Abstract
Background
Prior research, predominantly with adults, has shown that the serotonin transporter gene (5-HTTLPR) interacts with stress (GxE) to predict depressive symptoms; however, few GxE studies have been conducted with youth using rigorous methods, particularly a prospective design and contextual interview to assess stress. The current study examined the interaction between 5-HTTLPR and stress, both chronic and episodic, to predict longitudinal change in depressive symptoms among children and adolescents.
Methods
A general community sample of youth (N=200; 57% girls; mean age: 12.09 years old) were genotyped for 5-HTTLPR (rs 25531) at baseline. They were interviewed via contextual stress procedures to ascertain chronic family stress and episodic stressors and completed depressive symptoms questionnaires at baseline and 6 months later.
Results
A significant GxE showed that chronic family stress predicted prospective increases in depressive symptoms over 6 months among youth possessing the high risk S allele. This GxE was not found for episodic stressors occurring in the last 6 months. There was no moderation by sex or pubertal status.
Conclusions
These findings advance knowledge on GxE effects in depression among youth. This is the first study to show that chronic family stress, but not episodic stressors, when ascertained by rigorous stress interview, interacts with 5-HTTLPR to prospectively predict depressive symptoms among children and adolescents.
Keywords: children, adolescents, depression, genetics, environment, serotonin
Depression in children and adolescents is a serious and debilitating disorder [1]. Developmental epidemiological research clearly shows symptoms and episodes of depression increase markedly from childhood into adolescence [2, 3, 4], and the sex difference in depression emerges in early to middle adolescence [5]. Understanding etiological processes contributing to the development of youth depression is crucial as most individuals experience their first depressive episode in adolescence [3, 6, 7], and adolescent-onset depression substantially increases risk for continuity and recurrence of depression into adulthood [8]. While many vulnerabilities to depression exist and have been studied [9], much attention has been given to the study of gene-environment interactions (GxE). Interest in how GxE confers risk to depression has surged since the seminal publication by Caspi and colleagues [10] demonstrating that those who both experienced major negative events and carried at least one short allele of the serotonin transporter promoter gene (5-HTTLPR) experienced elevated depression (both symptoms and disorder) over time in early adulthood. Since then, numerous GxE studies of 5-HTTLPR and various environmental risks among adults have been conducted, with a recent and comprehensive meta-analysis showing a robust, significant GxE in adult depression [11].
Despite this recent meta-analysis demonstrating an overall significant GxE in adult depression, several limitations and unaddressed questions remain. First, many, but not all, of the adult studies demonstrated a significant GxE [e.g., 12]. Prior to Karg and colleagues’ [11] larger, more comprehensive and positive meta-analysis, Risch and colleague’s nonsignificant meta-analysis prompted several commentaries that may underlie the equivocal GxE findings. Several reviews have noted the inconsistencies in the GxE literature may stem from the use of environmental stress measures with unknown psychometric properties [e.g., 14, 15]. Indeed, Uher and McGuffin [14, 15] have demonstrated that studies utilizing more specific or interview based measures of stress were significantly more likely to obtain GxE effects in depression. Clearly, careful and rigorous measurement of environmental stress is necessary to accurately test GxE influences.
Additionally, there has been considerably less research investigating GxE in youth in contrast to the preponderance of adult GxE research. Initial studies have found evidence supporting an interaction between genes implicated in the 5HT system and environmental stress, such as maltreatment [16, 17], family environment [18, 19, 20], and general stressors [21, 22]. However, there are particular limitations to most of youth GxE studies. First, the majority of studies utilized cross-sectional designs (see [18, 22] for exceptions), which cannot tease apart the directionality of GxE on depression. Second, most studiesmeasured environmental stress with potentially subjective self-report stress checklists (see [18] as an exception). Reviewers of stress measurement have strongly advocated for contextual interviews as the gold-standard method to ascertain negative events more objectively relative to self-report checklists [e.g., 23, 24, 25, 15]. Other studies examined relatively infrequent or highly specific stressors (e.g., maltreatment [16, 17]) or included stressors that may not be developmentally appropriate for younger populations (e.g., financial difficulties [21]).
