Abstract
FACT is a roughly 180 kDa heterodimeric protein complex important for managing the properties of chromatin in eukaryotic cells. Chromatin is a repressive barrier that plays an important role in protecting genomic DNA and regulating access to it. This barrier must be temporarily removed during transcription, replication, and repair, but it also must be rapidly restored to the original state afterwards. Further, the properties of chromatin are dynamic and must be adjusted as conditions dictate. FACT was identified as a factor that destabilizes nucleosomes in vitro, but it has now also been implicated as a central factor in the deposition of histones to form nucleosomes, as an exchange factor that swaps the histones within existing nucleosomes for variant forms, and as a tether that prevents histones from being displaced by the passage of RNA polymerases during transcription. FACT therefore plays central roles in building, maintaining, adjusting, and overcoming the chromatin barrier. This review summarizes recent results that have begun to reveal how FACT can promote what appear to be contradictory goals, using a simple set of binding activities to both enhance and diminish the stability of nucleosomes.
Introductory FACTs
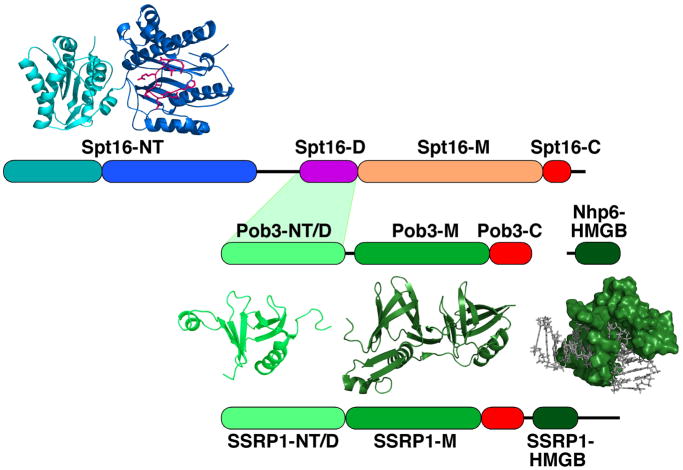
Human FACT is a heterodimer of hSpt16 and SSRP1 proteins that was first identified through its ability to allow RNA Pol II to transcribe DNA templates incorporated into nucleosomes, thus earning the name “facilitates chromatin transcription” [1–3]. FACT is highly conserved among eukaryotes, but in yeast and fungi the SSRP1 subunit is called Pob3 and lacks the HMGB DNA binding motif found at the C-terminus of SSRP1 [4, 5] (Figure 1). The DNA binding activity instead appears to be supplied by the separate protein Nhp6 [6–8]. Nhp6 is only loosely associated with Spt16-Pob3, is required in about a 10-fold molar excess over the heterodimer for in vitro assays, and promotes FACT function in vivo [6, 7, 9, 10]. Nhp6 also supports the activities of several other chromatin factors [8], so it appears to have a general role in promoting chromatin dynamics in yeast, as HMGB family members do in higher eukaryotes. FACT is essential for viability, but yeast cells can grow slowly without Nhp6, suggesting that the DNA binding function may not be a central component of FACT activity or that other proteins might substitute for Nhp6. FACT interacts physically and genetically with the site-specific DNA binding complex component Swi6 ([11]) and with the single-stranded DNA binding factor RPA ([12]), but these interactions appear to recruit FACT to specific promoters or to DNA replication intermediates, respectively. The precise mechanistic role of Nhp6 in FACT activity, the identities of potential substitutes, and how the single HMGB domain found in SSRP1 performs the tasks that require multiple Nhp6 molecules in yeast continue to be active areas of investigation.
Figure 1.
Domain structure of FACT proteins.
The NT (N-terminal), D (dimerization), M (middle), C (C-terminal), and HMGB domains of S. cerevisiae Spt16, Pob3, and Nhp6 and human SSRP1 are diagrammed to scale. The acidic domains are colored red. SSRP1 has a serine-rich C-terminal extension not found in yeast or fungal FACT. The Spt16-NT domain has two lobes as indicated in cyan and blue, with the loop enclosing the conserved putative peptide-binding domain indicated in magenta [30, 31]. Ribbon diagrams of the domains whose crystallographic structures are known are shown, as is a surface representation of Nhp6 bound to DNA determined by NMR [90]. Structures were rendered in PyMOL using PDB files 3BIQ (Spt16-NT) [31], 3F5R (Pob3-NT/D) [35], 2GCL (Pob3-M) [12], and 1J5N (Nhp6-DNA) [90].
FACT is essential for viability in a range of eukaryotes, although viable strains of fission yeast lacking the Pob3 subunit have been described [13–15]. Spt16 was identified in two genetic screens; one for factors responsible for enforcing the stringency of transcription initiation (either increased or decreased Spt16 activity suppressed insertion of a Ty1 transposon element in a reporter gene by allowing initiation to occur at inappropriate sites), and another for factors that control cell division cycle progression (decreased Spt16 activity caused lower rates of transcription of G1 cyclins, so it was also named Cdc68) [16–19]. While mutating POB3 also causes defects in transcription, Pob3 was first identified as a Pol1-binding factor physically associated with the catalytic subunit of DNA polymerase α in yeast [5], implicating FACT in DNA replication. Subsequent work has strengthened ties to transcription factors and to replication machinery [3, 13, 20–23], and has in addition suggested a role in centromere function [14]. These activities can all be ascribed to FACT’s core ability to alter the properties of chromatin by binding histones and nucleosomes, but the range of processes involved suggests that the acronym “FACT” should be reinterpreted to mean “facilitates chromatin transactions.”
FACT has also been implicated in DNA repair as a specificity factor for casein kinase II that causes it to phosphorylate serine residue 293 of human p53 in response to UV damage [24], and in mRNA transport as a factor that assists the loading of export machinery to messages [25]. These functions do not appear to involve histone chaperone activity, but may instead rely on the ability of FACT to interact with a broad range of proteins through its multiple binding motifs, as described below.
FACT is a histone chaperone with multiple binding domains
The structures of several domains of FACT have been described (Figure 1; reviewed recently in [26]), although the structure of the intact heterodimer alone or in complex with histones or nucleosomes remains elusive. Human FACT binds to histone H2A–H2B dimers so it has been considered to be an H2A–H2B histone chaperone [27–29]. Human SSRP1 was also reported to bind small amounts of H3–H4 [27], and recent work with the isolated N-terminal domain (NT domain) of Schizzosaccharomyces pombe Spt16 showed that it binds both the globular domains and the N-terminal tails of H3–H4 separately using distinct surfaces [30]. FACT is therefore able to chaperone both H2A–H2B and H3–H4 complexes in a free state, and it also binds intact nucleosomes with high affinity [7, 9, 10]. Current research is focused on determining how these different binding modules interact with one another to produce the core activities of FACT.
The NT domain of Spt16 adopts a “pita-bread” fold also found in a family of aminopeptidases with which Spt16 shares sequence similarity, although critical peptidase active site residues are missing and no peptidase activity has been detected with Spt16 or FACT [30, 31]. Yeast cells can tolerate deletion of the NT domain of Spt16 ([32]), but this deletion or substitution mutations involving any of several conserved residues within the putative peptide binding groove cause lethality when combined with a mutated allele of POB3 (see below, and [31]). The Spt16 NT domain therefore appears to be capable of binding peptides, and it performs an important function that may be redundant with Pob3. Further, intact FACT displays high affinity for peptides derived from the N-terminal tails of some histones, and proteolytic removal of all of the histone tails severely diminishes the affinity of FACT for nucleosomes [30, 31]. The obvious hypothesis is therefore that the conserved peptidase groove of the Spt16 NT domain is the binding site for the N-terminal tails of histones. However, current results do not support this simple model. The isolated S. pombe Spt16 NT domain does bind histone H3–H4 N-terminal peptides, but the tightest binding reported was for the H4 tail with a KD of 3 μM [30]. Intact Saccharomyces cerevisiae FACT was reported to bind H3 and H4 N-terminal tail peptides with a much higher affinity of 2–6 nM, but neither this binding nor the binding to intact nucleosomes required the Spt16 NT domain [31]. A conserved region of the S. pombe Spt16 NT domain interacted with the N-terminal tail of an adjacent monomer in a crystal lattice, but the binding surface did not coincide with the putative peptide-binding groove [30]. It therefore appears that the NT domain of Spt16 has multiple interaction surfaces, including regions that can interact separately with the globular domains and the N-terminal tails of H3–H4, that the NT domain has the capacity to bind to N-terminal peptides that may include both histones and other unknown proteins, and that FACT can interact tightly with histone N-terminal tails using domains other than the Spt16 NT domain.
