623. doi: 10.1113/jphysiol.2010.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 36.Welsh DG, Segal SS. Am J Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 37.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph V, Freeman BA. Circ Res. 2009;105:511–522. doi: 10.1161/CIRCRESAHA.109.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 40.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Am J Physiol Heart Circ Physiol. 2000;278:H762–H768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- 41.Ding H, Kubes P, Triggle C. Br J Pharmacol. 2000;129:1194–1200. doi: 10.1038/sj.bjp.0703144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldron GJ, Ding H, Lovren F, Kubes P, Triggle CR. Br J Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. Hypertension. 2001;38:833–839. doi: 10.1161/hy1001.092651. [DOI] [PubMed] [Google Scholar]
- 44.Chataigneau T, Feletou M, Huang PL, Fishman MC, Duhault J, Vanhoutte PM. Br J Pharmacol. 1999;126:219–226. doi: 10.1038/sj.bjp.0702300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceroni L, Ellis A, Wiehler WB, Jiang YF, Ding H, Triggle CR. Eur J Pharmacol. 2007;560:193–200. doi: 10.1016/j.ejphar.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, Roth I, Satta N, Dunoyer-Geindre S, Sorgen P, Taffet S, Kwak BR, Delmar M. Arterioscler Thromb Vasc Biol. 2010;30:827–834. doi: 10.1161/ATVBAHA.109.200816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ju H, Zou R, Venema VJ, Venema RC. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 50.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. J Biol Chem. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 51.Fra AM, Williamson E, Simons K, Parton RG. Proc Natl Acad Sci U S A. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 53.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Song KS, Koh SS, Kikuchi A, Lisanti MP. J Biol Chem. 1996;271:28647–28654. doi: 10.1074/jbc.271.45.28647. [DOI] [PubMed] [Google Scholar]
- 55.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. J Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gratton JP, Bernatchez P, Sessa WC. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 57.Schubert AL, Schubert W, Spray DC, Lisanti MP. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 61.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 62.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 63.Dudzinski DM, Michel T. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Gregoire V, Dessy C, Balligand JL, Feron O. Circ Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Mol Biol Cell. 2008;19:912–928. doi: 10.1091/mbc.E07-06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 68.Panchin YV. J Exp Biol. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 69.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 70.D'Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 71.MacVicar BA, Thompson RJ. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 73.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 74.Penuela S, Bhalla R, Nag K, Laird DW. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D'Hondt C, Ponsaerts R, De Smedt H, Vinken M, De Vuyst E, De Bock M, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G. Cell Signal. 2011;23:305–316. doi: 10.1016/j.cellsig.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 76.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pelegrin P, Surprenant A. Purinergic Signal. 2009;5:129–137. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni F, Ravichandran K, Penuela S, Laird DW, Isakson BE. Circulation Research. 2011;105:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bunse S, Schmidt M, Prochnow N, Zoidl G, Dermietzel R. J Biol Chem. 2010;285:38444–38452. doi: 10.1074/jbc.M110.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boassa D, Qiu F, Dahl G, Sosinsky G. Cell Commun Adhes. 2008;15:119–132. doi: 10.1080/15419060802013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson RJ, Zhou N, MacVicar BA. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. J Neurosci Res. 2008;86:2281–2291. doi: 10.1002/jnr.21675. [DOI] [PubMed] [Google Scholar]
- 83.Heeba G, Moselhy ME, Hassan M, Khalifa M, Gryglewski R, Malinski T. Br J Pharmacol. 2009;156:1256–1266. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takaya T, Hirata K, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, Alp NJ, Channon KM, Yokoyama M, Kawashima S. Arterioscler Thromb Vasc Biol. 2007;27:1632–1637. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
- 85.Tang EH, Vanhoutte PM. J Pharmacol Exp Ther. 2008;327:148–153. doi: 10.1124/jpet.108.140046. [DOI] [PubMed] [Google Scholar]
- 86.Billaud M, Marthan R, Savineau JP, Guibert C. PLoS One. 2009;4:e6432. doi: 10.1371/journal.pone.0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolin MS. Am J Physiol Heart Circ Physiol. 2009;296:H539–H549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 89.Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. Am J Pathol. 2009;175:916–924. doi: 10.2353/ajpath.2009.090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 91.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Circulation. 2010;121:123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 92.Boerma M, Forsberg L, Van Zeijl L, Morgenstern R, De Faire U, Lemne C, Erlinge D, Thulin T, Hong Y, Cotgreave IA. J Intern Med. 1999;246:211–218. doi: 10.1046/j.1365-2796.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 93.Yeh HI, Lu SK, Tian TY, Hong RC, Lee WH, Tsai CH. J Biomed Mater Res A. 2006;76:835–841. doi: 10.1002/jbm.a.30595. [DOI] [PubMed] [Google Scholar]
- 94.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 95.Desjardins F, Balligand JL. Acta Clin Belg. 2006;61:326–334. doi: 10.1179/acb.2006.052. [DOI] [PubMed] [Google Scholar]
- 96.Kato N, Sugiyama T, Morita H, Nabika T, Kurihara H, Yamori Y, Yazaki Y. Hypertension. 1999;33:933–936. doi: 10.1161/01.hyp.33.4.933. [DOI] [PubMed] [Google Scholar]
- 97.Mathew R, Huang J, Gewitz MH. Cardiol Rev. 2007;15:143–149. doi: 10.1097/01.crd.0000249381.49138.b9. [DOI] [PubMed] [Google Scholar]
- 98.Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. J Pharmacol Exp Ther. 2009;328:610–620. doi: 10.1124/jpet.108.143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeh HI, Lee PY, Su CH, Tian TY, Ko YS, Tsai CH. Am J Hypertens. 2006;19:129–135. doi: 10.1016/j.amjhyper.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 100.Dlugosova K, Mitasikova M, Bernatova I, Weismann P, Okruhlicova L. Physiol Res. 2008;57(Suppl 2):S23–S29. doi: 10.33549/physiolres.931548. [DOI] [PubMed] [Google Scholar]
- 101.de Wit C, Roos F, Bolz SS, Pohl U. Physiol Genomics. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 102.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 103.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 104.Rummery NM, McKenzie KU, Whitworth JA, Hill CE. J Hypertens. 2002;20:247–253. doi: 10.1097/00004872-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 105.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 106.Braunstein TH, Sorensen CM, Holstein-Rathlou NH. Apmis. 2009;117:268–276. doi: 10.1111/j.1600-0463.2009.02432.x. [DOI] [PubMed] [Google Scholar]
- 107.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 108.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 109.Zhang JH, Kawashima S, Yokoyama M, Huang P, Hill CE. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1000–1008. doi: 10.1002/ar.a.20369. [DOI] [PubMed] [Google Scholar]
- 110.Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C. Br J Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen WG, Zhu XF, Hou JQ, Pu JX, Yan CY. Zhonghua Nan Ke Xue. 2008;14:427–430. [PubMed] [Google Scholar]