Recently, Hammen and colleagues [18] found chronic family stress at age 15, but not acute stressors, interacted with 5-HTTLPR to predict depressive symptoms at age 20. This study importantly advanced knowledge in the GxE literature as it utilized a reliable and valid contextual stress interview to ascertain both chronic and acute family stress and evaluated which type of stress (i.e., chronic versus acute) interacted with 5-HTTLPR to predict depressive symptoms at age 20 in a representative sample. However, additional research is needed in order to both replicate these findings using contextual stress interview procedures assessing chronic and acute family stress in a GxE framework to predict depression and to address important remaining questions. In particular, chronic family stress was measured at age 15 whereas acute stressors, occurring between ages 15–19, were assessed at age 20. Furthermore, baseline levels of depressive symptoms at age 15 were not controlled for in GxE analyses to predict symptoms at age 20. Controlling for initial levels of symptoms is essential given strong continuity of symptoms over time as the best predictor of current depressive symptoms is past symptoms [26, 27]. This leaves open the possibility that processes other than GxE may have contributed to the prediction of depressive symptoms at age 20.
Finally, GxE research with youth has predominantly sampled and studied adolescents. Only Kaufman and colleagues [15] studied preadolescents (ages 5–15), yet they controlled for age which precludes an examination of whether and how GxE in depression changes across development. Given the clear developmental trends and surge in depression from childhood into adolescence, the lack of GxE research in samples of youth across different developmentally salient ages is a notable gap as it is unknown whether developmental processes, such as puberty, moderate expected GxE effects in depression. Pubertal status has been implicated in behavioral genetic studies as a possible moderator of GxE predicting depression in youth [28,29]; however, this has not yet been examined in molecular genetic research.
The current study aimed to extend GxE research among youth using a longitudinal design controlling for baseline depressive symptoms to enable prospective prediction of depressive symptoms as a function of 5-HTTLPR interacting with stress among a community sample of youth. Specifically, we examined whether chronic family stress and recent, acute (episodic) stressors interacted with 5-HTTLPR to predict youth depressive symptoms. We hypothesized that youth carrying a S/LG allele (in an additive genetic framework) and experiencing high levels of chronic family stress would exhibit the greatest prospective increase in depressive symptoms over time. Additionally, given the emergence of the sex difference in depression in early adolescence and mixed findings pertaining to GxE in girls versus boys (e.g., sex moderation in [18, 20, 21]; no moderation in the remaining studies), as well as a limited developmental focus in prior research, pubertal status and sex were examined as possible moderators.
Materials and Methods
Participants
Participants included 200 children and adolescents who were recruited from metropolitan Denver, Colorado school districts. Youth had to currently be in 3rd (age 7 to 9 years old), 6th (age 10 to 12 years old), or 9th (age 13 to 16 years old) grade. They were excluded if they had a severe learning or psychiatric problem (e.g., autism, psychosis) that was likely to interfere with completion of an extensive laboratory protocol. The participation rate was 72%, which is above the rate recommended for having a representative sample of the target population ([30, 31] see [22] for sampling details). The sample was approximately evenly divided by sex (males: 43%, females: 57%), grade (3rd grade: 31%, 6th grade: 38%, 9th grade: 32%), and of mixed ethnic origin (Caucasian: 67%, African American: 7%, Latino: 7%, Asian/Pacific Islander: 4%, Other/Mixed Race: 14%). Youth ranged in age from 7 to 16 years old (mean age= 12.09 years old, SD= 2.32).
Procedures
Each eligible parent and youth visited the laboratory for the baseline assessment. Parents provided informed written consent for their participation and for their child; youth provided written assent. Depressive symptoms were evaluated with a questionnaire and DNA was collected via saliva at the baseline assessment. Baseline episodic and chronic family stress was also evaluated. Follow up assessment evaluating depressive symptoms and episodic stressors occurred 6 months after the baseline visit (retention rate of 96%). Institutional Review Board approved all procedures. Youth were reimbursed for their participation.