The structure of the middle domain of Pob3 reveals two pleckstrin homology folds (a double PH motif; [12]). This domain has sequence and structural similarity with the H3–H4 chaperone Rtt106 [33, 34], consistent with the suggestion that Pob3/SSRP1 is an H3–H4 chaperone. The N-terminal domain of Pob3 contains a single PH fold [35], and the sequence of the middle domain of Spt16 suggests that it may also have at least one of these folds [26]. The PH motif is a compact structural core that is associated with domains that bind a broad range of ligands, including proteins [12]. The large number of known and putative PH folds within FACT therefore suggests that FACT is a modular assembly of domains that could bind to a variety of ligands, allowing multiple flexible contacts with histones or other proteins simultaneously.
Both Spt16 and Pob3/SSRP1 contain C-terminal domains with a high percentage of acidic residues (Figure 1). This is a common feature of histone chaperones, and deletion of either acidic domain from FACT is lethal [27, 36, 37]. However, the mechanistic function of these acidic domains remains unknown. One proposal is that the acidic residues assist in binding the basic regions of histones, either by non-specific charge neutralization or by providing a specific binding interface, but this model has not been rigorously tested. The precise role of these acidic domains in histone chaperone function therefore remains an unsolved puzzle.
Yeast cells can tolerate mutations that delete the NT domain of Spt16, that change residue Q308 in Pob3 to lysine or arginine, or that destabilize the interface between H2A and the H3–H4 surface in the docking domain region of the nucleosome (the docking domain is the extended C-terminal region of H2A that fits into a groove formed by H3–H4; see Fig 2 and [38]). However, combining any two of these mutations is lethal [31]. This suggests that the components of nucleosomes are tethered together through multiple partially redundant contacts, including histone-FACT contacts, histone-histone contacts, and probably histone-DNA contacts. The modular nature of FACT subunits easily accommodates such a system of multiple, independent, simultaneous interactions with different components of the nucleosome. In this view, the synthetic lethality described above shows that loss of a single tethering contact in this network is tolerable, but loss of multiple contacts is not. If this interpretation is correct, then preventing dispersal of nucleosomal components through tethering is an essential function of FACT.
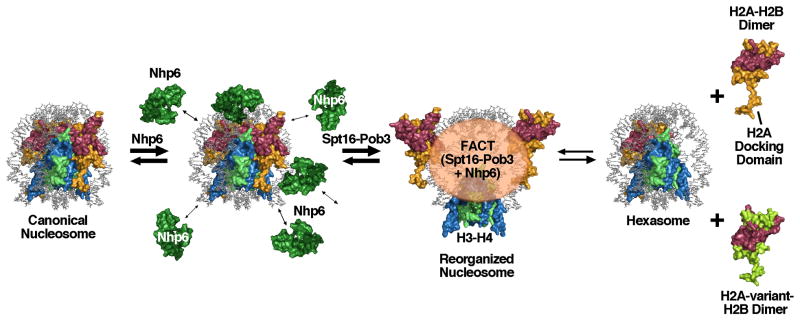
Figure 2.
Model for nucleosome reorganizing activities
Nhp6 binds to DNA at the surface of a canonical nucleosome, bending the DNA and destabilizing histone-DNA contacts [39]. Repeated binding-bending-release cycles within a short window of time render the nucleosome less stable and more suitable for reorganization by Spt16-Pob3. Reorganization involves disruption of the contacts between H2A–H2B and H3–H4, but the components of the nucleosome remain tethered together by FACT in a form in which the DNA is largely accessible. This dynamic form loses H2A–H2B dimers at a higher frequency than canonical nucleosomes do, but in the “global accessibility” model, dimer loss is an optional outcome of reorganization, not an obligate product. In the “dimer displacement” model FACT activity proceeds directly to displacement of an H2A–H2B dimer to form a hexasome. FACT might promote insertion of variant forms of H2A–H2B by chaperoning them and swapping them into either the reorganized form or hexasomes. The extended C-terminal tail of H2A that forms the docking domain that is partially responsible for dimer-tetramer stability in the core octamer is labeled; this domain may retain contact with H3–H4 during reorganization by bending at the point where it reaches the globular domain, unlike the rigid-body movement modeled here.
FACT reorganizes nucleosomes
FACT binds nucleosomes in vitro with an apparent KD of about 10 nM [9]. This binding is associated with increased global accessibility of the nucleosomal DNA to some endonucleases [7, 9, 10]. Some restriction enzymes digest their recognition sites within nucleosomal DNA at roughly half the rate observed with DNA free of histones, which is about 50 times faster than the digestion of these sites in the absence of FACT [39]. The enhanced digestion rate was observed at all locations within the nucleosomes that were tested, including sites near the central dyad of symmetry associated with H3–H4 proteins and sites near the entry/exit points of the DNA associated with H2A–H2B. In contrast, nucleosomal DNA was protected from digestion by other enzymes whether or not FACT was present. These results indicate that in the presence of FACT, nucleosomes are in a more open configuration in which the DNA is dramatically more accessible than it is in canonical nucleosomes, but is still more restricted than it is in the absence of histones (Figure 2). The DNA is globally affected, suggesting a dynamic structure in which the components of the nucleosome are tethered together but capable of moving so that different regions of the DNA are available at different times. This “global access” model for FACT function differs from the original “dimer displacement” proposal that postulated removal of one H2A–H2B dimer from octameric nucleosomes to form hexasomes [27]; these two models are discussed further below.
ATP-dependent chromatin remodeling enzymes also increase the accessibility of nucleosomal DNA, in most cases by translocating the histone octamer core along the DNA to move the recognition site in the DNA out of a nucleosomal context [40]. FACT does not hydrolyze ATP, does not share sequence similarity with ATP-dependent remodeling enzymes, and does not translocate histone cores relative to DNA [1, 10]. FACT’s ability to enhance accessibility of nucleosomal DNA to nucleases therefore results from a mechanism different from ATP-dependent remodeling, and has been called “nucleosome reorganization” to distinguish the two distinct ways of altering nucleosomes. FACT can interact with nucleosome remodelers, collaborating with Swi/Snf during activation of the HO promoter ([41]) and opposing the function of Chd1 ([42]). It is likely that nucleosome remodeling and nucleosome reorganization interact extensively with one another in vivo, but little is currently known about this interplay.
The role of H2A–H2B dimer displacement in reorganization
Immobilized nucleosomes lost about half of their complement of H2A–H2B dimers during a one hour incubation with human FACT [27]. Together with the H2A–H2B binding activity of FACT, this suggested the “dimer displacement” model for FACT activity in which the principle function of FACT is to create hexasomes (Figure 2). Supporting this interpretation, FACT was unable to promote transcription when histones were crosslinked to prevent expulsion of H2A–H2B dimers [2]. However, recent results with yeast FACT suggest that while reorganization does require disruption of the interface between H2A–H2B dimers and (H3–H4)2 tetramers, the increased nuclease sensitivity and sensitivity to hydroxyl radical damage observed in the DNA during reorganization of nucleosomes by FACT do not require full eviction of the dimer [39]. Instead, the “global accessibility” model proposes that reorganization involves equilibration between a closed canonical form and an open reorganized form of nucleosomes (Figure 2). Each form has the same composition, but the reorganized form is inherently looser and more prone to displacement of H2A–H2B dimers.
