Abstract
Purpose
Human cell lines are useful for studying cancer biology and pre-clinically modeling cancer therapy, but can be misidentified and cross contamination is unfortunately common. The purpose of this study was to develop a panel of validated head and neck cell lines representing the spectrum of tissue sites and histologies that could be used for studying the molecular, genetic, and phenotypic diversity of head and neck cancer.
Methods
A panel of 122 clinically and phenotypically diverse head and neck cell lines from head and neck squamous cell carcinoma (HNSCC), thyroid cancer, cutaneous squamous cell carcinoma, adenoid cystic carcinoma, oral leukoplakia, immortalized primary keratinocytes, and normal epithelium, was assembled from the collections of several individuals and institutions. Authenticity was verified by performing short tandem repeat (STR) analysis. Human papillomavirus (HPV) status and cell morphology were also determined.
Results
Eighty-five of the 122 cell lines had unique genetic profiles. HPV-16 DNA was detected in 2 cell lines. These 85 cell lines included cell lines from the major head and neck primary tumor sites, and close examination demonstrates a wide range of in vitro phenotypes.
Conclusion
This panel of 85 genomically validated head and neck cell lines represents a valuable resource for the head and neck cancer research community that can help advance understanding of the disease by providing a standard reference for cell lines that can be utilized for biological as well as preclinical studies.
Keywords: head and neck cancer, short tandem repeat (STR) profiling, head and neck squamous cell carcinoma, thyroid cancer, human papillomavirus
INTRODUCTION
Head and neck squamous cell cancer (HNSCC) consistently ranks among the 6 most frequently diagnosed cancers in the world (1). In 2010, there were an estimated 49,260 new head and neck (oral cavity, pharynx, and larynx) cancers diagnosed in the United States, representing approximately 3.2% of all cancers (2). Excluding cancer of the thyroid gland, squamous cell histology accounts for over 90% of all head and neck cancers (3). The 5-year survival rate of patients with HNSCC has not significantly improved over the past several decades, likely because of the aggressive nature of the tumor when it is diagnosed at an advanced stage (4). Local and regional recurrences are the most common form of treatment failure, but our understanding of the mechanisms of treatment failure and regional metastasis of HNSCC is still very limited. The thyroid and salivary glands are the 2 other major sites in the head and neck region where cancers frequently occur. Thyroid cancer is the most common endocrine neoplasm in the United States (2), and it was estimated that 44,670 new thyroid cancers would be diagnosed in the United States in 2010 (2). Salivary gland tumors comprise 3–10% of all cancers in the head and neck region (5).
Human tumor cell lines are extremely useful reagents with which to study the biology of cancer and are widely used both in vitro and in vivo to model tumor growth and therapy. Large panels of cell lines have been utilized by many research groups, the National Cancer Institute, and pharmaceutical companies in studies of different types of tumors. These cell-line panels have been shown to reflect much of the molecular, genetic, and phenotypic heterogeneity of the corresponding tumor types (6).
Human tumor cell lines are susceptible to cross-contamination by other cell lines during routine culture, leading to cell line mixtures or inadvertent misidentification. This issue has been reported for more than 45 years, and it is estimated that 15–36% of all cell lines are mixed with or mistaken for other cancer cell lines (7, 8). Therefore, it has been recommended that validation of the integrity of cell lines is an essential first step when establishing any laboratory stock. Indeed, the National Institutes of Health has issued a notice that grant applications for studies involving cell lines must include cell line authentication (9), and journals are beginning to require authentication prior to publication of research articles (10).
Compared to the number of cell lines for many other tumor types, the reported number of head and neck cancer cell lines is rather large (11, 12). More than 300 HNSCC cell lines have been described in the literature. Many of these cell lines have been used for several decades and have been widely distributed among investigators (11). Although many head and neck cancer cell lines have been reported and distributed, there has been only one report publishing the genotype for a panel of HNSCC cells (13). To date, a large panel of head and neck cancer cell lines collected from multiple investigators that represent all of the major subtypes of head and neck cancer has not been assembled and genotyped.
In this study, we sought to identify and characterize a panel of authentic head and neck cell lines derived from a spectrum of anatomic subsites that could be used for studies of the molecular, genetic, and phenotypic diversity of head and neck cancer. To do this, we assembled a panel of 122 cell lines from HNSCC, thyroid cancer, skin SCC, adenoid cystic carcinoma, leukoplakia, immortalized primary keratinocytes, and normal epithelium and subjected them to short tandem repeat (STR) genomic profiling for authentication. Once we established which lines were validated with high confidence, we sought to characterize the origins, HPV status, and in vitro morphology to demonstrate the diversity of this authenticated cell line panel.
MATERIALS AND METHODS
A panel of 122 human head and neck cell lines was assembled from a number of different researchers, institutions, and suppliers. This panel was chosen to represent each of the major HNSCC sites: oral cavity, oropharynx, hypopharynx, and larynx. Also chosen for study were anaplastic and papillary thyroid cancer, adenoid cystic carcinoma cell lines, and cell lines derived from lymph node metastases. In some cases isogenic cell line pairs were obtained, which included cells derived from both the primary tumor and lymph node metastases from the same patient. Also included were cell lines from cutaneous SCC, leukoplakia, immortalized primary keratinocytes, and normal epithelium.
Cell Lines and Culture Conditions
A total of 85 unique head and neck cell lines were used in our research, including 61 HNSCC cell lines, 11 thyroid cancer cell lines, 3 cutaneous SCC cell lines, 5 immortalized normal keratinocyte cell lines, 3 immortalized normal oral epithelial cell lines, and 2 leukoplakia cell lines. Information regarding each cell line and appropriate culture media is presented in Tables 1 and 2. The FaDu, CAL-27, Detroit562, SCC-4, SCC-9, SCC-15, and SCC-25 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The OSC-19 cell line was obtained from the Health Science Research Resource Bank (Osaka, Japan). The B-CPAP cell line was obtained from the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; Braunschweig, Germany). The NHEK cell line was purchased from Lonza Rockland, Inc., (Rockland, ME). The Nthy-ori 3-1 cell line was obtained from the European Collection of Cell Cultures (Wiltshire, United Kingdom). The sources for the other cell lines were as listed in Tables 1 and 2.
Table 1.
Primary site, source, and clinical features of tumors used to derive 87 HNSCC cell lines used in this study.*
| Cell line | Primary site | Age | Sex | TNM stage | Culture media † | Primary source ¶ |
|---|---|---|---|---|---|---|
| HN4 | REC (L) | 57 | M | T2N0M0 | DMEM, 10% FBS | Dr. D. M. Easty, Ludwig Institute for Cancer Research, London |
| HN5 | REC (OC) | 73 | M | T2N0M0 | DMEM/F12, 10% FBS | Dr. D. M. Easty, Ludwig Institute for Cancer Research, London |
| HN30 | P | - | - | - | DMEM, 10% FBS | Dr. John Ensley, Wayne State Univ. |
| HN31 | LN (HN30) | - | - | - | DMEM, 10% FBS | Dr. John Ensley, Wayne State Univ. |
| UM-SCC-1 | REC (OC) | 73 | M | T2N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-2 | REC (OC) | 64 | F | T2N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan ¶¶ |
| UM-SCC-3 | LN (nose) | 73 | F | T1N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-4 | OP | 47 | F | T3N2aM0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-6 | OP | 37 | M | T2N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-10A | L | 57 | M | T3N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-10B | LN (UMSCC10A) | 57 | M | T3N1M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-11A | L | 65 | M | T2N2aM0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-11B | L | 65 | M | T2N2aM0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-14A | REC (OC) | 58 | F | T1N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-14B | REC (UMSCC14A) | 58 | F | T1N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-17A | L | 47 | F | T1N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-17B | EXT (UMSCC17A) | 47 | F | T1N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-19 | OP | 67 | M | T2N1M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-22A | HP | 58 | F | T2N1M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-22B | LN (UMSCC22A) | 58 | F | T2N1M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-25 | LN (L) | 50 | M | T3N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-33 | LN (maxillary sinus) | 47 | F | T4N3aM0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-47 | OC | 53 | M | T3N1M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| UM-SCC-85 | REC (Nose) | 58 | M | T4N0M0 | DMEM, 10% FBS | Dr. Thomas E. Carey, Univ. of Michigan |
| JHU011 | REC (L) | - | M | T3N0 | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU012 | LN (OC) | 70 | F | T1N2b | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU013 | LN | - | - | - | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU019 | OP | - | - | - | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU022 | LN (L) | - | M | T3N2b | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU028 | OC | - | - | - | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| JHU029 | L | - | M | T4N0 | RPMI 1640, 10% FBS | Dr. David Sidransky, Johns Hopkins Univ. |
| MDA686TU | OP | 48 | M | T3N3b | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA686TU | OP | 48 | M | T3N3b | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University ¶¶ |