Measures
Depression
The Children’s Depression Inventory (CDI [32]) was used to assess depressive symptoms in youth at both baseline (Time 1) and 6 month follow-up (Time 2). The CDI is the most commonly used measure of depressive symptoms in youth and possesses good reliability and validity [33]. Internal consistency (α) was above .80 at both Time 1 and Time 2. The range of scores from this community sample (Time 1: M = 6.64; SD = 5.47, range 0–35; Time 2: M = 4.0; SD = 3.71; range: 0–20) were comparable to published norms [34] and prior research with general community samples [4].
Chronic family stress
The youth version of the UCLA Chronic Stress Interview (CSI [35]), a semi-structured contextual stress interview, assessed youths’ ongoing stress. The CSI has demonstrated excellent reliability and validity [36, 37, 38]. For this study, the parent-child and household domains were used to create an index for chronic family stress. The parent-child domain assesses the quality of the relationship between youth and parent figures. The household domain assesses the quality of the youths’ relationship with others in the household (e.g. siblings, grandparents). Interviewers ascertained from youth the duration that the quality of the parent-child and household relationships had been as described. Severity and duration information on parent-child and household stress were presented to a team of 3 or more blind raters, who came to an agreed upon severity score on a scale from 1 (little/no stress) to 5 (severe stress) and chronicity score on a scale from 1 (less than 6 months) to 5 (5 years or more). Severity and chronicity ratings were recoded and multiplied to create a composite stress score that weighted each severity score by its duration (see [39] for details). The parent-child and household domains’ combined severity/chronicity scores were moderately correlated (r = .57, p < .001), so they were averaged together to form the chronic family stress score.
Episodic stress
Episodic stressors were evaluated with the UCLA Life Stress Interview (LSI [40, 41]). The LSI utilizes the contextual threat method pioneered by Brown and Harris [23]. At Times 1 and 2, interviewers obtained information on occurrence and circumstances (e.g. objective changes) of episodic stressors in the preceding 6 months. Information on spontaneously discussed events and specifically-probed stressors was presented to the stress rating team (see above). Raters came to a consensus on the severity score of the event using a 5-point scale (1 = no impact to 5 = extremely severe impact). Episodic stress scores were obtained by summing severity scores across all events coded for the particular time point. Measurement of episodic stress via the LSI procedures is reliable and valid [e.g., 38, 42, 43].
Pubertal Development
All youth were administered the Pubertal Development Scale (PDS [44]), which includes five questions about physical development, scored from 1 (no) to 4 (development complete). Reliability and validity of the PDS is high [44, 45]. PDS scores are strongly associated with physical examination for pubertal development [45]. We followed standard PDS scoring to create prepubertal and postpubertal groups separately for girls and boys.
Genotyping
Children provided buccal cells for DNA collection via OrageneR kits from DNA Genotek (Ottawa, Ontario, Canada) and genomic DNA was collected and isolated using standard salting out and solvent precipitation methods. The 5-HTTLPR alleles were assayed [46] and modified by using primers reported by Hu et al. [47]. There was high success rate (98% of samples) for DNA extraction and genotyping. The rs25531 SNP genotypes (LA vs. LG) were obtained by incubating the PCR products with MspI [48]. Samples were analyzed on an ABI PRISMR 3130xl Sequencer. Three groups of participants were formed based on their genotyping: children homozygous for the lower expressing S or LG alleles (i.e., S/S), those heterozygous (i.e., S/L), and those homozygous for the higher expressing LA allele (i.e., L/L).
Results
Preliminary Analyses
Means and standard deviations for all primary variables overall and separated by sex and puberty are presented in Table 1. Post-pubertal youth had significantly more episodic stress than pre-pubertal youth; no other sex or puberty differences were noted. Table 2 shows Pearson correlations among all primary variables. 5-HTTLPR polymorphisms were in Hardy-Weinberg equilibrium. Genotype frequencies for 5-HTTLPR were 24% LA homozygotes, 48% heterozygotes, and 27% S/LG homozygotes. Genotype did not vary significantly by race (χ2 < 6.62).
Table 1.