The modular nature of FACT would allow it to keep the components of the nucleosome associated with one another in a flexible manner, so the reorganized form may not be a unique structure but a dynamic, loose association of proteins and DNA that are poised to coalesce into a canonical nucleosome. Each region of the DNA would be exposed at different times in this dynamic structure, which would explain the global increase in restriction endonuclease sensitivity observed throughout nucleosomes [39]. At the same time, with FACT and the components of the nucleosome remaining tethered together, the reorganized form would retain some structure, explaining the partial or complete protection of the DNA observed with different probes [39].
The global access model is compatible with all of the observations that supported the dimer displacement model, as it also requires disruption of the dimer-tetramer interface and can result in loss of dimers from nucleosomes. It can also explain observations that are less readily accounted for by the dimer displacement model, such as the global nuclease sensitivity discussed above and the lack of correlation between the amount of dimer loss under different reaction conditions and the level of DNA accessibility [39]. Figure 2 summarizes the models, with the difference being that in the global access model the reorganized form is an important intermediate and dimer displacement is an optional outcome, while the dimer displacement model does not require a formal reorganized intermediate and dimer displacement is the obligate outcome.
Nucleosome dynamics and FACT activity
FACT could promote DNA accessibility by binding to canonical nucleosomes and inducing them to adopt a more open configuration. Alternatively, nucleosomes might be in equilibrium between the canonical form and a reorganized form and FACT preferentially binds to and stabilizes the open state. Recent single-molecule FRET experiments provide support for the latter model, revealing a dynamic equilibrium between canonical nucleosomes and a form in which the H2A–H2B dimers remain associated with the DNA but are farther away from the dyad of symmetry than expected from the crystal structure [43]. This form was observed at low levels under physiological conditions, and might correspond to the reorganized form postulated above to be the binding partner for FACT.
Further evidence suggesting an important role for dynamic changes in nucleosome structure during FACT activity have been provided by a recent genetic study. Mutations in histone genes that suppress the growth defects caused by the spt16-11 allele in yeast were found to cluster in H2A and H2B at their interface with H3–H4 [44]. Nucleosomes reconstituted in vitro with some of these suppressor mutations in the histones displayed increased sensitivity to restriction endonuclease digestion, which was interpreted to mean that the mutated histones promoted increased levels of the open, reorganized form of nucleosomes. In contrast, purified FACT containing the spt16-11 mutation was less able to support accessibility of nucleosomal DNA to endonuclease digestion. Taken together, these results suggest that nucleosomes are inherently dynamic structures that equilibrate between the canonical closed state and a more open reorganized form. FACT either promotes the open state or stabilizes it so that nucleosomes spend a greater fraction of their time in the accessible state. The genetic suppression is therefore the result of combining a FACT mutant that is less efficient at maintaining a population of reorganized nucleosomes with nucleosomes that are easier to reorganize due to a destabilized dimer-tetramer interface. The key role of the dimer-tetramer interface revealed by these results is consistent with both dimer displacement and global access models for FACT activity, and shows that the models derived from in vitro studies are likely to be relevant in vivo. Other interpretations of the results remain possible, but this model provides testable hypotheses for probing the mechanism of FACT activity in vivo and in vitro, and also provides a new perspective on the importance of dynamic changes in nucleosome structure in living cells.
Effect of FACT on RNA Pol II
FACT was initially purified using its ability to promote RNA Pol II elongation through nucleosomes in vitro as an assay [1], and further insight into this activity has been gained recently [27, 45, 46]. Pol II pauses principally at two sites, about 15 and 45 nucleotides into the nucleosome, and FACT primarily promotes progression through the pause at 45 nts. The pause at 15 nts is due to contact between H2A–H2B and DNA, while the pause at 45 nts marks the transition to H3–H4:DNA contacts, suggesting that FACT primarily helps the polymerase overcome tetramer:DNA bonds. Pausing at this site is also reduced by removal of the H2A–H2B N-terminal tails, and by mutations in H3–H4 that weaken DNA binding near this same site [46, 47]. FACT could therefore alleviate pausing by altering the stability of tetramer association with DNA or by altering the internal dynamics of nucleosomes by removing H2A–H2B dimers. In either case, these results show that the primary effect of FACT in vitro on RNA Pol II transcription does not occur as the polymerase approaches the nucleosome but instead occurs after the polymerase has transcribed well into the nucleosomal boundary.
FACT can promote nucleosome eviction
Chromatin is inherently repressive, so activation of transcription can involve eviction of nucleosomes to allow assembly of transcription complexes on promoter DNA sites that are otherwise inaccessible. Histone chaperones such as Asf1 have been implicated in nucleosome eviction [48], although it is currently unclear whether chaperones play an active role in initiating disassembly of nucleosomes or a passive one in carrying histones away from sites where other factors such as ATP-dependent remodelers are functioning. As a histone chaperone that can destabilize nucleosomes, FACT seems capable of playing both roles. Consistent with this possibility, FACT has been shown to be required for nucleosome eviction during activation of several inducible or cell-cycle regulated yeast promoters including GAL1, HO, PHO5, and CLN2 [11, 39, 41, 49]. The mechanism of nucleosome eviction appears to vary with the promoter, as both Asf1 and FACT contribute to eviction from PHO5 and HO promoters, FACT but not Asf1 contributes to eviction from CLN2, and FACT is not required for the nucleosome eviction that is observed during activation of the cell-cycle regulated promoter of PIR1. Nucleosome eviction is therefore accomplished through different pathways under different circumstances, some involving FACT and some independent of it.
FACT is needed for rapid induction of the GAL1 promoter in yeast, as FACT deficiency caused inefficient eviction of nucleosomes from this promoter and diminished levels of transcription [39, 42]. The dimer displacement model predicts that H2A–H2B should be displaced by FACT prior to removal of H3–H4, but the ratio of H3 to H2B in the GAL1 promoter remained constant during induction. This does not rule out the dimer displacement model, as H3–H4 removal could follow loss of H2A–H2B too rapidly to allow detection of intermediates, but it does suggest that hexasomes are not long-lived intermediates in promoters being acted upon by FACT.
The HO promoter in yeast is under unusually stringent control, and studies of HO activation have revealed distinct mechanisms for evicting nucleosomes from different regions of the promoter at different times. The HO promoter extends over about 2000 bp, encompassing about 10 nucleosomes whose level of occupancy varies during activation [41]. Nucleosome eviction in the most distal region of the promoter begins before RNA polymerase is recruited, and depends on recognition of sites in the DNA by Swi5, followed by recruitment of the coactivator complexes Swi/Snf, SAGA, and Mediator. This initial eviction does not require FACT or Asf1, but a second wave of eviction of nucleosomes more proximal to the transcription start site requires FACT, and a third wave adjacent to the second requires Asf1. Repopulation of histones over the Swi5 binding sites begins even while the later evictions are occurring, and this reassembly requires both FACT and Asf1. Studies of HO therefore show that nucleosome eviction is not a simple binary switch between fully closed and fully open promoter states. Instead, DNA binding proteins, ATP-dependent remodelers, and histone modifying enzymes all collaborate with histone chaperones to specify which regions of chromatin are altered, and different sets of these “keys” are needed to unlock different nucleosomes at different times. This layered, sequential mechanism decreases spurious activation by making it improbable that the multiple independent barriers can be overcome simultaneously by a random series of events. However, it is not yet obvious how each nucleosome acquires a distinct enough personality that eviction machinery that is effective for one set of nucleosomes is ineffective for removing adjacent nucleosomes. In other words, it is not clear how eviction affects one or a small number of nucleosomes without allowing the destabilization to spread into neighboring regions until additional factors like FACT or Asf1 are recruited. Learning how FACT and other histone chaperones are recruited and regulated so that they act only within specific boundaries at specific times remains an important unsolved step in understanding the role of chromatin in regulating gene expression.