| MDA686LN | LN (MDA686TU) | 48 | M | T3N3b | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA686LN | LN (MDA686TU) | 48 | M | T3N3b | DMEM, 10% FBS | Dr Peter G. Sacks, New York University ¶¶ |
| MDA886LN | LN (L) | 64 | M | T3N3a | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1186 | L | - | M | T3N1 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1386TU | HP | 72 | M | T4N3bM0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1386LN | LN (MDA1386TU) | 72 | M | T4N3bM0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1586 | L | - | M | - | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1686 | Cheek | - | M | T3N0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1986LN | LN (OC) | - | - | T2N2b | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| MDA1986LN | LN (OC) | - | - | - | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University ¶¶ |
| PCI-3 | OP | 50 | M | T3N0M0 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC ¶¶ |
| PCI-13 | OC | 50 | M | T4N1M0 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC |
| PCI-15A | HP | 69 | M | T2N1M0 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC |
| PCI-15B | LN (PCI-15A) | 69 | M | T2N1M0 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC |
| PCI-22A | OC | 59 | M | T4N1M0 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC ¶¶ |
| PCI-22B | LN (PCI-22A) | 59 | M | T4N1M1 | DMEM, 10% FBS | Dr. Theresa Whiteside, UPMC ¶¶ |
| PCI-24 | OC | 81 | M | T2N0M0 | DMEM, 10% FBS | Drs. Theresa Whiteside, UPMC |
| SCC-4 | OC | 55 | M | T3N0M0 | DMEM/F12, 10% FBS‡ | ATCC |
| SCC-9 | OC | 25 | M | T2N1 | DMEM/F12, 10% FBS‡ | ATCC |
| SCC-15 | OC | 55 | M | T4N1M0 | DMEM/F12, 10% FBS‡ | ATCC |
| SCC-25 | OC | 70 | M | T1N1M0 | DMEM/F12, 10% FBS‡ | ATCC |
| SCC-61 | OC | - | - | T4N2b | DMEM, 20% FBS‡‡ | Dr. Ralph Weichselbaum, Univ. of Chicago |
| FaDu | HP | 56 | M | - | DMEM, 10% FBS | ATCC |
| OSC-19 | LN (OC) | 61 | M | - | DMEM, 10% FBS | HSRRB |
| OSC-19LN1§ | OSC-19 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| OSC-19LN2§ | OSC-19LN1 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| OSC-19LN3§ | OSC-19LN2 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| OSC-19LN4§ | OSC-19LN3 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| OSC-19LN5§ | OSC-19LN4 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| TR146 | REC (OC) | 67 | F | - | DMEM, 10% FBS | Dr. Thomas Rupniak, Imperial Cancer Research Fund, London |
| Tu-138 | OC | 53 | M | T3N0M0 | DMEM/F12, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-158LN | OP | 44 | M | T2N2aM0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-159 | OP | 44 | M | T3N0M0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-167 | OC | 70 | F | T4N2bM0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-182 | OP | 40 | F | T3N2bM0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-212 | HP | 60 | M | T2N2cM0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| Tu-212LN | LN (TU212) | 60 | M | T2N2cM0 | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| T404 | OC | - | - | - | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| T406 | OC | - | - | - | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| T409 | OC | - | - | - | DMEM, 10% FBS | Dr. Gary Clayman, MDACC |
| DM12 | Tu167 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| DM14 | Tu167 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| JMAR | Tu167 | - | - | - | DMEM, 10% FBS | Dr. Jeffrey Myers, MDACC |
| SqCC/Y1 | OC | - | - | - | DMEM/F12, 10% FBS** | Dr. Alan C. Sartorelli, Yale Univ. |
| CAL-27 | OC | 56 | M | - | DMEM, 10% FBS | ATCC |
| MSK-922 | REC (L) | 68 | M | - | DMEM, 10% FBS | Dr. Stimson Schantz, Memorial Sloan-Kettering Cancer Center |
| Ca9-22 | OC | - | - | - | DMEM, 10% FBS | HSRRB ¶¶ |
| PE/CA-PJ34 | OC | 60 | M | - | DMEM, 10% FBS | ECACC |
| Detroit 562 | pleural effusion (P) | - | F | - | DMEM, 10% FBS | ATCC |
| 183 | OP | 54 | M | T3N0M0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| 183 | OP | 54 | M | T3N0M0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University ¶¶ |
| 1483 | OC | 66 | M | T2N1M0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
| 1483 | OC | 66 | M | T2N1M0 | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University ¶¶ |
| 584A2 | L | - | M | - | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
Abbreviations: ATCC, American Type Culture Collection; ECACC, European Collection of Cell Cultures, Wiltshire, United Kingdom; DMEM, Dulbecco’s modified Eagle’s medium; EXT, extension to adjacent tissue; FBS, fetal bovine serum; HP, hypopharynx; HSRRB, Health Science Research Resource Bank (Japan Health Sciences Foundation), Osaka, Japan; L, larynx; LN, lymph node; MDACC, The University of Texas MD Anderson Cancer Center; N, Neck tissue; OC, oral cavity; OP, oropharynx; P, pharynx; REC, recurrence; Univ., University; UM-SCC, University of Michigan Squamous Cell Carcinoma; UPMC, University of Pittsburgh Medical Center.
Highlighted lines indicate cell lines with 1 or more problems in their STR profiling results.
Media conditions: FBS, fetal bovine serum (Sigma, St. Louis, MO). DMEM, Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen Corporation, Carlsbad, CA). DMEM/F12, Dulbecco's modified Eagle’s medium: Nutrient Mixture F-12 (Mediatech, Inc., Herndon, VA). DMEM, 10% FBS supplemented with penicillin-streptomycin, vitamin solution (Thermo Scientific, Rockford, IL), L-glutamine, nonessential amino acids (Lonza Rockland, Inc., Rockland, ME), and sodium pyruvate (Gibco). DMEM/F12, 10% FBS supplemented with penicillin-streptomycin and L-glutamine. RPMI 1640 (Mediatech), 10% FBS supplemented with penicillin-streptomycin, L-glutamine, sodium pyruvate, and nonessential amino acids.
Supplemented with L-glutamine, sodium pyruvate, penicillin-streptomycin, 1.2 mg/ml Sodium Bicarbonate (Gibco), 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 0.4 µg/ml hydrocortisone (Sigma).
Supplemented with 0.4 µg/ml hydrocortisone.
DMEM/F12 low glucose medium (Mediatech Inc.) 10% FBS supplemented supplemented with penicillin-streptomycin, L-glutamine
OSC-19 LN-series were established from a cervical lymph node metastasis of a mouse whose tongue was injected with OSC-19 or OSC-19 LN-series cells orthotopically.
Cells were established by or purchased from these sources.
Cells were from a secondary source. See details in Supplementary Table S1.
Table 2.
Primary site, source, and clinical features of tisuues used to derive 35 thyroid cancer, skin, adenoid cystic carcinoma, normal keratinocyte, normal epitheliual, and leukoplakic lesion cell lines used in this study.*
| Cell line | Primary site | Age | Sex | Culture media† | Primary source ¶ |
|---|---|---|---|---|---|
| Thyroid | |||||
| KAT18 | Anaplastic | - | - | RPMI 1640, 10% FBS | Dr. K. B. Ain, Univ. of Kentucky Medical Center |
| KAT4 | Anaplastic | - | F | RPMI 1640, 10% FBS | Dr. K. B. Ain, Univ. of Kentucky Medical Center |
| DRO | Anaplastic | - | - | RPMI 1640, 10% FBS | Dr. G. F. J. Julliard, UCLA |
| ARO | Anaplastic | - | - | RPMI 1640, 10% FBS | Dr. G. F. J. Julliard, UCLA |
| U-Hth7 | Anaplastic | 74 | F | MEM, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| U-Hth74-clone7 | Anaplastic | 73 | F | MEM, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| U-Hth83 | Anaplastic | 66 | M | RPMI 1640, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| U-Hth104 | Anaplastic | 72 | F | RPMI 1640, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| U-Hth112 | Anaplastic | 82 | M | MEM, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| SW1736 | Anaplastic | 77 | F | MEM, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| 8505C | Anaplastic | - | - | MEM, 10% FBS | ECACC |
| C643 | Anaplastic | 76 | M | MEM, 10% FBS | Dr. Nils Erik Heldin, Univ. of Uppsala, Sweden |
| RPTC-1 | Papillary | - | - | RPMI 1640, 10% FBS | Dr. Gary Clayman, MDACC |
| BHP2-7 | Papillary | - | F | RPMI 1640, 10% FBS | Dr. Jerome Hershman, GLA |
| BHP5-16 | Papillary | - | - | RPMI 1640, 10% FBS | Dr. Jerome Hershman, GLA |
| NPA87 | Papillary | - | - | RPMI 1640, 10% FBS | Dr. Collin Weber, Columbia Univ. |
| TPC-1 | Papillary | - | - | RPMI 1640, 10% FBS | Dr. Junji Tanaka, Kanazawa Univ., Kanazawa, Japan |
| B-CPAP | Papillary | - | F | RPMI 1640, 10% FBS | DSMZ |
| K2 | Papillary | - | - | DMEM/F12, 10% FBS | Dr. Cēcile Challeton, Institut Gustave Roussy, Villejuif, France |
| Skin | |||||
| Colo16 | CSCC | 59 | F | DMEM/F12, 10% FBS | Dr. G. E. Moore, Denver General Hospital |
| SRB-1 | CSCC | - | - | DMEM/F12, 10% FBS | Dr. Isaiah J. Fidler, MDACC |
| SRB-12 | CSCC | - | - | DMEM/F12, 10% FBS | Dr. Isaiah J. Fidler, MDACC |
| Adenoid cystic carcinoma | |||||
| ACC2 | Hard palate | 28 | F | RPMI 1640, 10% FBS | Dr. Rong-Gen He, Shanghai Jiao Tong Univ., China |
| ACC3 | Parotid gland | 49 | M | RPMI 1640, 10% FBS | Dr. Rong-Gen He, Shanghai Jiao Tong Univ., China |
| ACCM | ACC2 | 28 | F | RPMI 1640, 10% FBS | Dr. Xiao-Fang Guan, Shanghai Second Medical University, China |
| Immortalized normal keratinocyte | |||||
| HOK16B | OC | - | - | K-SFM, 5% FBS | Dr. No-Hee Park, UCLA school of dentistry |
| NOM9/TK | OC | 59 | M | KGM | Dr. Jerry Shay, UTSMC |
| NOM9/TKp53 | OC | 59 | M | KGM | Dr. Jerry Shay, UTSMC |
| HaCaT | Epidermal | - | - | DMEM/F12, 10% FBS | Dr. Petra Boukamp, German Cancer Research, Germany |
| NHEK | Epidermal | - | - | K-SFM, 5% FBS | Lonza Rockland, Inc. (Rokcland, ME) |
| Immortalized normal epithelia | |||||
| OKF6/TERT-1 | OC | 57 | M | K-SFM, 5% FBS | Dr. James Rhienwald, Harvard Medical School |
| OKF6/TERT-2 | OC | 57 | M | K-SFM, 5% FBS | Dr. James Rhienwald, Harvard Medical School |
| Nthy-ori 3-1 | Thyroid | - | - | RPMI 1640, 10% FBS | ECACC |
| Leukoplakia lesion | |||||
| MSKleuk1 | OC | - | - | KGM | Dr. Peter G. Sacks, New York University |
| MSKleuk1S | OC | - | - | DMEM, 10% FBS | Dr. Peter G. Sacks, New York University |
Abbreviations: CSCC, cutaneous squamous cell carcinoma; DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; ECACC, European Collection of Cell Cultures, Wiltshire, United Kingdom; GLA, VA Greater Los Angeles Healthcare System, Los Angeles, CA; MDACC, The University of Texas MD Anderson Cancer Center; OC, oral cavity; UCLA, University of California, Los Angeles, Los Angeles, CA.