Descriptive statistics overall and by sex and pubertal status.
| Full Sample | Girls | Boys | Pre-Pubertal | Post-Pubertal | |
|---|---|---|---|---|---|
| Variable | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) |
| CDI | 4.00(3.71) | 4.04(4.02) | 3.78(4.03) | 3.87(4.02) | 4.26(4.42) |
| LSI | 2.75(3.71) | 3.55(4.24) | 2.79(2.96) | 2.41(3.38) | 3.54(4.29)* |
| CSI | 1.01(1.51) | 1.05(1.47) | .97(1.56) | .97(1.49) | 1.08(1.56) |
Note: CDI=Children’s Depression Inventory at Time 2; LSI= Life Stress Interview at Time 2; CSI= Chronic Stress Interview at Time 2. Episodic Stress scores at Time 2 were computed by summing severity ratings across stressors and range from 0 to24; Chronic family stress scores at Time 1 are a composite of severity and chronicity ratings and range from 0 to 7.50.
p < .05
Table 2.
Bivariate associations among primary variables
| Variable | 1. | 2. | 3. | 4. |
|---|---|---|---|---|
| 1. 5-HTTLPR | - | |||
| 2. Chronic Family Stress | −.05 | - | ||
| 3. Episodic Stress | .08 | .15* | - | |
| 4. CDI | .11 | .24*** | .21** | - |
p<.05
p <.01
p<.001
Data Analytic Plan
Hierarchical regression was used to test GxE (chronic family stress or episodic stressors) as predictor of prospective change in depressive symptoms. The dependent variable was CDI at Time 2. Time 1 CDI was entered in step 1 to control for covariance between baseline symptoms and main effects of genotype and stress and to enable prediction of residual change in depressive symptoms over time (from T1 to T2). We also entered ethnicity in step 1 to address potential concerns regarding population stratification [49]. All main effects were entered in step 2. Continuous variables were centered prior to testing for interactions. Higher order interactions (e.g., 5-HTTLPR × chronic family stress) were then examined. For all regression models, non-significant interactions were removed, and models were reconducted. Post hoc analyses of interactions were conducted to test simple slopes at different levels of genotype [50, 51].
Effect of chronic family stress
Table 2 shows there was no significant gene-environment correlation (rGE) between 5-HTTLPR and chronic family stress. To test GxE, we first included both puberty (pre- and post-puberty) and sex as possible moderators of the chronic family stress by 5-HTTLPR interaction. Neither puberty (b = .68, SE = 1.26, t = .54, p = .59) nor sex (b = .38, SE = .60, t = .58, p = .56) nor the interaction between sex and puberty (b = −.39, SE = 1.45, t = −.27, p = .79) moderated chronic family stress × 5-HTTLPR, so all higher order interactions were removed. Analyses (Table 3) revealed a significant interaction between chronic family stress and 5-HTTLPR (rs25531). This GxE effect is shown in Figure 1 with chronic family stress depicted at 1 SD above and below the mean. Post-hoc analyses showed the steepest slope for those with the S/S genotype (b = .98, SE = .35, t = 2.82, p =.005) and a significant, but less steep slope for those with the S/L genotype (b = .66, SE = .20, t = 3.27, p = .001). The slope for the L/L genotype group was not significant (b = .34, SE = .37, t = .91, p = .37). This supports an additive genetic model with youth possessing the S allele exhibiting prospective elevations in depressive symptoms over time. Given work questioning functionality of rs25531 [52] and many studies [e.g., 10] utilizing the standard VNTR for 5-HTTLPR (i.e., coding for S and L versus S, LA, and LG), we also analyzed the data in this manner. This significant GxE was comparable when the standard VNTR for 5-HTTLPR × chronic family stress was analyzed (b = .53). Last, the GxE effect was comparable when the largest ethnic group of only White youth was analyzed (b = .53).
Table 3.