FACT promotes reassembly of nucleosomes during transcription
FACT clearly destabilizes nucleosomes in vitro, and as described above it can participate in nucleosome eviction, suggesting that FACT can also destabilize nucleosomes in vivo. However, canonical nucleosomes can be recovered after reorganization by FACT ([39]) so FACT might also participate in nucleosome assembly by promoting the “reverse” of the reorganization reaction in Figure 2. Supporting this view, FACT can deposit core histones onto free DNA in vitro [27], and several lines of evidence now suggest that FACT promotes nucleosome formation in vivo.
Transcription is associated with elevated rates of histone replacement, partly because of nucleosome eviction from some promoters during activation, and partly because of destabilization of nucleosomes by the transcription machinery during elongation [50, 51]. In the dimer displacement model, FACT deficiency would lead to decreased turnover of H2A–H2B, as less FACT activity would lead to less dimer displacement and therefore less replacement would be required. Alternatively, if FACT’s normal function is to tether nucleosomal components together as in the global access model, then FACT deficiency would lead to increased turnover of histones in transcribed regions as less FACT activity would lead to increased nucleosome disruption by RNA Pol II.
Strong evidence in support of the latter prediction has been provided by experiments in yeast that measured histone replacement rates in normal cells and in cells with diminished FACT activity [52]. In these experiments, the rates of histone turnover in the presence of FACT were low, but increased dramatically when FACT was depleted. The turnover rates increased for both H2A–H2B dimers and (H3–H4)2 tetramers, heavily transcribed genes displayed larger effects, and inhibition of transcription decreased the rate of turnover. One physiological role of FACT is therefore to prevent transcription from disrupting nucleosomes, presumably by preventing histones from dispersing or by promoting rapid reassembly of the nucleosomes using the original components so that new histones do not need to be recruited.
A second line of evidence linking FACT to efficient nucleosome stabilization comes from examination of cryptic promoter activation. Sequences with promoter activity can be found within some transcription units; these sites normally do not support initiation of transcription because of the repressive effects of the local chromatin structure, but they can be activated by defects that weaken the chromatin barrier [53, 54]. Factors that promote reassembly of nucleosomes disrupted by transcription or that reverse the activating marks that support initiation of transcription are therefore needed to prevent these cryptic promoters from being activated. Mutations in the genes that encode histone chaperones such as FACT and Spt6 activate cryptic promoters [55, 56], demonstrating that these chaperones have an important role in promoting nucleosome reassembly after transcription.
Cryptic promoter activation is an aberrant outcome of a defect in nucleosome reassembly, but normal regulation of transcription of the SER3 gene involves a similar mechanism [57]. In this case, the non-coding gene SRG1 is located upstream of SER3, and high levels of constitutive transcription of SRG1 cause nucleosomes to be positioned over sequences in the SER3 promoter that must be accessible to allow it to be activated. SRG1 transcription therefore leads to repression of SER3, but only if nucleosomes are redeposited efficiently after passage of RNA Pol II. Some FACT defects allow normal transcription of SRG1 but cause decreased nucleosome occupancy in the SER3 promoter and inappropriate activation of this gene [57]. FACT is known to promote initiation and elongation of transcription but defects in these activities would not be expected to cause activation of SER3 without decreasing SRG1 transcription. These results therefore once again implicate FACT in nucleosome reassembly following transcription.
FACT prevents excess histone displacement
Free histones can bind to chromatin and form aberrant structures that interfere with the regulation of transcription and initiation of DNA replication [58, 59]. Excess histones are instead bound by chaperones, phosphorylated by the protein kinase Rad53, and degraded by the proteasome [60]. The abnormally high rates of displacement of histones from chromatin observed in FACT mutants should therefore trigger these free histone buffering and degradation mechanisms. Supporting this model, decreased FACT activity caused increased levels of free histones associated with the chaperones Asf1 and Nap1, and the maximal effect required ongoing transcription [61]. Complexes of both H2A–H2B and H3–H4 with chaperones were observed, indicating that FACT deficiency leads to complete dissociation of histone octamers. The excess non-nucleosomal histones caused repression of transcription of the CLN3 gene, delaying the completion of G1, presumably as a result of aberrant chromatin formed in the promoter of this gene [61]. This could explain the G1 arrest observed in early studies of SPT16/CDC68 [16, 19]. This can also explain the synthetic defects observed when spt16 mutations are combined with asf1 or rad53 mutations [41, 61], as loss of a histone chaperone reduces the free histone buffering capacity of the cell and loss of Rad53 function prevents degradation of the excess histones.
The increased histone displacement in cells lacking normal levels of FACT activity might also explain the synthetic lethality observed previously between FACT defects and loss of components of the Hir/Hpc complex [62]. The Hir/Hpc complex participates in repression of transcription of the histone genes outside of S phase, but it is also implicated in deposition of nucleosomes independent of DNA replication [63]. The aberrant displacement of histones that occurs in cells lacking normal levels of FACT activity would create high levels of chromatin damage as transcribed regions become depleted of nucleosomes. This might be tolerated in the presence of a robust repair mechanism involving the Hir/Hpc complex, but is lethal in the absence of this mechanism.
FACT can therefore participate in nucleosome destabilization, but it also contributes to nucleosome stability by minimizing the disruption of chromatin during transcription. This seems paradoxical, but can be explained by the reversibility of the mechanisms proposed in Figure 2.
FACT has a role in histone exchange
Reorganized nucleosomes are prone to displacement of H2A–H2B dimers, making reorganization a likely intermediate in pathways that remove the existing dimers to incorporate variants. This appears to be the case for the DNA double strand break repair variant H2A.X in mammalian cells, as introduction of this form into chromatin is inhibited by knockdown of FACT expression [64]. FACT appears to be both chaperone and exchange factor for this reaction, as FACT copurified with soluble H2A.X and FACT also promoted both the removal of H2A.X from nucleosomes and insertion of this variant into canonical nucleosomes [64, 65]. The activity was specific, as FACT did not exchange the H2A.Z variant in these experiments, and the histone chaperone Nap1 did not promote exchange of either variant. These results suggest that FACT can act as a specific exchange factor, perhaps by using a combination of general destabilization of nucleosomes and specific chaperoning of histone variants. FACT mutants have not been reported to have defects in DNA repair in yeast, but this might reflect a lack of alleles affecting this specific activity or a difference in the use of histone variants, as yeasts do not encode an H2A.X variant.
FACT has been implicated in the function of the H2A.Z variant in yeast, where it is called Htz1. Deletion of HTZ1 in yeast enhances the defects caused by FACT mutations, causing severe synthetic growth defects or lethality [42, 66]. This suggests that FACT activity is enhanced by incorporation of Htz1 into nucleosomes, so that loss of Htz1 increases the requirement for efficient FACT function. Alleles of SPT16 have also been described that suppress defects caused by the loss of Htz1, which has been interpreted to indicate that the role of Htz1 in promoting transcription elongation by RNA Pol II can be replaced by enhancing the activity of FACT in this process [66]. The mechanisms underlying these observations remain unexplored but these alleles of SPT16 provide new tools for studying FACT’s functions.
Although human FACT did not promote exchange of H2A.Z in vitro [64], evidence for a direct role for yeast FACT as an H2A.Z chaperone has been described in vivo, especially in the absence of the primary yeast H2A.Z chaperone Chz1 or the deposition factor Swr1 [67]. FACT has also been implicated in the deposition and removal of yeast Htz1in the nucleosomes flanking the SUP4 gene, even in cells containing normal Chz1 and Swr1 [68]. In this case, FACT is responsible for repressing transcription by RNA Pol III, and other studies underscore the importance of FACT for both activating and repressing transcription by RNA Pol I, Pol II, and Pol III in a variety of organisms [69–71]. The general rule appears to be that FACT activity is needed for establishing and maintaining the repressive chromatin barrier, so defects in FACT can lead to derepression of transcription. At the same time, FACT is needed to overcome this barrier, so defects in FACT can lead to a failure to activate transcription. As noted above, this paradox can be resolved by the reversibility of the core activity of FACT (Figure 2).