Highlighted lines indicate cell lines with 1 or more problems shown in their STR profiling results.
Media conditions: FBS, fetal bovine serum (Sigma). DMEM, Dulbecco’s modified Eagle’s medium (Gibco). MEM, minimum essential medium (Mediatech Inc.). KGM, keratinocyte growth medium (Lonza). RPMI 1640 (Mediatech), 10% FBS supplemented with penicillin-streptomycin (Thermo Scientific), sodium pyruvate (Gibco), L-glutamine, and nonessential amino acids (Lonza). MEM, 10% FBS supplemented with penicillin-streptomycin, L-glutamine, and nonessential amino acids. DMEM/F12, 10% FBS supplemented with penicillin-streptomycin and L-glutamine. K-SFM, 5% FBS supplemented with bovine pituitary extract (Gibco) and epidermal growth factor (Gibco). KGM package kit (Lonza) contains keratinocyte basal medium supplemented with bovine pituitary extract, insulin, epidermal growth factor, hydrocortisone, and gentamicin/amphotericin-B. DMEM, 10% FBS supplemented with penicillin-streptomycin, vitamin solution, L-glutamine, nonessential amino acids, and sodium pyruvate.
Cells were established by or purchased from these sources.
Adherent monolayer cultures were maintained on plastic and incubated at 37°C in 5% carbon dioxide and 95% air. To maintain the integrity of the collections, we carefully maintained the cell lines in culture and stored stocks of the early-passage cells. The cultures were free of Mycoplasma species and were maintained for no longer than 12 weeks after recovery from frozen stocks in culture.
DNA Extraction
Nuclear DNA was extracted from cells using a genomic DNA purification kit (Qiagen Inc., Chatsworth, CA). Briefly, 0.5 × 106 to 1 × 106 cells were plated in a 60-mm culture dish to finally achieve approximately 70% cellular confluence. Cells were scraped and resuspended in 10–20 µl of culture medium. Three hundred microliters of cell lysis solution was added to the resuspended cells, and cells were incubated at 37°C until the solution was homogeneous. The cell lysates were mixed with 1.5 µl of RNase A solution by 25 inversions of the test tube and incubated at 37°C for 5 minutes. After 100 µl of protein precipitation solution was added to the RNase A-treated cell lysates, the samples were mixed vigorously by vortex at high speed for 20 seconds and then centrifuged at 13,000 × g for 1 minute. The supernatants containing the DNA were transferred to a new tube with 300 µl of 100% isopropanol, mixed by 50 inversions of the test tube, and centrifuged at 13,000 × g for 1 minute. DNA pellets were washed with 300 µl of 70% ethanol, air dried, and resuspended in 20–50 µl of DNA hydration solution. The samples were incubated for 1 hour at 65°C and overnight at room temperature.
STR Profiling
For each cell line, 200 ng of DNA was suspended in 10 µl of double-distilled water and analyzed by STR profiling (14) at the Fragment Analysis Facility at Johns Hopkins University (Baltimore, MD). The system uses a Promega Powerplex 1.2 kit (Promega, Madison, WI) to amplify specific polymorphic and minimal artificial regions by polymerase chain reaction (PCR) and resolve amplified regions using fluorescent dyes and high throughput. This is the kit that ATCC uses to obtain the information in its public database of STR profiles. The selected markers included 8 Combined DNA Index System (CODIS) core STR loci (CSF1PO; 5q33.3–34, D13S317; 13q22–q31, D16S539; 16q24-qter, D5S818; 5q21–q31, D7S820; 7q, TH01; 11p15.5, TPOX; 2p23-2pter, and vWA; 12p12-pter) and 1 sex Chromosome locus, amelogenin (Xp22.10–22.3 and Y). All STR profiling results for our 122 cell lines were compared to the STR profiles for these cell lines in the ATCC STR profiling database (15). For data comparison, we used well-characterized and validated reference data provided by ATCC and others (13, 16).
Human Papillomavirus (HPV) Detection
DNA was extracted from 62 head and neck cell lines as described above. To detect HPV-16 and HPV-18, regions of E6 and E7 were amplified by PCR using specific primers. The sequences of primers were as follows: HPV-16-E6 sense, GCAATGTTTCAGGACCCACA; HPV-16-E6 antisense, CGCAGTAACTGTTGCTTGCAGT; HPV-16-E7 sense, TTGTTGCAAGTGTGACTCTACGC; HPV-16-E7 antisense, CCTAGTGTGCCCATTAACAGGTC; HPV-18-E6 sense, TCACAACATAGCTGGGCACTA; HPV-18-E6 antisense, CTTGTGTTTCTCTGCGTCGTT; HPV-18-E7 sense, ATGAAATTCCGGTTGACCTTC; HPV-18-E7 antisense, GTCGGGCTGGTAAATGTTGAT; β-actin sense, GGCATCCTCACCCTGAAGTA; β-actin antisense, AGGTGTGGTGCCAGATTTTC. The DNA of Siha cells containing 9.2 copies of HPV-16 DNA per 5 µl was used as a positive control for HPV-16, and the DNA of pBR322 plasmid with 104.5 copies of HPV-18 DNA per 5 µl was used as a positive control for HPV-18. HPV-negative DNA was isolated from MDA686TU cells and served as a negative control. The PCR mixture without template DNA was used as a control for PCR, and a housekeeping gene, β-actin, was used as an internal control. A total reaction of 15 µl comprised 100 ng of DNA, 0.1 µM each primer, 0.1 mM dNTP, and 1X PCR buffer with 2.5 mM MgCl2 and 0.375 µl HotStar Tag (Sigma, St. Louis, MO; catalog number D6558-1.5KU). PCR cycling conditions were denaturation at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 30 seconds, annealing at 56°C (HPV-16) or 60°C (HPV-18) for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 5 minutes.
Cell Morphology Examination
For each cell line, 2–3 million cells were plated in a 10-cm dish and incubated for 48 hours in full serum medium in order to finally achieve approximately 70% cellular confluence in each dish. Eighty-five unique cell lines were photomicrographed using an Olympus IX71 microscope (Olympus America, Melville, NY) with magnifications of 40× and 100× to examine their morphology.
Mycoplasma Treatment and Detection
2 million cells were plated in a 10-cm dish and incubated for 24 hours in full serum medium in the absence of antibiotics. 12.5–25ug/ml of plasmocin (InvivoGen, Catalog # ant-mpt) was added for another 24 hours. The medium was then replaced (the cells were split if needed) with fresh plasmocin every 3–4 days for 10 to 14 days. Mycoplasma levels were evaluated using the MycoAlert® Mycoplasma Detection Kit –Lonza (Catalog # LT07–318).
RESULTS
STR Profiling
The STR profiles of the FaDu, CAL-27, Detroit562, SCC-4, SCC-9, SCC-15, and SCC-25 cell lines, which we acquired from ATCC, were identical to the STR profiles of those cell lines in the ATCC database. Another 78 cell lines were found to be unique as compared to one another and to all of the other cell lines listed in the ATCC database. In total, we identified unique or appropriate genetic profiles for 85 head and neck cell lines (Table 3). The verified cell lines included 61 HNSCC cell lines, 11 thyroid cancer cell lines, 3 cutaneous SCC cell lines, 2 leukoplakia cell lines, 5 immortalized primary keratinocyte cell lines, and 3 immortalized primary epithelium cell lines.
Table 3.