Prediction of Depressive Symptoms from Genotype and Chronic Family Stress (Final Statistics)
| Predictor | ΔR2 | b (SE b) | β | t |
|---|---|---|---|---|
| Step 1 | ||||
| CDI Time 1 | .42(.04) | .57 | 9.56*** | |
| Ethnicity | .02(.13) | .01 | .13 | |
| Step 2 | .06 | |||
| Puberty | .01(.50) | .001 | .02 | |
| Gender | −.93(.46) | −.12 | −2.02 | |
| 5-HTTLPR | .29(.37) | .05 | .90 | |
| Chronic Family Stress | −.03(.30) | −.01 | −.11 | |
| Step 3 | .02 | |||
| 5-HTTLPR × Chronic Family Stress | .58(.24) | .26 | 2.41* | |
p<.001
p = .02
Model R2 = .39, F(7, 188) = 16.42, p < .001
Figure 1.
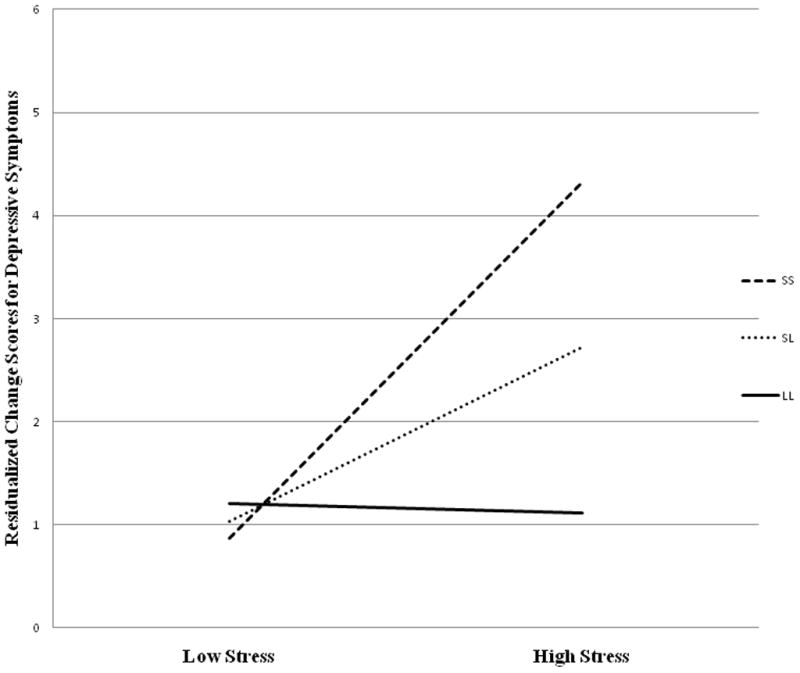
Interaction between 5-HTTLPR and chronic family stress predicting prospective elevations in depressive symptoms over time.
Effect of episodic stress
There was no significant rGE between 5-HTTLPR and episodic stress (Table 2). The regression model testing 5-HTTLPR × episodic stress was identical to chronic stress above, except episodic stressors ascertained at baseline (i.e., those occurring in 6 months prior to Time 1) were also controlled for in step 1 to enable a rigorous test of 5-HTTLPR × episodic stress over 6 month follow-up predicting change in symptoms. Neither puberty (b = .46, SE = .45, t = 1.03, p = .31) nor sex (b = −.28, SE = .31, t = −.90, p = .37) nor the interaction between sex and puberty (b = −.28, SE = .50, t = −.55, p = .58) moderated the interaction between episodic stress and 5-HTTLPR, so all higher order interactions were removed. Episodic stress in the last 6 months did not interact with 5-HTTLPR to predict prospective change in depressive symptoms (b = −.01, SE = .09, t = −.15, p = .88). Likewise, there was no significant interaction between 5-HTTLPR and episodic stress when Time 1 episodic stress was not controlled (b = −.06, SE = .10, t = −.57, p = .57). The GxE effect was comparable when 5-HTTLPR was analyzed with the standard VNTR method (b = −.04).