FACT is linked to assembly of new nucleosomes during replication
Chromatin must be doubled along with the DNA during replication, requiring rapid assembly of a large number of nucleosomes. Several histone chaperones have been implicated in this process, including Asf1, CAF-1, and Rtt106 [28, 72]. Deleting the genes encoding these factors in yeast causes phenotypes reflecting inefficient deposition of chromatin, but the cells remain viable. These factors therefore contribute to chromatin formation, but other factors must also be capable of performing this function, albeit less efficiently, in their absence. FACT is a likely candidate for a central role in nascent nucleosome formation because it is physically associated with the DNA replication machinery, it is essential for viability, it can deposit core histones onto DNA [27], and it appears to be able to convert loosely associated complexes of histones and DNA into canonical nucleosomes (Figure 2) [39].
Another line of evidence supporting a role for FACT in nucleosome deposition during replication comes from analysis of synthetic genetic defects between FACT gene mutations and histone gene mutations. A strain with the pob3-Q308K allele was viable when the only source of histone H4 was H4-K8R, K16R, but it was inviable when only H4-K5R, K12R was available [12]. All four of these lysine residues are in the unstructured N-terminal tail of H4 and are acetylated under different conditions, with K8 and K16 being associated primarily with transcriptional activation and K5 and K12 being the sites acetylated in the newly synthesized histones used for deposition during replication. Substituting arginine for lysine preserves the overall basic nature of the N-terminal tails, but prevents acetylation. The synthetic lethality therefore suggests that when Pob3 is defective, cells cannot tolerate another mutation that also disturbs nucleosome deposition. Cells lacking normal FACT also fail to tolerate loss of the histone acetyltransferase Gcn5 [62], which has been linked to chromatin deposition [73]. However, this observation illustrates the standard difficulty in interpreting genetic effects with FACT as Gcn5 is also important for regulating transcription, so it is not clear whether the synthetic defect observed on growth rates reflects a problem in replication, a problem in transcription, or both.
Regulation of FACT
FACT has roles in several distinct processes, and these roles can at least appear to be contradictory. It therefore seems likely that FACT’s activity is regulated to produce the appropriate outcome in specific circumstances. Examples of regulation by localization and post-translational modification of FACT or its histone substrates have been reported, but this remains largely unexplored territory.
FACT is an abundant complex, with about 25,000 heterodimers per haploid yeast cell, or roughly one complex per three nucleosomes [13]. It therefore seems likely that sufficient amounts of FACT would be readily available for any process without recruiting it to specific sites, but the amount of FACT associated with some promoters and replication origins has been observed to increase during activation [11, 39, 41, 74]. Swi6 binds directly to FACT, providing a mechanism for recruiting FACT to yeast promoters recognized by the DNA binding proteins Swi4 and Mbp1 that form complexes with Swi6 [11]. FACT is less abundant relative to nucleosomes in higher organisms, making it likely that targeting mechanisms become more important. The HP1c protein in Drosophila melanogaster appears to be involved in FACT recruitment, as it interacts with FACT and promotes its localization to sites of RNA Pol II transcription [75]. It is not known whether these interactions simply increase the local concentration of FACT to allow it to perform its general activity in a specific location, or if instead these interactions regulate FACT so that it performs specific functions only in local contexts.
Genetic interactions show that FACT activity in vivo is strongly influenced both positively and negatively by acetylation, methylation, and ubiquitylation of histones [7, 12, 42, 62, 76–78]. FACT itself is also subject to post-translational modifications, and these can change its functional properties [64, 74, 79]. The Spt16 subunit of FACT in yeast can be ubiquitylated by an E3 ligase based on the cullin Rtt101, and this modification appears to target a population of FACT molecules for a function in DNA replication [74]. Ubiquitylation of Spt16 is associated with enhanced localization of both FACT and the MCM helicase with replication origins, supporting previous results showing that FACT has an important role at replication origins [23], and that FACT and MCM collaborate to bind to origins [80]. Ubiquitylation appears to have a role in origin firing, as some FACT defects and loss of Rtt101 each caused diminished origin function [74]. The mechanistic role of FACT at origins has not been determined, but by analogy with its roles in transcription initiation, it might promote recognition of DNA sequences in chromatin by specific origin-binding proteins by evicting nucleosomes, it could enhance establishment of initiation complexes by direct recruitment of factors, it could ease progression of polymerases after initiation, or it could assist the deposition of nucleosomes after genome duplication. In any case, these results show that FACT activity can be regulated by post-translational modifications of nucleosomes or of FACT itself.
FACT’s role in transcription elongation and termination
FACT appears to have a specific function during termination of transcription, although the nature of this role remains puzzling. FACT and other elongation factors that travel with RNA Pol II dissociate from the complex in at least two distinct locations near the 3′ ends of transcription units, implying an orderly transition of the polymerase from elongation to termination states [81], with FACT being released during the first transition. Mutations in histone H3 can interfere with the transition involving FACT, and therefore cause FACT and RNA Pol II to accumulate inappropriately in this region [82–84]. Specific alleles of SPT16 suppress the defects caused by these H3 mutations, suggesting a direct physical association of FACT with H3 at this step, or perhaps a functional interaction in which the properties of H3 and some specific function of FACT are interdependent. The role of this interaction in transcription termination remains unknown, pointing to an important gap in our understanding of this process.
FACT was first purified as a transcription elongation factor, and its ability to destabilize nucleosomes, to bind to other elongation factors, and to associate with RNA Pol II throughout transcription units are all consistent with this role. However, FACT deficiency did not cause a decrease in the rate of RNA Pol II progression in yeast cells in tests using an inducible promoter and a long transcription unit [42, 85]. Other factors such as TFIIS and PAF1 have been shown to promote progression of RNA Pol II through chromatin [45–47, 86], so these factors might be redundant with FACT, or FACT might not be the rate-limiting elongation factor under the conditions tested in yeast cells. Notably, the effects of FACT depletion are different for different transcription units, and the requirement for FACT appears to correlate with the stability of nucleosome positioning in the transcribed region [87, 88]. Cells lacking normal levels of FACT fail to activate some inducible or cell cycle regulated promoters, but they maintain relatively normal transcription of constitutively active genes [11, 41, 42, 52]. This confusing set of observations can be resolved if FACT functions globally, but is only rate-limiting locally. For example, FACT might be required to overcome particular nucleosomes but not others, so activation of some promoters or elongation through some templates displays a strong requirement for FACT, but expression of other genes is less dependent on it. FACT therefore appears to have a relatively simple core activity, but reducing the level of this activity in a cell has many consequences that can appear contradictory because of the way that the single core activity is applied in different circumstances.
FACT’s role in replication elongation
FACT binds to DNA polymerase α [5], and copurifies with DNA replication elongation complexes [89]. The histone mutations that suppress defects caused by the spt16-11 mutation also provide indirect evidence that FACT has an important role promoting progression of replication complexes [44]. The spt16-11 mutation causes both the Spt− phenotype and sensitivity to the replication toxin hydroxyurea (HU), but the histone mutations only suppressed the HU sensitivity. The Spt− phenotype reveals a defect in transcription initiation and, while more complex, HU sensitivity can indicate a defect in DNA polymerase elongation. One interpretation of the suppression of the HU sensitivity without suppression of the Spt− phenotype is that the histone mutations allow replication complexes to proceed at a normal rate through chromatin because they reduce the need for normal levels of FACT activity to keep nucleosomes in a reorganized form ahead of the replication fork. The delays normally associated with the diminished deoxynucleoside triphosphate pools caused by HU treatment are therefore not exacerbated by additional delays due to inappropriately stable chromatin on the template. The histone mutations therefore ease progression of DNA replication, but do not solve the problem caused by defective FACT’s inability to assemble or reassemble normal chromatin, so the transcription defect revealed by the Spt− phenotype remains. If correct, this interpretation reveals an important role for FACT in promoting progression of replication forks.