STR profiles of 85 unique cell lines.*
| Cell line | Amelogenin | CSF1PO | D13S317 | D16S539 | D5S818 | D7S820 | THO1 | TPOX | vWA |
|---|---|---|---|---|---|---|---|---|---|
| HNSCC | |||||||||
| HN4 | X X ** | 10 11 | 13 13 | 9 12 | 11 12 | 10 10 | 7 9 | 11 11 | 16 17 |
| HN5 | X ** | 11 13 | 11 | 11 13 | 13 | 11 | 9 | 8 11 | 15 18 19 |
| HN30 | X Y | 10 12 | 11 12 | 11 12 | 12 13 | 10 10 | 7 9 | 6 11 | 15 18 |
| HN31 | X Y | 10 12 | 11 12 | 11 12 | 12 13 | 10 10 | 7 9 | 6 11 | 15 18 |
| UM-SCC-1 | X ** | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| UM-SCC-3 | X X | 10 12 | 11 13 | 11 12 | 11 13 | 9 10 | 6 9.3 | 8 8 | 17 17 |
| UM-SCC-4 | X | 11 | 12 | 12 | 11 | 9 10 | 7 | 9 10 | 17 18 |
| UM-SCC-6 | X Y | 10 | 13 | 9 12 | 12 | 10 11 | 6 9.3 | 11 | 15 16 |
| UM-SCC-10A | X ** | 10 11 | 9 | 11 | 12 13 | 9 | 6 9 | 8 11 | 19 |
| UM-SCC-10B | X ** | 10 11 | 9 | 11 | 12 13 | 9 | 6 | 8 11 | 19 |
| UM-SCC-11A | X ** | 7 | 14 | 12 14 | 11 | 11 | 7 | 8 | 16 17 18 |
| UM-SCC-11B | X ** | 7 | 14 | 12 14 | 11 | 11 | 7 | 8 11 | 16 17 18 |
| UM-SCC-14A | X | 10 | 12 | 11 12 | 11 14 | 9 10 | 6 8 | ? | 14 18 |
| UM-SCC-14B | X X | 10 10 | 12 12 | 11 12 | 11 14 | 9 10 11 | 6 8 | 8 8 | 14 18 |
| UM-SCC-17A | X | 10 11 | 11 13 | 10 11 | 11 | 13 | 6 8 | 10 11 | 14 17 |
| UM-SCC-17B | X | 10 11 | 11 13 | 10 11 | 11 | 13 | 6 8 | 10 11 | 14 17 |
| UM-SCC-19 | X Y | 11 11 | 11 11 | 9 13 | 11 11 | 9 9 | 6 7 | 8 11 | 16 19 |
| UM-SCC-22A | X X | 10 10 | 8 12 | 9 11 | 12 12 | 8 9 | 6 6 | 8 11 | 18 18 |
| UM-SCC-22B | X | 10 | 8 12 | 9 11 | 12 | 8 9 | 6 | 8 11 | 15 18 |
| UM-SCC-25 | X Y | 10 15 | 11 11 | 10 12 | 12 12 | 12 13 | 6 9 | 8 9 | 19 19 |
| UM-SCC-33 | X X | 13 13 | 11 11 | 10 10 | 10 10 | 10 10 | 9.3 9.3 | 8 8 | 14 18 |
| UM-SCC-47 | X Y | 11 13 | 8 11 | 8 13 | 11 12 | 11 | 7 9.3 | 10 11 | 18 |
| UM-SCC-85 | X X ** | 11 11 | 11 12 | 12 12 | 11 12 | 9 9 | 7 9.3 | 8 11 | 17 17 |
| JHU011 | X * | 10 12 | 12 | 9 14 | 9 12 | 11 | 6 9 | 8 9 | 16 17 |
| JHU022 | X Y | 10 | 11 | 12 | 12 13 | 9 10 | 6 | 8 | 14 16 |
| JHU029 | X Y | 8 12 | 12 13 | 10 13 | 13 15 | 10 11 | 8 | 9 11 | 15 |
| MDA686TU | X X ** | 12 12 | 11 12 | 8 9 | 12 12 | 10 10 | 7 8 | 10 11 | 15 16 |
| MDA686LN | X X ** | 12 12 | 12 12 | 8 9 | 12 12 | 10 10 | 7 8 | 10 11 | 15 16 |
| MDA886LN | X Y | 10 10 | 9 9 | 11 11 | 12 12 | 10 10 | 9 9 | 8 8 | 17 19 |
| MDA1186 | X Y | 12 12 | 9 10 | 12 13 | 12 12 | 11 12 | 6 9.3 | 8 10 | 17 18 |
| MDA1386TU | X X ** | 11 11 | 11 11 | 9 13 | 12 12 | 8 12 | 9 9 | 8 8 | 20 20 |
| MDA1386LN | X X ** | 11 11 | 11 11 | 9 13 | 12 12 | 8 12 | 9 9 | 8 8 | 20 20 |
| MDA1586 | X Y | 8 9 | 12 12 | 10 10 | 11 12 | 8 10 | 7 9 | 10 11 | 16 17 |
| MDA1686 | X Y | 12 12 | 8 12 | 12 13 | 13 13 | 8 11 | 7 10 | 8 8 | 14 16 |
| MDA1986LN | X X | 11 14 | 13 13 | 9 12 | 9 11 | 8 9 | 7 7 | 8 8 | 17 19 20 |
| PCI-13 | X Y | 10 11 | 10 11 | 12 13 | 12 | 12 13 | 6 7 | 8 9 | 16 18 |
| PCI-15A | X Y | 11 11 | 11 11 | 11 11 | 11 11 | 11 11 | 6 6 | 8 8 | 18 18 |
| PCI-15B | X Y | 11 11 | 11 11 | 11 11 | 11 11 | 11 11 | 6 6 | 8 8 | 18 18 |
| PCI-24 | X Y | 10 12 | 11 | 9 12 | 11 12 | 8 11 | 9 9.3 | 8 | 15 18 |
| SCC-4 | X Y | 11 11 | 11 13 | 12 12 | 13 13 | 9 11 | 9.3 9.3 | 8 8 | 15 17 |
| SCC-9 | X Y | 11 11 | 9 9 | 10 11 | 12 12 | 8 8 | 8 9 | 9 11 | 17 17 |
| SCC-15 | X Y | 10 13 | 9 14 | 12 15 | 12 12 | 10 11 | 9 9.3 | 8 8 | 15 17 |
| SCC-25 | X X ** | 10 10 | 13 13 | 11 12 | 12 12 | 12 12 | 8 8 | 8 12 | 17 19 |
| SCC-61 | X Y | 10 12 | 10 12 | 9 11 | 12 13 | 8 12 | 7 9 | 8 | 16 17 |
| FaDu | ? | 12 12 | 8 9 | 11 11 | 12 12 | 11 12 | 8 8 | 11 11 | 15 17 |
| OSC-19 | X Y | 12 12 | 12 12 | 9 12 | 10 13 | 9 11 | 9 9 | 11 11 | 14 18 |
| OSC-19LN1 | X Y | 12 | 12 12 | 9 | 10 13 | 9 11 | 9 | 11 | 14 18 |
| OSC-19LN2 | X Y | 12 | 12 12 | 9 | 10 13 | 9 11 | 9 | 11 | 14 18 |
| OSC-19LN3 | X Y | 12 | 12 12 | 9 | 10 13 | 9 11 | 9 | 11 | 14 18 |
| OSC-19LN4 | X Y | 12 | 12 12 | 9 | 10 13 | 9 11 | 9 | 11 | 14 18 |
| OSC-19LN5 | X Y | 12 | 12 12 | 9 | 10 13 | 9 11 | 9 | 11 | 14 18 |
| TR146 | X | 11 13 | 11 14 | 13 | 10 11 | 10 12 | 6 9 | 8 9 | 15 18 |
| Tu-138 | X ** | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| SqCC/Y1 | X | 9 11 | 11 | 11 13 | 12 13 | 8 11 | 7 9.3 | 8 | 15 16 |
| CAL-27 | X X ** | 10 12 | 10 11 | 11 12 | 11 12 | 10 10 | 6 9.3 | 8 8 | 14 17 |
| MSK-922 | X X ** | 10 12 | 12 12 | 9 11 | 11 11 | 11 11 | 8 8 | 8 8 | 17 17 |
| PE/CA-PJ34 | X X ** | 10 10 | 12 12 | 9 13 | 10 12 | 9 10 | 6 6 | 8 8 | 18 18 |
| Detroit562 | X X | 11 13 | 12 12 | 11 11 | 11 12 | 8 10 | 8 9 | 8 10 | 16 16 |
| 183 | X Y | 10 11 | 11 12 | 13 13 | 10 13 | 9 9 | 6 6 | 8 8 | 18 18 |
| 1483 | X X ** | 10 10 | 10 12 | 9 11 | 13 13 | 7 10 | 9 9.3 | 11 11 | 14 18 |
| 584A2 | X Y | 10 12 | 6 12 | 11 13 | 12 12 | 11 11 | 6 9 | 11 11 | 16 18 |
| Thyroid cancer | |||||||||
| U-Hth74-clone7 | X X | 10 12 | 12 13 | 11 11 | 11 12 | 8 9 | 8 9 | 11 11 | 17 19 |
| U-Hth83 | X Y | 11 11 | 11 13 | 11 12 | 12 12 | 12 12 | 6 9 | 8 8 | 16 19 |
| KAT18 | X X | 11 12 | 11 11 | 11 12 | 10 12 | 10 10 | 7 9.3 | 8 11 | 16 16 |
| SW1736 | X X | 12 12 | 11 12 | 11 12 | 12 13 | 8 11 | 6 ? | 11 11 | 16 19 |
| U-Hth7 | X X | 12 12 | 11 14 | 9 13 | 11 11 | 8 11 | 9 9.3 | 11 11 | 14 ? |
| U-Hth104 | X X | 12 12 | 12 12 | 8 9 12 | 12 12 | 10 10 | 6 9.3 | 8 11 | 14 16 |
| 8505C | X | 12 13 | 13 | 12 | 10 11 | 10 | 6 9 | 11 | 17 19 |
| C643 | X Y | 10 11 | 8 10 | 9 13 | 11 12 | 9 12 | 9.3 10 | 11 12 | 15 17 |
| B-CPAP | X X | 13 13 | 12 12 | 11 12 | 10 11 | 10 10 | 6 9.3 | 8 11 | 14 17 |
| TPC-1 | X X | 11 12 | 11 12 | 9 9 | 8 10 | 11 11 | 9 9 | 11 11 | 14 18 |
| K2 | X Y | 11 12 | 11 14 | 11 12 | 10 11 | 11 | 6 9 | 8 | 17 18 |
| Skin cancer | |||||||||
| Colo16 | X X | 12 12 | 11 12 | 12 13 | 10 13 | 8 11 | 8 8 | 8 8 | 15 18 |
| SRB-1 | X X | 10 11 | 11 12 | 9 9 | 12 13 | 10 10 | 6 9 | 8 8 | 14 17 |
| SRB-12 | X X | 12 12 | 11 11 | 14 14 | 11 11 | 9 10 | 9 9.3 | 8 8 | 16 16 |
| Normal cells | |||||||||
| HOK16B | X X | 10 11 | 9 12 | 12 12 | 9 10 | 12 12 | 8 9 | 8 11 | 17 19 |
| NOM9/TK | X Y | 10 12 | 11 12 | 11 11 | 11 13 | 11 11 | 8 9.3 | 8 11 | 17 18 |
| NOM9/TKp53 | X Y | 10 12 | 11 12 | 11 11 | 11 13 | 11 11 | 8 9.3 | 8 11 | 17 18 |
| HaCaT | X X | 9 11 | 10 12 | 9 12 | 12 12 | 9 11 | 9.3 9.3 | 11 12 | 16 17 |
| NHEK | X X | 11 15 | 10 12 | 12 14 | 11 12 | 11 12 | 8 9 | 8 8 | 16 19 |
| OKF6/TERT-1 | X X ** | 11 12 | 11 11 | 11 12 | 12 13 | 8 10 | 6 9.3 | 9 11 | 14 15 |
| OKF6/TERT-2 | X Y | 11 12 | 11 11 | 11 12 | 12 13 | 8 10 | 6 9.3 | 9 11 | 14 15 |
| Nthy-ori 3-1 | X | 12 | 11 | 12 13 | 11 | 7 12 | 7 | 9 | 16 18 |
| MSKleuk1 | X X | 10 13 | 11 14 | 12 12 | 12 12 | 10 11 | 6 7 | 8 10 | 17 18 |
| MSKleuk1S | X X | 10 13 | 11 14 | 12 12 | 12 12 | 10 11 | 6 7 | 8 10 | 17 18 |
Every single number correlates to the number of repeats for any given marker. “?” means failed to amplify, and 1 allele call means the sample is homozygous.