Discussion
Recent meta-analytic research supports a GxE predicting depression [11], yet there still remain several questions regarding GxE in depression, specifically of stress type using rigorous stress measurement, prospective prediction of symptoms among youth samples, and possible moderation by sex and pubertal status from childhood into adolescence. The current study utilized a validated and developmentally appropriate measure of environmental stress, directly compared recent episodic stressors and chronic family stress, and sampled youth from the community across developmentally salient periods to test a GxE predicting prospective increases in depressive symptoms. Results demonstrated that chronic family stress, but not recent episodic stressors, predicted prospective elevations in depressive symptoms over 6 months among youth who possessed the S allele of the 5-HTTLPR gene (in an additive manner). This effect was found to be equivalent in both boys and girls and across pubertal development.
This study contributes to the GxE literature in depression and replicated Hammen and colleague’s [18] findings. We directly examined the differential impact of chronic versus episodic stress in youth with a well-established assessment of stress (i.e. contextual stress interview), which has been validated in prior research [e.g., 36, 38] and allowed for more precise measurement and understanding of each participant’s life circumstances. This is the preferred approach to assessing stress and has shown stronger GxE effects in depression [13, 14]. Prior reviews of the GxE literature in depression noted concerns with appropriate measurement of environmental stress, which reduces sensitivity when testing GxE [15, 53].
Our results are consistent with previous GxE findings in youth that used measures of chronic family stress [16, 17, 18]. Chronic social stress is a major risk factor for depression [54], especially when experienced during susceptible developmental periods. Additionally, chronic stress may be a critical factor at the cellular level as suggested by recent research reviewing the link between stress and epigenetics [55]. Chronic stress may contribute to epigenetic changes which may endure via altered cellular “memory.” Chronic stress has a marked impact on 5-HTT gene expression, especially in those possessing an S allele [56, 57]. Although previous GxE studies have found significant results when measuring acute and/or recent stressors [e.g., 10, 21, 22], it is possible that these studies were, in fact, capturing chronic stress exposure given they did not explicitly ascertain and separately test chronic and acute stress. The use of a contextual stress interview in the present study and Hammen and colleague’s work enables more precise measurement and distinction between chronic and episodic stress as well as direct comparison of the two types of stress exposure.
Furthermore, the current study explicitly sampled youth across different developmentally salient periods to begin to study whether the GxE was moderated by pubertal status. Most previous studies did not examine whether their findings differed across developmental periods. Results showed that pubertal status did not moderate the GxE.
Also, there was no sex moderation of the GxE effect. Prior research has been mixed regarding sex moderation of GxE in youth [16, 17, 18, 20, 21]. One possible explanation for this discrepancy may be that the three studies finding sex moderation utilized samples of older adolescents or young adults [16, 18, 20]. Developmental epidemiological research shows that sex differences in depression emerge after age 13 [3, 58]. As the current study’s average age was approximately 12 years old, this may not have been the optimal age range for investigating sex moderation in the context of GxE in youth.
There were various strengths and limitations to this study. In addition to the contextual stress interview to carefully ascertain important aspects of stress, the longitudinal design enabled a more stringent test of GxE in depression. We controlled for initial levels of depressive symptoms that overlap with both stress and genetic risk to enable prediction of prospective elevations of depressive symptoms and establish temporal precedence of GxE predicting depressive symptoms. Finally, we examined GxE effects with a community sample of youth, which have been shown to be more generalizable and provide more accurate statistical tests compared with clinical samples [59, 60].
Limitations of the current study provide opportunities and suggestions for future research. The relatively small sample size could have affected the ability to detect sex and puberty moderation of the GxE effect; therefore, future studies should aim to use larger sample sizes to examine these effects. Additionally, the current study investigated elevations in depressive symptoms and not clinical depression. We assessed depressive symptoms given research demonstrating that subclinical depressive symptoms predict later disorder [61, 62] and are on a continuum with clinical depression [63]. Nevertheless, utilizing diagnostic interviews in future research would clarify whether these findings would apply to clinical levels of depression. Although the longitudinal aspect of the current study is a strength given very few studies have examined the effects of GxE prospectively (see [18] and [22] for exceptions), future studies should examine change in depression over longer follow-up time frames as the current study’s timeframe was relatively short (6 months). As the youth were of mixed ethnic background, population stratification may be a concern. However, ethnicity was controlled for in analyses providing a more rigorous statistical test of the GxE [49] and the GxE effect was comparable among the sub-sample of White youth. Finally, although there was no evidence of an rGE between 5-HTTLPR and episodic or chronic family stress, future studies should investigate the possibility of other parent or youth rGE or perhaps even gene by gene interactions (GxG) that could be related to 5-HTTLPR and environmental stress.