Summary and Future Directions
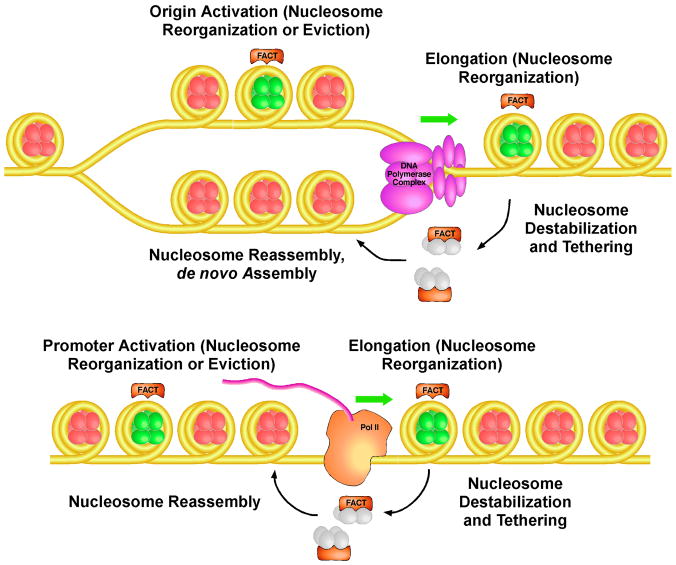
FACT has been implicated in processes that use chromatin as a substrate and therefore have to overcome the repressive barrier posed by nucleosomes. Perhaps surprisingly, it has now also been firmly linked to establishing and maintaining that barrier through a role in nucleosome deposition during DNA replication and by maintaining the integrity of nucleosomes during transcription. The broad range of roles of FACT includes activating promoters and replication origins, promoting progression of RNA and DNA polymerases on chromatin templates, and building or restoring nucleosomes after polymerase passage, as summarized in Figure 3. The mechanism or mechanisms FACT uses to participate in these diverse functions remain somewhat obscure but can be ascribed to a single core activity of reversibly stabilizing an open, reorganized nucleosome conformation.
Figure 3.
FACT’s potential physiological roles
Canonical, repressive nucleosomes are indicated in red, with reorganized nucleosomes shown in green. In DNA replication (upper panel), FACT’s ability to promote nucleosome reorganization appears to contribute to activation of replication origins by providing access to the DNA, by promoting progression of the replication complex through chromatin by interacting with the MCM helicase and by destabilizing nucleosomes, by tethering of histones during replication, and by assisting the assembly/reassembly of nucleosomes after replication. Similar functions are proposed in transcription (lower panel). FACT collaborates at all steps with other histone chaperones, such as CAF-1, Asf1, Rtt106, and possibly Nap1, as well as ATP-dependent remodelers and chromatin modifying factors.
Open questions regarding FACT include whether it induces changes in nucleosome structure or traps nucleosomes in the reorganized configuration, what the complex of FACT with nucleosomes looks like, and whether its ability to stabilize and destabilize nucleosomes are regulated. It will also be of interest to determine whether FACT’s many binding interactions permit it to be recruited to various contexts to perform the same basic activity or if these interactions aler FACT so that it only performs certain actions in certain contexts. Similarly, the discovery that ubiquitylation appears to affect the partitioning of FACT into subpopulations dedicated to different tasks raises the question of how many other post-translational modifications affect FACT activity and for what purposes. FACT’s roles in transcription are becoming clearer, but the contribution it makes to DNA replication, especially its potentially central role in nucleosome deposition, remain largely speculative. We may have a good grasp of most of the facts of life, but much clearly remains to be learned about the life of FACTs!
Highlights.
FACT has a modular domain organization, allowing simultaneous contact with multiple histones or other proteins
FACT either induces or captures a reorganized nucleosome form that is more open than the canonical structure
Nucleosome reorganization promotes initiation and elongation of transcription and replication
FACT can destabilize nucleosomes but it also tethers the components together for reassembly, promoting nucleosome stability
FACT may have a central role in de novo nucleosome assembly during DNA replication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 2.Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises the human SPT16/CDC68 and SSRP1 proteins. Nature. 1999;400:284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- 3.Reinberg D, Sims RJ., 3rd de FACTo nucleosome dynamics. J Biol Chem. 2006;281:23297–23301. doi: 10.1074/jbc.R600007200. [DOI] [PubMed] [Google Scholar]
- 4.Brewster NK, Johnston GC, Singer RA. Characterization of the CP complex, an abundant dimer of Cdc68 and Pob3 proteins that regulates yeast transcriptional activation and chromatin repression. J Biol Chem. 1998;273:21972–21979. doi: 10.1074/jbc.273.34.21972. [DOI] [PubMed] [Google Scholar]
- 5.Wittmeyer J, Formosa T. The Saccharomyces cerevisiae DNA polymerase alpha catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol Cell Biol. 1997;17:4178–4190. doi: 10.1128/mcb.17.7.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster NK, Johnston GC, Singer RA. A bipartite yeast SSRP1 analog comprised of Pob3 and Nhp6 proteins modulates transcription. Mol Cell Biol. 2001;21:3491–3502. doi: 10.1128/MCB.21.10.3491-3502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formosa T, Eriksson P, Wittmeyer J, Ginn J, Yu Y, Stillman DJ. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. Embo J. 2001;20:3506–3517. doi: 10.1093/emboj/20.13.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stillman DJ. Nhp6: a small but powerful effector of chromatin structure in Saccharomyces cerevisiae. Biochim Biophys Acta. 2010;1799:175–180. doi: 10.1016/j.bbagrm.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruone S, Rhoades AR, Formosa T. Multiple Nhp6 Molecules are Required to Recruit Spt16-Pob3 to Form yFACT Complexes and Reorganize Nucleosomes. J Biol Chem. 2003;278:45288–45295. doi: 10.1074/jbc.M307291200. [DOI] [PubMed] [Google Scholar]
- 10.Rhoades AR, Ruone S, Formosa T. Structural features of nucleosomes reorganized by yeast FACT and its HMG box component, Nhp6. Mol Cell Biol. 2004;24:3907–3917. doi: 10.1128/MCB.24.9.3907-3917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahata S, Yu Y, Stillman DJ. The E2F functional analogue SBF recruits the Rpd3(L) HDAC, via Whi5 and Stb1, and the FACT chromatin reorganizer, to yeast G1 cyclin promoters. EMBO J. 2009;28:3378–3389. doi: 10.1038/emboj.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanDemark AP, Blanksma M, Ferris E, Heroux A, Hill CP, Formosa T. The structure of the yFACT Pob3-M domain, its interaction with the DNA replication factor RPA, and a potential role in nucleosome deposition. Molecular cell. 2006;22:363–374. doi: 10.1016/j.molcel.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Formosa T. FACT and the reorganized nucleosome. Molecular BioSystems. 2008;4:1085–1093. doi: 10.1039/b812136b. [DOI] [PubMed] [Google Scholar]
- 14.Lejeune E, Bortfeld M, White SA, Pidoux AL, Ekwall K, Allshire RC, Ladurner AG. The chromatin-remodeling factor FACT contributes to centromeric heterochromatin independently of RNAi. Curr Biol. 2007;17:1219–1224. doi: 10.1016/j.cub.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Lijsebettens M, Grasser KD. The role of the transcript elongation factors FACT and HUB1 in leaf growth and the induction of flowering. Plant Signal Behav. 2010;5:715–717. doi: 10.4161/psb.5.6.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley A, Singer RA, Johnston G. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol Cell Biol. 1991;11:5718–5726. doi: 10.1128/mcb.11.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark-Adams CD, Norris D, Osley MA, Fassler JS, Winston F. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 1988;2:150–159. doi: 10.1101/gad.2.2.150. [DOI] [PubMed] [Google Scholar]
- 18.Malone EA, Clark CD, Chiang A, Winston F. Mutation in SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5710–5717. doi: 10.1128/mcb.11.11.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast JA, Murray LE, Rowley A, Carruthers DR, Singer RA, Johnston GC. Size selection identifies new genes that regulate Saccharomyces cerevisiae cell proliferation. Genetics. 1990;124:81–90. doi: 10.1093/genetics/124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Squazzo SL, Costa PJ, Lindstrom D, Kumer KE, Simic R, Jennings JL, Link AJ, Arndt KM, Hartzog G. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 2002;21:1764–1774. doi: 10.1093/emboj/21.7.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Wang TS. A coordinated temporal interplay of nucleosome reorganization factor, sister chromatin cohesion factor, and DNA polymerase alpha facilitates DNA replication. Mol Cell Biol. 2004;24:9568–9579. doi: 10.1128/MCB.24.21.9568-9579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambus A, van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, Calzada A, Labib K. A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase alpha within the eukaryotic replisome. EMBO J. 2009;28:2992–3004. doi: 10.1038/emboj.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu LR, Seki M, Watanabe N, Murofushi H, Furukohri A, Waga S, Score AJ, Blow JJ, Horikoshi M, Enomoto T, Tada S. Biphasic chromatin binding of histone chaperone FACT during eukaryotic chromatin DNA replication. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller DM, Lu H. p53 serine 392 phosphorylation increases after UV through induction of the assembly of the CK2.hSPT16.SSRP1 complex. J Biol Chem. 2002;277:50206–50213. doi: 10.1074/jbc.M209820200. [DOI] [PubMed] [Google Scholar]