Possible loss of Y chromosome in the cells.
Thirty-seven cell lines were found to have 1 or more inconsistencies in their STR profile (Table 4). Most of these inconsistencies were likely the result of cell line cross-contamination and/or misidentification. In many cases, STR profiling alone could not determine the exact cause of the inconsistencies. All of the inconsistencies we identified are presented in Table-4. The following results detail several examples of the types of cell line aberrations we encountered after STR profiling:
Table 4.
STR profiles of 37 misidentified and cross-contaminated cell lines.*
| Cell line | Amelogenin | CSF1PO | D13S317 | D16S539 | D5S818 | D7S820 | THO1 | TPOX | vWA |
|---|---|---|---|---|---|---|---|---|---|
| PCI-3 | X | 11 | 11 | 11 | 13 | 8 11 | 7 | 8 9 | 17 |
| JHU019 | X | 11 | 11 | 11 | 13 | 8 11 | 6 7 | 8 9 | 17 |
| PC-3** | X | 11 | 11 | 11 | 13 | 8 11 | 6 7 | 8 9 | 17 |
| JHU028 | X | 10 12 | 11 | 11 12 | 13 | 8 11 | 8 | 8 11 | 14 |
| A549** | X Y | 10 12 | 11 | 11 12 | 11 | 8 11 | 8 9.3 | 8 11 | 14 |
| DRO | X X | 11 12 | 11 14 | 9 9 | 12 12 | 9 9 | 8 8 | ? 8 10 | 16 17 |
| A-375** | X | 11 12 | 11 14 | 9 | 12 | 9 | 8 | 8 10 | 16 17 |
| ARO | X X | 11 12 | 11 11 | 11 12 | 11 12 | 10 10 | 6 6 | 8 9 | 17 19 |
| KAT4 | X X | 11 12 | 11 13 | 9 11 12 | 11 12 | 8 10 12 | 6 7 | 8 9 11 | 16 17 18 19 |
| HT-29** | X | 11 12 | 11 12 | 11 12 | 11 12 | 10 | 6 9 | 8 9 | 17 19 |
| BHP5-16 | X | 11 | 12 | 9 13 | 11 12 | 8 10 | 6 7 | 8 11 | 16 18 |
| NPA87 | X | 11 | 12 | 9 13 | 11 12 | 8 | 6 7 | 8 11 | 16 18 |
| MDA-MB-435S** | X | 11 | 12 | 13 | 12 | 8 10 | 6 7 | 8 11 | 16 18 |
| ACC2 | X | 9 10 | 12 13.3 | 9 10 | 11 12 | 8 12 | 7 | 8 12 | 16 18 |
| ACC3 | X | 9 10 | 12 13.3 | 9 10 | 11 12 | 8 12 | 7 | 8 12 | 17 18 |
| ACCM | X | 9 10 | 12 13.3 | 9 10 | 11 12 | 8 12 | 7 | 8 12 | 17 18 |
| HeLa** | X | 9 10 | 12 13.3 | 9 10 | 11 12 | 8 12 | 7 | 8 12 | 16 18 |
| DM12 | X | 10 11 12 14 | 8 11 13 | 9 12 13 | 9 11 13 | 8 9 12 | 6 7 | 8 11 | 15 18 20 |
| DM14 | X | 10 11 12 14 | 8 11 13 | 9 12 13 | 9 11 13 | 8 9 12 | 6 7 | 8 11 | 15 17 18 20 |
| JMAR | X | 10 12 13 | 8 11 12 | 8 9 12 13 | 10 12 13 | 9 10 12 | 6 8 | 8 10 11 | 15 16 18 |
| MDA1986LN† | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| 1483† | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| MDA686LN† | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| T409 | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| Tu-167 | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| UM-SCC-1*** | X | 10 12 | 8 11 | 12 13 | 10 13 | 9 12 | 6 | 8 11 | 15 18 |
| MDA686TU† | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| 183† | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| T404 | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| T406 | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| Tu-158LN | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| Tu-159 | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| Tu-182 | X X | 12 13 | 12 12 | 8 9 | 12 12 | 10 10 | 8 8 | 10 11 | 15 16 |
| Tu-212 | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| Tu-212LN | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| Tu-138*** | X | 12 13 | 12 | 8 9 | 12 | 10 | 8 | 10 11 | 15 16 |
| PCI-22A | X | 10 | 8 12 | 9 11 | 12 | 8 9 | 6 | 8 11 | 18 |
| PCI-22B | X | 10 12 | 12 | 9 | 10 12 | 10 11 | 6 | 8 11 | 17 |
| Ca9-22 | X X | 10 12 | 12 12 | 9 11 | 11 11 | 11 11 | 8 8 | 8 8 | 17 17 |
| MSK-922 | X X | 10 12 | 12 12 | 9 11 | 11 11 | 11 11 | 8 8 | 8 8 | 17 17 |
| Ca9-22§ | X Y | 12 | 11 | 9 10 | 12 | 11 13 | 6 | 8 11 | 16 |
| JHU012 | X Y | 10 | 11 | 12 | 12 13 | 9 10 | 6 | 8 | 14 16 |
| JHU022*** | X Y | 10 | 11 | 12 | 12 13 | 9 10 | 6 | 8 | 14 16 |
| JHU013 | ? | 12 | 8 9 | 11 | 12 | 11 12 | 8 | 11 | 15 17 |
| FaDu*** | ? | 12 12 | 8 9 | 11 11 | 12 12 | 11 12 | 8 8 | 11 11 | 15 17 |
| UM-SCC-2 | X Y | 11 | 12 | 9 | 11 13 | 13 | 6 9.3 | 8 | 14 17 |
| UM-SCC-2‡ | X | 11 13 | 12 | 8 12 | 16 | ||||
| RPTC-1 | X | 11 12 | 11 12 | 9 | 8 10 | 11 | 9 | 11 | 14 18 |
| BHP2-7 | X | 11 12 | 11 12 | 9 | 8 10 | 11 | 9 | 11 | 14 18 |
| TPC-1*** | X | 11 12 | 11 12 | 9 9 | 8 10 | 11 11 | 9 9 | 11 11 | 14 18 |
| U-Hth112 | X X | 12 12 | 11 11 | 12 12 | 11 11 | 8 10 | 6 6 | 8 9 | 19 19 |
| U-Hth112** | X Y | ? | 12 | ? | 12 | 8 10 | ? | ? | 20 |
Highlighted lines indicate cell lines with 1 or more problems in their STR profiling results. Every single number correlates to the number of repeats for any given marker. “?” means failed to amplify, and 1 allele call means the sample is homozygous.
Cells obtained from Dr. Gary Clayman, The University of Texas MD Anderson Cancer Center.
STR profiles from HSRRB(16).
STR profile from (13).
STR profiles from ATCC.
STR profiles from Dr. Jeffrey Myers, The University of Texas MD Anderson Cancer Center.