In sum, the current study demonstrated that youth possessing the S allele who experienced more chronic family stress exhibited greater increases in depressive symptoms over time. This GxE effect was not seen when utilizing episodic stressors occurring in the preceding 6 months as a measure of environment. These findings suggest that chronic exposure to family stress has a significant impact on the development of depressive symptoms, especially in youth at measured genetic risk.
Acknowledgments
This work was supported by NIMH grant 5R01 MH077195 (Hankin and Abela). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
References
- 1.Birmaher B, Ryan N, Williamson D. Mood disorders across the life span. New York, NY US: Wiley-Liss; 1996. Depression in children and adolescents: Clinical features and pathogenesis; pp. 51–81. [Google Scholar]
- 2.Avenevoli S, Knight E, Kessler R, Merikangas K. Handbook of depression in children and adolescents. New York, NY US: Guilford Press; 2008. Epidemiology of depression in children and adolescents; pp. 6–32. [Google Scholar]
- 3.Hankin B, Abramson L, Moffitt T, et al. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Petersen A, Compas B, Brooks-Gunn J, et al. Depression in adolescence. Am Psychol. 1993;48(2):155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychol Bull. 2001;127(6):773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- 6.Costello JE, Mustillo S, Erkanli A, et al. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 7.Kim-Cohen J, Caspi A, Moffitt TE, et al. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-up of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60(7):709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 8.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 9.Abela J, Hankin B. Handbook of depression in children and adolescents. New York, NY US: Guilford Press; 2008. [Google Scholar]
- 10.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTTLPR gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 11.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 14.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 15.Monroe SM, Reid MW. Gene-environment interactions in depression research: Genetic polymorphisms and life-stress polyprocedures. Psychological Science. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman J, Yang B, Douglas-Palumberi H, et al. Brain-derived neurotrophic factor 5-HHTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: Depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- 18.Hammen C, Brennan P, Keenan-Miller D, et al. Chronic and acute stress, gender, and serotonin transporter gene environment interactions predicting depression symptoms in youth. J Child Psychol Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobile M, Rusconi M, Bellina M, et al. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. J Child Psychol Psychiatry. 2009;50(3):317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 20.Sjöberg R, Nilsson K, Nordquist N, et al. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9(4):443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 21.Eley TC, Sugden K, Corsico A, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9(10):908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 22.Hankin BL, Jenness J, Abela JRZ, Smolen A. Interaction of 5-HTTLPR and idiographic stressors predicts prospective depressive symptoms specifically among youth in a multi-wave design. J Clin Child Adolesc Psychol. 2011;40(4):572–585. doi: 10.1080/15374416.2011.581613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown GW, Harris T. Social origins of depression: A study of psychiatric disorder in women. London: Tavistock Publications; 1978. [Google Scholar]
- 24.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1(1):293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 25.Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annu Rev Clin Psychol. 2008;4:433–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- 26.Lewinsohn PM, Zeiss AM, Duncan EM. Probability of relapse after recovery from an episode of depression. J Abnorm Psychol. 1989;98:107–166. doi: 10.1037//0021-843x.98.2.107. [DOI] [PubMed] [Google Scholar]
- 27.Tram JM, Cole DA. A multimethod examination of the stability of depressive symptoms in childhood and adolescence. J Abnorm Psychol. 2006;115:674–686. doi: 10.1037/0021-843X.115.4.674. [DOI] [PubMed] [Google Scholar]
- 28.Rice F, Harold GT, Thapar A. Negative life events as an account of age-related differences in the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2003;44(7):977–987. doi: 10.1111/1469-7610.00182. [DOI] [PubMed] [Google Scholar]
- 29.Silberg JL, Pickles A, Rutter M, et al. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 199;56(3):225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- 30.Tolonen H. Standardization and Quality Control. National Public Health Institute A27/2005. Helsinki: 2005. Towards the high quality of population health surveys. Available from: http://www.ktl.fi/attachments/suomi/julkaisut/julkaisusarja_a/2005/2005a27.pdf. [Google Scholar]