- 25.Hautbergue GM, Hung ML, Walsh MJ, Snijders AP, Chang CT, Jones R, Ponting CP, Dickman MJ, Wilson SA. UIF, a New mRNA export adaptor that works together with REF/ALY, requires FACT for recruitment to mRNA. Curr Biol. 2009;19:1918–1924. doi: 10.1016/j.cub.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler DD, Luger K. The histone chaperone Fact: Structural insights and mechanisms for nucleosome reorganization. J Biol Chem. 2011 doi: 10.1074/jbc.R110.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301:1090–1093. doi: 10.1126/science.1085703. [DOI] [PubMed] [Google Scholar]
- 28.Ransom M, Dennehey BK, Tyler JK. Chaperoning Histones during DNA Replication and Repair. Cell. 2010;140:183–195. doi: 10.1016/j.cell.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corpet A, Almouzni G. Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol. 2009;19:29–41. doi: 10.1016/j.tcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Stuwe T, Hothorn M, Lejeune E, Rybin V, Bortfeld M, Scheffzek K, Ladurner AG. The FACT Spt16 “peptidase” domain is a histone H3–H4 binding module. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8884–8889. doi: 10.1073/pnas.0712293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanDemark AP, Xin H, McCullough L, Rawlins R, Bentley S, Heroux A, Stillman DJ, Hill CP, Formosa T. Structural and Functional Analysis of the Spt16p N-terminal Domain Reveals Overlapping Roles of yFACT Subunits. J Biol Chem. 2008;283:5058–5068. doi: 10.1074/jbc.M708682200. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell AF, Brewster NK, Kurniawan J, Minard LV, Johnston GC, Singer RA. Domain organization of the yeast histone chaperone FACT: the conserved N-terminal domain of FACT subunit Spt16 mediates recovery from replication stress. Nucleic Acids Res. 2004;32:5894–5906. doi: 10.1093/nar/gkh922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Huang H, Zhou BO, Wang SS, Hu Y, Li X, Liu J, Zang J, Niu L, Wu J, Zhou JQ, Teng M, Shi Y. Structural analysis of Rtt106p reveals a DNA binding role required for heterochromatin silencing. J Biol Chem. 2010;285:4251–4262. doi: 10.1074/jbc.M109.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, Zhang Z. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell. 2008;134:244–255. doi: 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan K, Xu X, Cui H, Savchenko A, Edwards A, Joachimiak A. M.C.f.S. Genomics, The crystal structure of a subunit of the heterodimeric. FACT complex (Spt16p-Pob3p) 2008 PDB ID: 3F5R. [Google Scholar]
- 36.Evans DR, Brewster NK, Xu Q, Rowley A, Altheim BA, Johnston GC, Singer RA. The yeast protein complex containing Cdc68 and Pob3 mediates core-promoter repression through the Cdc68 N-terminal domain. Genetics. 1998;150:1393–1405. doi: 10.1093/genetics/150.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlesinger MB, Formosa T. POB3 is required for both transcription and replication in the yeast Saccharomyces cerevisiae. Genetics. 2000;155:1593–1606. doi: 10.1093/genetics/155.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White CL, Suto RK, Luger K. Structure of the yeast nucleosome core particle reveals fundamental changes in internucleosome interactions. Embo J. 2001;20:5207–5218. doi: 10.1093/emboj/20.18.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin H, Takahata S, Blanksma M, McCullough L, Stillman DJ, Formosa T. yFACT induces global accessibility of nucleosomal DNA without H2A–H2B displacement. Molecular cell. 2009;35:365–376. doi: 10.1016/j.molcel.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 41.Takahata S, Yu Y, Stillman DJ. FACT and Asf1 Regulate Nucleosome Dynamics and Coactivator Binding at the HO Promoter. Molecular cell. 2009;34:405–415. doi: 10.1016/j.molcel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswas D, Dutta-Biswas R, Mitra D, Shibata Y, Strahl BD, Formosa T, Stillman DJ. Opposing roles for Set2 and yFACT in regulating TBP binding at promoters. Embo J. 2006;25:4479–4489. doi: 10.1038/sj.emboj.7601333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohm V, Hieb AR, Andrews AJ, Gansen A, Rocker A, Toth K, Luger K, Langowski J. Nucleosome accessibility governed by the dimer/tetramer interface. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullough L, Rawlins R, Olsen AE, Xin H, Stillman DJ, Formosa T. Insight into the Mechanism of Nucleosome Reorganization from Histone Mutants that Suppress Defects in the FACT Histone Chaperone. Genetics. 2011 doi: 10.1534/genetics.111.128769. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondarenko VA, Steele LM, Ujvari A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM. Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II. Molecular cell. 2006;24:469–479. doi: 10.1016/j.molcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Ujvari A, Hsieh FK, Luse SW, Studitsky VM, Luse DS. Histone N-terminal tails interfere with nucleosome traversal by RNA polymerase II. J Biol Chem. 2008;283:32236–32243. doi: 10.1074/jbc.M806636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh FK, Fisher M, Ujvari A, Studitsky VM, Luse DS. Histone Sin mutations promote nucleosome traversal and histone displacement by RNA polymerase II. EMBO Rep. 2010;11:705–710. doi: 10.1038/embor.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adkins MW, Howar SR, Tyler JK. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Molecular cell. 2004;14:657–666. doi: 10.1016/j.molcel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Ransom M, Williams SK, Dechassa ML, Das C, Linger J, Adkins M, Liu C, Bartholomew B, Tyler JK. FACT and the proteasome promote promoter chromatin disassembly and transcriptional initiation. J Biol Chem. 2009;284:23461–23471. doi: 10.1074/jbc.M109.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulaeva OI, Gaykalova DA, Studitsky VM. Transcription through chromatin by RNA polymerase II: Histone displacement and exchange. Mutat Res. 2007;618:116–129. doi: 10.1016/j.mrfmmm.2006.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SK, Tyler JK. Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev. 2007;17:88–93. doi: 10.1016/j.gde.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Jamai A, Puglisi A, Strubin M. Histone chaperone spt16 promotes redeposition of the original h3–h4 histones evicted by elongating RNA polymerase. Molecular cell. 2009;35:377–383. doi: 10.1016/j.molcel.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science. 2003;301:1096–1099. doi: 10.1126/science.1087374. [DOI] [PubMed] [Google Scholar]