Cross-contamination of HNSCC Cell Lines by Non-HNSCC Cell Lines
One of the most important findings of this study is the identification of head and neck cell lines that were cross-contaminated by cell lines of other tumor types (Table 4). We determined that PCI-3 and JHU019 were cross-contaminated by PC-3 prostate adenocarcinoma cells, JHU028 was cross-contaminated by A549 lung cancer cells, DRO was cross-contaminated by A-375 melanoma cells, ARO and KAT4 were cross-contaminated by HT-29 colon cancer cells, BHP5–16 and NPA87 were cross-contaminated by MDA-MB-435S melanoma cells, and ACC2, ACC3, and ACCM were cross-contaminated by HeLa cervical adenocarcinoma cells. Each of these STR profile matches was identified on the basis of the ATCC database of STR profiles. Not all of the HNSCC cell lines demonstrated complete identity to the potentially cross-contaminating cell line in the ATCC database. However, the preponderance of markers strongly implicated cross-contamination, and we are no longer comfortable using these cell lines for studies of HNSCC.
T409, Tu-167, MDA1986LN, 1483, and MDA686LN cell lines are genetically identical to the UM-SCC-1 cell line
The cell line UM-SCC-1 was obtained from the laboratory of Dr. Thomas E. Carey, and STR profiling showed results consistent with published data (for D13S317, D5S818, D7S820, vWA, and amelogenin) (13). We found that T409, Tu-167, MDA1986LN, 1483, and MDA686LN, which we obtained from Dr. Gary Clayman’s laboratory, exhibited an STR profile identical to that of UM-SCC-1, indicating that they are likely cross-contaminated by UM-SCC-1. However, early-passage stocks of MDA1986LN, MDA686LN and 1483 acquired from the laboratory of Dr. Peter Sacks, who originally established these lines had unique genotypes and we support their continued used.
DM12, DM14, and JMAR cell lines have multiple alleles identical to those of the UM-SCC-1 cell line
We found 8 UM-SCC-1 markers in the JMAR STR profile and 7 UM-SCC-1 markers in the DM12 and DM14 profiles. Only the D5S818 marker was not shared with UM-SCC-1. In addition to the UM-SCC-1 markers, we found other unknown alleles in these 3 cell lines. Generally, each locus should have no more than 2 alleles, so any additional alleles suggest contamination. We thus conclude that these 3 cell lines are likely to be cross-contaminated not only by UM-SCC-1 but also by other cell lines, and we no longer use these cells.
MDA686TU, 183, T404, T406, Tu-158LN, Tu-159, Tu-182, Tu-212, Tu-212LN, and Tu-138 HNSCC cell lines have identical STR profiles
We found another group of cell lines that had identical STR profiles. This group included MDA686TU, 183, T404, T406, Tu-158LN, Tu-159, Tu-182, Tu-212, Tu-212LN, and Tu-138. It should be noted that early-passage stocks of MDA686TU and 183 acquired from the laboratory of Dr. Peter Sacks have unique genotypes and we recommend their use.
The PCI-22A cell line does not match the PCI-22B cell line
PCI-22A and PCI-22B were obtained from a single patient; thus, the 2 cell lines should have identical STR profiles. However, the STR profiles did not match, except for 2 markers (THO1 and TPOX). Therefore, we were unable to verify the authenticity of either cell line, and these cell lines should not be considered as an isogenic pair of cells.
The Ca9–22 cell line is genetically identical to the MSK-922 HNSCC cell line
We obtained an early-passage stock of the MSK-922 line from the laboratory who established this cell line. Ca9–22 was obtained from another laboratory and found to have an identical STR profile to MSK-922. Additionally, we compared this profile to the original STR profile of the Ca9–22 cell line collected by the Japan Health Sciences Foundation (JHSF) (http://cellbank.nibio.go.jp/celldata/jcrb0625.htm). We found that our profile for Ca9–22 was completely different in all the 9 loci (Table 4) from the profile provided by JHSF. We conclude that our STR profile is that of MSK-922, and we recommend that anyone utilizing the Ca9–22 cell line should confirm its STR profile with that on the JHSF website since several laboratories have misidentified stocks of this line.
The JHU012 cell line is genetically identical to the JHU022 cell line, and the JHU013 cell line is genetically identical to the FaDu cell line
JHU013 was reported to be derived from JHU012, and these cell lines should thus have identical STR profiles; however, their STR profiles are not identical. Also, we found that the STR profile of JHU013 is identical to the STR profile of the FaDu cell line; therefore, JHU013 appears to be cross-contaminated by FaDu. In addition, we have found that JHU012 and JHU022 have identical STR profiles. Therefore, we recommend discontinuing the use of the JHU12 and JHU13 cell lines.
The UM-SCC-2 cell line used in some laboratories is not identical to the original UM-SCC-2 cell line
We obtained the UM-SCC-2 cell line from another laboratory that was found to be genetically different from the original UM-SCC-2 cell line (13), indicating that UM-SCC-2 from this group was likely cross-contaminated by other unknown cells. It is recommended that investigators who have this cell line in their collection perform STR genotyping and compare the results to those published here and in reference 12.
The RPTC-1, and BHP2–7 cell lines are genetically identical to the TPC-1 papillary thyroid cancer cell line
STR analysis revealed that the RPTC-1, and BHP2–7 were genetically identical to the TPC-1 papillary thyroid cancer cell line at all 8 loci. Importantly, it has previously been reported that BHP2–7 is genetically identical to TPC-1 (16). These results indicate that RPTC-1 is also genetically identical to TPC-1. Continued use of the RPTC-1 and BHP2–7 is discouraged.
Data for the U-Hth112 cell line do not match published data on this cell line
We obtained U-Hth112 cells from Dr. Nils Erik Heldin who originally prepared this line. The STR profiling data for U-Hth112 are very close to, but not identical to those previously published (16). It is not clear whether the small difference observed here is significant and thus whether this cell line is valid or not. Therefore, investigators using this line should be aware of the slight discrepancy, and consideration should be given to using other completely validated anaplastic thyroid carcinoma cell lines when possible.
Characterization of a Panel of 85 STR-Validated Head and Neck Cell Lines
STR profiling of 122 cell lines identified 37 invalid cell lines, which were removed from our panel. The STR genotypes of the remaining 85 validated cell lines are summarized in Table 3.
Clinical Characteristics
We collected the age, sex, primary tumor site, and TNM stage for all of the patients from whom lines were derived for which this information was available (Table 1). A summary of the results for the HNSCC cell lines is shown in Supplemental Table S3. Forty cell lines were from males (65.6%), which consistent with 72% of patients with HNSCC being male (2). The major primary tumor sites from which cells in this panel were derived include oral cavity (21.3%), oropharynx (8.2%), hypopharynx (6.6%), and larynx (13.1%). Twenty one cell lines (47.5%) were derived from metastases or recurrent disease. Among those with known tumor stage, Twenty-one cell lines were from patients with T1–2 tumors, and 23 were from patients with T3–4 tumors. Nineteen cell lines were from patients with N0 disease, 13 from patients with N1 disease, and 12 from patients with N2–3 disease. Additionally, 34 (55.7%) were from patients with stage III-IV disease.
Human Papillomavirus
We identified the presence of HPV-16 E6 and E7 in the HOK-16B cell line, which was anticipated since it was created from normal human oral keratinocytes immortalized by HPV-16 (E6/E7) transfection (17) (Table 5). We also identified HPV-16 E6 and E7 in the UM-SCC-47 cell line, consistent with previous reports (18, 19). While it has been reported that 1483 cell line is HPV-18 positive (18), MDA686LN is HPV-16 positive, and MDA1986LN is both HPV-16 and HPV-18 positive (20), we did not find evidence of HPV-16 or HPV-18 DNA in these cell lines. In addressing these discrepancies, we also performed western blot analyses to determine expression of p16 on HOK-16B, UM-SCC-47, 1483, MDA686LN and MDA1986LN cell lines, since a significant association has been reported between HPV-positivity and p16 overexpression (21). Although HOK16B and UM-SCC-47 over-expressed the p16 protein, no p16 expression was observed in 1483, MDA686LN or MDA1986LN cells (data not shown).
Table 5.