- 31.Tolonen H, Dobson A, Kulathinal S. Effect on trend estimates of the difference between survey respondents and non-respondents: Results from 27 populations in the WHO MONICA Project. Eur J Epidemiol. 2005;20:887–898. doi: 10.1007/s10654-005-2672-5. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs M. Rating scales to assess depression in school children. Acta Paedopsychiatr. 1981;46:305–315. [PubMed] [Google Scholar]
- 33.Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. J Clin Child Adolesc Psychol. 2005;34(3):412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 35.Hammen C, Adrian C, Gordon D, et al. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. J Abnorm Psychol. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- 36.Eberhart N, Hammen C. Interpersonal predictors of onset of depression during the transition to adulthood. Personal Relationships. 2006;13(2):195–206. [Google Scholar]
- 37.Hammen C, Brennan PA. Interpersonal dysfunction in depressed women: Impairments independent of depressive symptoms. J Affect Disord. 2002;72:145–156. doi: 10.1016/s0165-0327(01)00455-4. [DOI] [PubMed] [Google Scholar]
- 38.Shih JH, Eberhart N, Hammen C, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. J Clin Child Adolesc Psychol. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- 39.Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. J Consult Clin Psychol. 1993;61:354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- 41.Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol. 2000;68:782–787. [PubMed] [Google Scholar]
- 42.Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: tests of an interpersonal impairment hypothesis. J Consult Clin Psychol. 2001;69:284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Dev Psychopathol. 2007;19(2):497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 45.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2008;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anchordoquy HC, McGeary C, Liu L, et al. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behav Genet. 2003;33(1):73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- 47.Hu X, Oroszi G, Chun, et al. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 48.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 49.Jorm A, Easteal S. Assessing candidate genes as risk factors for mental disorders: The value of population-based epidemiological studies. Soc Psychiatry Psychiatr Epidemiol. 2000;35(1):1–4. doi: 10.1007/s001270050001. [DOI] [PubMed] [Google Scholar]
- 50.Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 51.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- 52.Wendland J, Martin B, Kruse M, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 53.Goodyer IM. Emanuel Miller Lecture: Early onset depressions-meanings, mechanisms and processes. J Child Psychol Psychiatry. 2008;49(12):1239–1256. doi: 10.1111/j.1469-7610.2008.01964.x. [DOI] [PubMed] [Google Scholar]
- 54.Rudolph K, Flynn M, Abaied J. Handbook of depression in children and adolescents. New York, NY US: Guilford Press; 2008. A developmental perspective on interpersonal theories of youth depression; pp. 79–102. [Google Scholar]
- 55.Johnstone S, Baylin S. Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nat Rev Genet. 2010;11(11):806–812. doi: 10.1038/nrg2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinnally EL, Capitanio JP, Leibel R, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain and Behav. 2010;9(6):575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philibert R, Madan A, Andersen A, et al. Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. Am J Med Genet B Neuropsychiatr Genet. 2007;114B(1):101–105. doi: 10.1002/ajmg.b.30414. [DOI] [PubMed] [Google Scholar]
- 58.Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort difference on the children’s depression inventory: A meta-analysis. J Abnorm Psychol. 2002;111(4):578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- 59.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41:1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 60.Goodman SH, Lahey BB, Fielding B, et al. Representativeness of clinical samples of youths with mental disorders: A preliminary population-based study. J Abnorm Psychol. 1997;106(1):3–14. doi: 10.1037//0021-843x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 61.Klein DN, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: Predictors of escalation to full-syndrome depressive disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pine D, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? Am J Psychiatry. 1999;156(1):133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 63.Hankin BL, Fraley RC, Lahey BB, Waldman I. Is youth depressive disorder best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. J Abnorm Psychol. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]