- 54.Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23:8323–8333. doi: 10.1128/MCB.23.22.8323-8333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia WJ, Anderson S, Yates J, Washburn MP, Workman JL. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 56.Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, Winston F. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol. 2008;6:e277. doi: 10.1371/journal.pbio.0060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hainer SJ, Pruneski JA, Mitchell RD, Monteverde RM, Martens JA. Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev. 2011;25:29–40. doi: 10.1101/gad.1975011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrews AJ, Chen X, Zevin A, Stargell LA, Luger K. The histone chaperone Nap1 promotes nucleosome assembly by eliminating non-nucleosomal histone DNA interactions. Molecular cell. 2010;37:834–842. doi: 10.1016/j.molcel.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holzen TM, Sclafani R. Genetic interaction of RAD53 protein kinase with histones is important for DNA replication. Cell Cycle. 2010;9:117–129. doi: 10.4161/cc.9.23.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunjan A, Paik J, Verreault A. The emergence of regulated histone proteolysis. Curr Opin Genet Dev. 2006;16:112–118. doi: 10.1016/j.gde.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Morillo-Huesca M, Maya D, Munoz-Centeno MC, Singh RK, Oreal V, Reddy GU, Liang D, Geli V, Gunjan A, Chavez S. FACT prevents the accumulation of free histones evicted from transcribed chromatin and a subsequent cell cycle delay in G1. PLoS Genet. 2010;6:e1000964. doi: 10.1371/journal.pgen.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Formosa T, Ruone S, Adams MD, Olsen AE, Eriksson P, Yu Y, Rhoades AR, Kaufman PD, Stillman DJ. Defects in SPT16 or POB3 (yFACT) in Saccharomyces cerevisiae Cause Dependence on the Hir/Hpc Pathway. Polymerase passage may degrade chromatin structure. Genetics. 2002;162:1557–1571. doi: 10.1093/genetics/162.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray-Gallet D, Quivy JP, Scamps C, Martini EM, Lipinski M, Almouzni G. HIRA Is Critical for a Nucleosome Assembly Pathway Independent of DNA Synthesis. Molecular cell. 2002;9:1091–1100. doi: 10.1016/s1097-2765(02)00526-9. [DOI] [PubMed] [Google Scholar]
- 64.Heo K, Kim H, Choi SH, Choi J, Kim K, Gu J, Lieber MR, Yang AS, An W. FACT-mediated exchange of histone variant H2AX regulated by phosphorylation of H2AX and ADP-ribosylation of Spt16. Molecular cell. 2008;30:86–97. doi: 10.1016/j.molcel.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Du YC, Gu S, Zhou J, Wang T, Cai H, Macinnes MA, Bradbury EM, Chen X. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol Cell Proteomics. 2006;5:1033–1044. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Santisteban MS, Hang M, Smith MM. Histone Variant H2A.Z and RNA Polymerase II Transcription Elongation. Mol Cell Biol. 2011;31:1848–1860. doi: 10.1128/MCB.01346-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luk E, Vu ND, Patteson K, Mizuguchi G, Wu WH, Ranjan A, Backus J, Sen S, Lewis M, Bai Y, Wu C. Chz1, a nuclear chaperone for histone H2AZ. Molecular cell. 2007;25:357–368. doi: 10.1016/j.molcel.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Mahapatra S, Dewari PS, Bhardwaj A, Bhargava P. Yeast H2A.Z, FACT complex and RSC regulate transcription of tRNA gene through differential dynamics of flanking nucleosomes. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkq1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birch JL, Tan BC, Panov KI, Panova TB, Andersen JS, Owen-Hughes TA, Russell J, Lee SC, Zomerdijk JC. FACT facilitates chromatin transcription by RNA polymerases I and III. EMBO J. 2009;28:854–865. doi: 10.1038/emboj.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denninger V, Fullbrook A, Bessat M, Ersfeld K, Rudenko G. The FACT subunit TbSpt16 is involved in cell cycle specific control of VSG expression sites in Trypanosoma brucei. Mol Microbiol. 2010;78:459–474. doi: 10.1111/j.1365-2958.2010.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallastegui E, Millan-Zambrano G, Terme JM, Chavez S, Jordan A. Chromatin reassembly factors are involved in transcriptional interference promoting HIV latency. J Virol. 2011;85:3187–3202. doi: 10.1128/JVI.01920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 73.Burgess RJ, Zhou H, Han J, Zhang Z. A role for Gcn5 in replication-coupled nucleosome assembly. Molecular cell. 2010;37:469–480. doi: 10.1016/j.molcel.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J, Li Q, McCullough L, Kettelkamp C, Formosa T, Zhang Z. Ubiquitylation of FACT by the cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev. 2010;24:1485–1490. doi: 10.1101/gad.1887310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon SH, Florens L, Swanson SK, Washburn MP, Abmayr SM, Workman JL. Heterochromatin protein 1 (HP1) connects the FACT histone chaperone complex to the phosphorylated CTD of RNA polymerase II. Genes Dev. 2010;24:2133–2145. doi: 10.1101/gad.1959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biswas D, Yu Y, Prall M, Formosa T, Stillman DJ. The yeast FACT complex has a role in transcriptional initiation. Mol Cell Biol. 2005;25:5812–5822. doi: 10.1128/MCB.25.14.5812-5822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Molecular cell. 2008;31:57–66. doi: 10.1016/j.molcel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 78.Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D. Histone H2B Monoubiquitination Functions Cooperatively with FACT to Regulate Elongation by RNA Polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 79.Tsunaka Y, Toga J, Yamaguchi H, Tate SI, Hirose S, Morikawa K. Phosphorylated intrinsically disordered region of FACT masks its nucleosomal DNA binding elements. J Biol Chem. 2009;284:24610–24621. doi: 10.1074/jbc.M109.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan BC, Chien CT, Hirose S, Lee SC. Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication. Embo J. 2006;25:3975–3985. doi: 10.1038/sj.emboj.7601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayer A, Lidschreiber M, Siebert M, Leike K, Soding J, Cramer P. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol. 2010;17:1272–1278. doi: 10.1038/nsmb.1903. [DOI] [PubMed] [Google Scholar]
- 82.Duina AA, Rufiange A, Bracey J, Hall J, Nourani A, Winston F. Evidence that the localization of the elongation factor Spt16 across transcribed genes is dependent upon histone H3 integrity in Saccharomyces cerevisiae. Genetics. 2007;177:101–112. doi: 10.1534/genetics.106.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lloyd A, Pratt K, Siebrasse E, Moran MD, Duina AA. Uncoupling of the patterns of chromatin association of different transcription elongation factors by a histone H3 mutant in Saccharomyces cerevisiae. Eukaryot Cell. 2009;8:257–260. doi: 10.1128/EC.00348-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myers CN, Berner GB, Holthoff JH, Martinez-Fonts K, Harper JA, Alford S, Taylor MN, Duina AA. Mutant Versions of the S. cerevisiae Transcription Elongation Factor Spt16 Define Regions of Spt16 That Functionally Interact with Histone H3. PLoS One. 2011;6:e20847. doi: 10.1371/journal.pone.0020847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwabish MA, Struhl K. Evidence for Eviction and Rapid Deposition of Histones upon Transcriptional Elongation by RNA Polymerase II. Mol Cell Biol. 2004;24:10111–10117. doi: 10.1128/MCB.24.23.10111-10117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J, Guermah M, Roeder RG. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell. 2010;140:491–503. doi: 10.1016/j.cell.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jimeno-Gonzalez S, Gomez-Herreros F, Alepuz PM, Chavez S. A gene-specific requirement for FACT during transcription is related to the chromatin organization of the transcribed region. Mol Cell Biol. 2006;26:8710–8721. doi: 10.1128/MCB.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pelechano V, Jimeno-Gonzalez S, Rodriguez-Gil A, Garcia-Martinez J, Perez-Ortin JE, Chavez S. Regulon-specific control of transcription elongation across the yeast genome. PLoS Genet. 2009;5:e1000614. doi: 10.1371/journal.pgen.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gambus A, van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, Calzada A, Labib K. A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase alpha within the eukaryotic replisome. EMBO J. 2009;28 doi: 10.1038/emboj.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masse JE, Wong B, Yen YM, Allain FH, Johnson RC, Feigon J. The S. cerevisiae architectural HMGB protein NHP6A complexed with DNA: DNA and protein conformational changes upon binding. J Mol Biol. 2002;323:263–284. doi: 10.1016/s0022-2836(02)00938-5. [DOI] [PubMed] [Google Scholar]