HPV status and cell morphology of unique head and neck cell lines.
| Cell line | HPV-16-E6 | HPV-16-E7 | HPV-18-E6 | HPV-18-E7 | Cell line morphology |
|---|---|---|---|---|---|
| HNSCC | |||||
| HN4 | - | - | - | - | TC |
| HN5 | - | - | - | - | R |
| HN30 | - | - | - | - | TC |
| HN31 | - | - | - | - | TC |
| UM-SCC-1 | - | - | - | - | TC |
| UM-SCC-3 | - | - | - | - | R |
| UM-SCC-4 | - | - | - | - | WC |
| UM-SCC-6 | - | - | - | - | VTC |
| UM-SCC-10A | - | - | - | - | WC |
| UM-SCC-10B | - | - | - | - | R |
| UM-SCC-11A | - | - | - | - | R |
| UM-SCC-11B | - | - | - | - | CF |
| UM-SCC-14A | - | - | - | - | CF |
| UM-SCC-14B | - | - | - | - | WC |
| UM-SCC-17A | - | - | - | - | VTC |
| UM-SCC-17B | - | - | - | - | TC |
| UM-SCC-19 | - | - | - | - | TC |
| UM-SCC-22A | - | - | - | - | CR |
| UM-SCC-22B | - | - | - | - | CR |
| UM-SCC-25 | - | - | - | - | WC |
| UM-SCC-33 | - | - | - | - | CF |
| UM-SCC-47 | + | + | - | - | CR |
| UM-SCC-85 | - | - | - | - | TC |
| JHU011 | - | - | - | - | WC |
| JHU022 | - | - | - | - | CR |
| JHU029 | - | - | - | - | R |
| MDA686TU | - | - | - | - | WC |
| MDA686LN | - | - | - | - | CR |
| MDA886LN | - | - | - | - | CF |
| MDA1186 | - | - | - | - | CF |
| MDA1386TU | - | - | - | - | F |
| MDA1386LN | - | - | - | - | F |
| MDA1586 | - | - | - | - | CR |
| MDA1686 | - | - | - | - | WC |
| MDA1986LN | - | - | - | - | TC |
| PCI-13 | - | - | - | - | TC |
| PCI-15A | - | - | - | - | CF |
| PCI-15B | - | - | - | - | WC |
| PCI-24 | - | - | - | - | TC |
| SCC-4 | - | - | - | - | WC |
| SCC-9 | - | - | - | - | WC |
| SCC-15 | - | - | - | - | TC |
| SCC-25 | - | - | - | - | TC |
| SCC-61 | - | - | - | - | R |
| FaDu | - | - | - | - | CF |
| OSC-19 | - | - | - | - | TC |
| OSC-19LN1 | NE | NE | NE | NE | TC |
| OSC-19LN2 | NE | NE | NE | NE | TC |
| OSC-19LN3 | NE | NE | NE | NE | TC |
| OSC-19LN4 | NE | NE | NE | NE | TC |
| OSC-19LN5 | NE | NE | NE | NE | TC |
| TR146 | - | - | - | - | WC |
| TU-138 | - | - | - | - | CF |
| SqCC/Y1 | - | - | - | - | TC |
| CAL-27 | - | - | - | - | CF |
| MSK-922 | - | - | - | - | TC |
| PE/CA-PJ34 | - | - | - | - | TC |
| Detroit562 | - | - | - | - | VTC |
| 183 | - | - | - | - | VTC |
| 1483 | - | - | - | - | VTC |
| 584A2 | - | - | - | - | TC |
| Thyroid cancer | |||||
| Hth74 | NE | NE | NE | NE | TC |
| Hth83 | NE | NE | NE | NE | TC |
| KAT18 | NE | NE | NE | NE | CF |
| SW1736 | NE | NE | NE | NE | R |
| Hth7 | NE | NE | NE | NE | F |
| Hth104 | NE | NE | NE | NE | R |
| 8505C | NE | NE | NE | NE | F |
| C643 | NE | NE | NE | NE | CR |
| B-CPAP | NE | NE | NE | NE | CF |
| TPC-1 | NE | NE | NE | NE | WC |
| K2 | NE | NE | NE | NE | WC |
| Skin cancer | |||||
| Colo16 | NE | NE | NE | NE | CR |
| SRB-1 | NE | NE | NE | NE | TC |
| SRB-12 | NE | NE | NE | NE | CR |
| Normal cells | |||||
| HOK16B | + | + | - | - | TC |
| NOM9/TK | - | - | - | - | R |
| NOM9/TKp53 | - | - | - | - | R |
| HaCaT | - | - | - | - | TC |
| NHEK | NE | NE | NE | NE | F |
| OKF6/TERT-1 | NE | NE | NE | NE | F |
| OKF6/TERT-2 | NE | NE | NE | NE | R |
| Nthy-ori 3-1 | NE | NE | NE | NE | WC |
| MSKleuk1 | - | - | - | - | CR |
| MSKleuk1S | - | - | - | - | WC |
Abbreviations: CF, colonies, fibroblast-like stretched cells; CR, colonies, round; F, fibroblast-like (long-stretched cells, few loose attachments); NE, not evaluated; R, round (round-flat cells, few loose attachments); TC, tight colonies; VTC, very tight colonies; WC, weak colonies.
Morphology
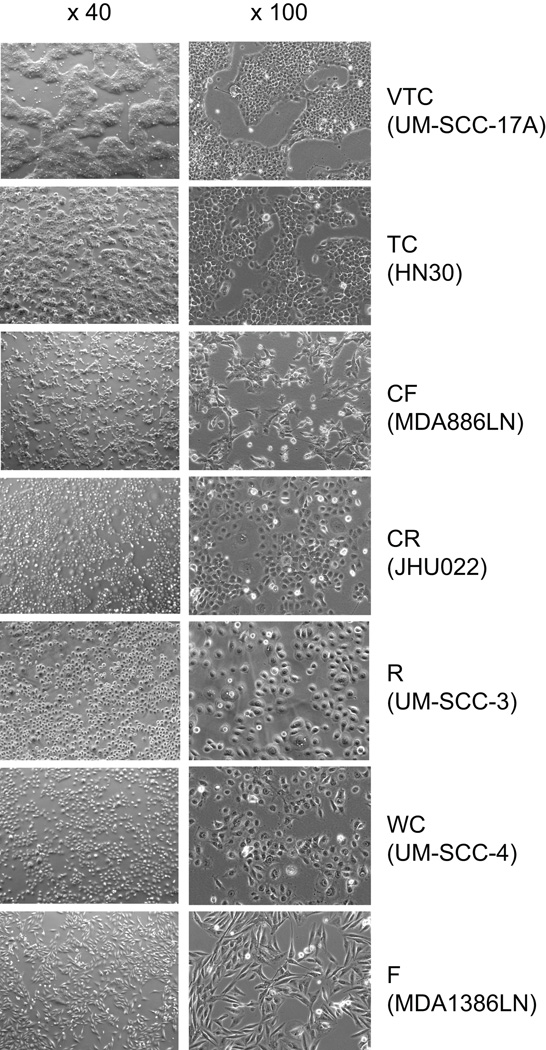
To document the morphology of the 85 unique head and neck cell lines in vitro, we photographed each of the cell lines at 2 magnifications (Supplementary Fig S1). These images begin to demonstrate the phenotypic heterogeneity present in the panel of cell lines. We categorized the cell lines into 7 groups on the basis of their morphologies (Fig 1, Supplementary Fig S1, and Table 5). These qualitative groups incorporated epithelial and mesenchymal aspects of the morphologies, including cellular shape and attachment to adjacent cells. For example, the “very tight colonies” group contains cell lines that have a more typical epithelial morphology. All “very tight colonies” cells appear tightly attached to other cells. In contrast, the “fibroblast-like” group contains mesenchymal-like cell lines. The “fibroblast-like” cells are more spindle-shaped and make few contacts with adjacent cells. The other groups demonstrate a range of phenotypes between these extremes. The number of cells in each group is shown in Supplementary Table S4.
Figure 1. Cell morphology of head and neck cell lines.
Each of the cell lines was photographed at 2 magnifications (× 40 and × 100), and cell lines were categorized into 7 groups on the basis of their morphologies. VTC, very tight colonies; TC, tight colonies; CF, colonies, fibroblast-like stretched cells; CR, colonies, round; R, round (round-flat cells, few loose attachments); WC, weak colonies; F, fibroblast-like (long-stretched cells, few loose attachments)
Mycoplasma
All the 85 unique cell lines were tested for mycoplasma contamination and if positive they were treated until a mycoplasma negative result was obtained.
DISCUSSION
In this study, we assembled a panel of 85 head and neck cell lines which were authenticated by STR profiling of 122 cell lines from multiple tissues of the head and neck region, including oral cavity, pharynx, larynx, nose, skin, thyroid, and parotid gland tissues; samples of leukoplakia lesions; and primary keratinocytes and normal oral epithelium. The verified cell lines included 61 HNSCC cell lines, 11 thyroid cancer cell lines, 3 cutaneous SCC cell lines, 2 leukoplakia lesion cell lines, 5 immortalized primary keratinocyte cell lines, and 3 immortalized primary epithelial cell lines.
We also identified 37 cell lines, which were either misidentified or cross-contaminated. The misidentification and cross-contamination of mammalian cell cultures continues to be a widespread problem in research, even though awareness of this problem dates back more than 45 years. It has been estimated that the incidence of research papers that report the use of cell cultures that were misidentified or cross-contaminated is 15–36% (7, 8), and a number of recent articles and editorials have highlighted the significance of this problem for the global research community (16, 22–25). This study identified authentication problems with 30% of the cell lines that were analyzed. If these problems had remained undetected, these invalid cell lines could have resulted in false conclusions about head and neck cancers that could delay progress in understanding and treating these deadly diseases. We hope that the data presented here can serve as a standard reference for head and neck cancer researchers and facilitate the validation of the cell lines in their own laboratories. Proof of cell line authentication is now required by many journals and is a relatively simple and inexpensive technique.
STR profiling has been reported to be an efficient and reproducible method to verify the true origin of human cell lines (26). In this study, we performed STR profiling using 9 different genetic markers also utilized by the ATCC (http://www.atcc.org) in order to facilitate comparison of our data to data in the ATCC’s STR database. STR profiling without appropriate controls can occasionally lead to slightly ambiguous results. For example, the STR profile of JHU028 did not exactly match the profile of the A549 lung cancer cell line. Two alleles were found to be slightly different, amelogenin and THO1 (Table 4). The amelogenin marker is known be to somewhat unstable, and samples from males do not always display the Y amelogenin marker, which could be the case for the A549 cell line, which was derived from the tumor of man (27). Loss of the Y chromosome is common even in peripheral blood lymphocytes and frequently seen in HNSCC cells from older males (28). The discrepancy in the THO1 marker could have a number of possible explanations, including cross-contamination and genomic instability. Additionally, it is mathematically possible that 2 unique cell lines will have identical STR profiles when only 9 loci are analyzed.
While STR analysis provides an effective way for cell-line comparison and identification of cross contamination, it is almost impossible to determine whether or not a cell line originates from a specific source unless it matches the signature of the donor tissue. The most accurate way to authenticate a cell line is to perform STR profiling of control tissue from the tumor from which the cell line is derived or early-passage stocks of the cells. As these resources often do not exist for many older cell lines, it is often necessary to make judgments about the validity of a cell line based on comparisons to published data and those on the website of cell line collections such as the ATCC (15). Brenner et al. (13) reported STR profiles for 73 UM-SCC cell lines and, in some cases, either early-passage tumor cells or normal fibroblasts were available to confirm the genotyping. Of significance to the present study, there was concordance between the results in that study and the results with the same lines in this study. Many of the factors that influenced our decisions about cell line validity are described in the Results section. As we found that contamination can occur even during the stage of primary culture, a new cell line ideally should have a corresponding tumor signature recorded when it is established, and that data should be stored with de-identified clinical information including whether the cell line was derived from previously untreated tissue, or tumor tissue that resulted from treatment failures and the treatment rendered. Much of these data were unavailable during our analysis of these cell lines. In general, we erred on the side of caution and eliminated questionable cell lines from our collection. The 85 unique head and neck cell lines that we report here represent one of the largest panels of cell lines assembled for the head and neck cancer research community.
HPV infection plays an important role in the pathogenesis of oropharyngeal cancers, and HPV DNA is identified in approximately 40–80% of oropharyngeal cancers (29, 30). However, we found that only 1 of the 56 HNSCC lines evaluated for HPV status was HPV-16 positive. This finding may be due to the fact that only 5 cell lines were derived from oropharyngeal tissue and the fact that the majority of these cell lines were established before the observation of the HPV-associated oropharyngeal cancer epidemic (29). This finding could also reflect an inherent difference in the biology of HPV-positive oropharyngeal cancers. Patients with oropharyngeal squamous cell carcinoma associated with HPV has been shown in several reports to have improved survival, suggesting HPV-driven disease responds well to treatment and is perhaps less aggressive (31, 32). The lack of HPV-positive cell lines in our panel suggests that future efforts should be made to identify or derive HPV-positive cell lines in order to study this subset of HNSCC further given the growing importance of this subset of HNSCC (30). Another important driver of HNSCC is TP53 mutation (33, 34). We have concurrently examined this gene along with the in vivo growth characteristics of the HNSCC cell lines among our panel in a separate report (35), and this study exemplifies how the present report can serve as a foundation for large-scale examination of the biology of HNSCC.
Molecular and genetic heterogeneity have been identified in many tumor types, including breast, colon, and lung tumors. This heterogeneity influences the clinical course of the disease and can predict response to therapy. Cell line panels often reflect the heterogeneity present in primary tumors, and these panels can be used as tools to understand the clinical disease. We believe that the panel of unique head and neck cell lines that we have assembled will shed light on the clinical heterogeneity present in HNSCC. An indication of this heterogeneity can be seen in the range of in vitro morphologies that we observed. Mesenchymal tumors or so-called epithelial-mesenchymal transition indicates a poor prognosis in HNSCC and other tumor types (36, 37). We identified at least 18 cell lines with some indication of mesenchymal morphology. This panel should be useful for future studies on the role of mesenchymal characteristics in HNSCC. This panel may also improve our understanding of other aspects of HNSCC, and we and others are currently undertaking a number of studies of the molecular and genetic heterogeneity of this cell line panel and its in vitro and in vivo phenotypes.
In conclusion, we have identified a new cell line resource for the head and neck cancer research community. This panel of 85 unique cell lines represents many tissues of the head and neck region. Nearly 30% of the cell lines that were assayed during the validation process had been previously misidentificated or cross-contaminated. This finding should serve as a strong rationale to researchers that cell line authentication is critical to efficient and productive research on head and neck cancer. We hope that this panel of 85 genomically verified cell lines will improve our understanding of HNSCC and facilitate the identification of novel therapeutic interventions.
Statement of Translational Relevance.
Human cell lines are vital reagents for studying cancer biology, and pre-clinically modeling novel cancer therapeutics, but recent reports have revealed that many cell lines are either misidentified or cross-contaminated with other cell lines. This fact remains true in the case of head and neck cancer cells lines. To clarify the derivation, and lineage of head and neck cancer cell lines for investigators working in this field, we assembled and performed STR analysis on a large panel of such cell lines collected from multiple investigators. Using this method, we found that 85 of the 122 head and neck cell lines had unique genetic profiles. This panel of validated and characterized cell lines serves as a resource for the head and neck cancer research community, to help further our understanding of neoplastic diseases of the head and neck region and to improve our ability to prognosticate and treat them.
Supplementary Material
Acknowledgements
We would like to thank Laura Kasch for the authentication analysis of the cell lines, Chong Zhao for his assistance in the HPV analysis. We also thank Stephanie Deming for editing the manuscript, and three anonymous reviewers for their thorough review and highly appreciate the comments and suggestions, which significantly improved the quality of this paper.
Grant Support: This work was supported by the PANTHEON program, NIH Specialized Programs of Research Excellence grant P50CA097007, NIH Cancer Center Support Grant CA016672 and R01DE14613 NIH/NCI.
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors declare that they have no potential conflicts of interests.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: a cancer journal for clinicians. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Batsakis J. Tumors of the head and neck. Baltimore: Williams and Wilkins; 1979. [Google Scholar]
- 4.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. International journal of cancer. 2005;114:806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 5.Ansari MH. Salivary gland tumors in an Iranian population: a retrospective study of 130 cases. J Oral Maxillofac Surg. 2007;65:2187–2194. doi: 10.1016/j.joms.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 7.Drexler HG, Dirks WG, Matsuo Y, MacLeod RA. False leukemia-lymphoma cell lines: an update on over 500 cell lines. Leukemia. 2003;17:416–426. doi: 10.1038/sj.leu.2402799. [DOI] [PubMed] [Google Scholar]
- 8.Dirks WG, Drexler HG. Authentication of cancer cell lines by DNA fingerprinting. Methods in molecular medicine. 2004;88:43–55. doi: 10.1385/1-59259-406-9:43. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz Bravo N, Gottesman M. Notice regarding authentication of cultured cell lines #NOT-OD-08-017. National Institutes of Health. 2007 [Google Scholar]
- 10.AACR Journals: Instructions for Authors. [cited; Available from: http://cancerres.aacrjournals.org/site/misc/ifora.xhtml.
- 11.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head & neck. 2007;29:163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 12.Lansford C, Grenman R, Bier H, Somers KD, Kim SY, Whiteside TL, et al. Head and neck cancers. In: Masters JR, Palsson B, editors. Human cell culture, Vol 2, cancer cell lines, Part 2. Dordrecht: Kluwer Academic Publishers; 1999. pp. 185–255. [Google Scholar]
- 13.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head & neck. 2010;32:417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Type Culture Collection STR Profile Database. [cited; Available from: http://www.atcc.org/CulturesandProducts/CellBiology/STRProfileDatabase/tabid/174/Default.aspx.
- 16.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991;12:1627–1631. doi: 10.1093/carcin/12.9.1627. [DOI] [PubMed] [Google Scholar]
- 18.Min BM, Baek JH, Shin KH, Gujuluva CN, Cherrick HM, Park NH. Inactivation of the p53 gene by either mutation or HPV infection is extremely frequent in human oral squamous cell carcinoma cell lines. Eur J Cancer B Oral Oncol. 1994;30B:338–345. doi: 10.1016/0964-1955(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, et al. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928–933. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Storthz KA, Shillitoe EJ. Mutations in the long control region of human papillomavirus DNA in oral cancer cells, and their functional consequences. Cancer research. 1997;57:1614–1619. [PubMed] [Google Scholar]
- 21.Langendijk JA, Psyrri A. The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: implications for treatment strategies and future clinical studies. Ann Oncol. 2010;21:1931–1934. doi: 10.1093/annonc/mdq439. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix M. Persistent use of “false” cell lines. International journal of cancer. 2008;122:1–4. doi: 10.1002/ijc.23233. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, et al. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther. 2009;8:713–724. doi: 10.1158/1535-7163.MCT-08-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. International journal of cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 25.Dirks WG, MacLeod RA, Nakamura Y, Kohara A, Reid Y, Milch H, et al. Cell line cross-contamination initiative: an interactive reference database of STR profiles covering common cancer cell lines. International journal of cancer. 2010;126:303–304. doi: 10.1002/ijc.24999. [DOI] [PubMed] [Google Scholar]
- 26.Masters JR. HeLa cells 50 years on: the good, the bad and the ugly. Nature reviews. 2002;2:315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
- 27.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. Journal of the National Cancer Institute. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 28.Atlas of Genetics and Cytogenetics in Oncology and Haematology. [cited; Available from: http://atlasgeneticsoncology.org/Anomalies/YlossID1089.html.
- 29.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 30.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 33.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Human mutation. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 35.Sano D, Ow TJ, Xie TX, Zhao M, Pickering CR, Zhou G, et al. Disruptive TP53 Mutation is Associated with Aggressive Disease Characteristics in an Orthotopic Murine Model of Oral Tongue Cancer manuscript under review. doi: 10.1158/1078-0432.CCR-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang MH, Chang SY, Chiou SH, Liu CJ, Chi CW, Chen PM, et al. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene. 2007;26:1459–1467. doi: 10.1038/sj.onc.1209929. [DOI] [PubMed] [Google Scholar]
- 37.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.