Abstract
Background
Allergic diseases affect large population. Pollen, a ubiquitous allergen, is the trigger of seasonal rhinitis, conjunctivitis and asthma, as well as an exacerbating factor of atopic dermatitis. However, the underlying mechanism by which pollen induces thymic stromal lymphopoietin (TSLP) triggered allergic inflammation via epithelial innate immunity is largely unknown.
Objective
To explore whether short ragweed (SRW) pollen induces TSLP/OX40L/OX40 signaling via Toll-like receptor (TLR) 4-dependent pathways in allergic disease.
Methods
Three models were used for this study, a well-characterized murine model of allergic conjunctivitis induced by SRW pollen, a topical challenge model on mouse ocular surface, and a culture model of primary human corneal epithelium exposed to aqueous extract of defatted SRW pollen (SRWe).
Results
The topical challenges with SRW pollen generated a typical allergic conjunctivitis in BALB/c mice. Clinical signs, stimulated TSLP/OX40L/OX40 signaling and Th2 cytokines in ocular mucosa and draining cervical lymph nodes were significantly reduced or eliminated in TLR4 deficient (Tlr4-d) or MyD88 knockout (MyD88−/−) mice, compared with their wild type littermates. SRWe stimulated TSLP production by ocular epithelia in wild type, but not Tlr4-d or MyD88−/− mice. SRWe stimulated TSLP was blocked by TLR4 antibody and NF-kB inhibitor in mouse and human corneal epithelia.
Conclusion
We for the first time uncovered a novel phenomenon that SRW pollen, acting as a functional TLR4 agonist, initiates TLR4-dependent TSLP/OX40L/OX40 signaling that triggers Th2-dominant allergic inflammation. These findings shed light on the understanding of mucosal epithelial innate immunity, and create new therapeutic targets to cure allergic diseases.
Keywords: Allergy, pollen, conjunctivitis, Toll-like receptor, thymic stromal lymphopoietin, innate immunity
INTRODUCTION
Allergic diseases like seasonal allergy, asthma, atopic dermatitis, affect up to 20–30% of the population in industrialized countries, and up to 50% of these individuals reporting ocular allergic manifestations.1–3 The incidence of allergies has increased steadily over the past 30 years. Th2-dominant hypersensitivity is a major contributor to allergic inflammatory diseases, but the underlining mechanism for initiation of this adaptive immune disorder by mucosal epithelia remains a relative mystery. The molecular triggers for Th2 allergic inflammation were not clear until studies identified a novel epithelium-derived pro-allergic cytokine, thymic stromal lymphopoietin (TSLP), which activates myeloid dendritic cells (DCs) to produce OX40 ligand (OX40L) that triggers a Th2 inflammatory response. Compelling evidence demonstrates that TSLP represents one of the master switches initiating allergic inflammation at the interface between epithelial cells and dendritic cells, and has a determinant role in atopic dermatitis and asthma.4–6 The role of TSLP in ocular allergic inflammation was also reported, including ours.7,8 However, it is not clear whether TSLP is induced by environmental allergens, such as pollen, in allergic diseases.
Pollen, a ubiquitous allergen, is the trigger of seasonal rhinitis, conjunctivitis and asthma, as well as an exacerbating factor of atopic dermatitis. However, the underlying molecular mechanism by which pollen induces Th2-dominant allergic inflammation via epithelial innate immunity pathways is largely unknown. Substantial evidence now indicates that epithelial cells are central participants in innate and adaptive immune responses.9–11 The innate immune response relies on evolutionarily ancient germline-encoded receptors, the pattern-recognition receptors (PRRs),12 which recognize highly conserved microbial component. A breakthrough in understanding the ability of innate immune system to rapidly recognize pathogens is the discovery of Toll-like receptors (TLRs), the most important family among the PRRs. A variety of TLRs has been identified in skin and mucosal epithelia, including ocular surface epithelium (see review articles13,14) while the role of ligand-stimulated epithelial TLR signaling in regulating innate and adaptive immunity remains to be elucidated.
Based on the observation that we and other groups have made15–17 that TSLP is mainly induced via TLR mediated innate response in epithelia exposed to microbial products, we hypothesized that pollen, such as Ambrosia short ragweed (SRW), may serve as a functional TLR4 agonist that induces production of TSLP, an epithelium-derived proallergic cytokine, via innate immunity signaling to trigger Th2-dominant allergic inflammation. The ocular surface is the most common site for developing allergic inflammation, because allergens can easily deposit directly on to the exposed mucosal surface of the eye. To uncover the novel molecular signaling pathways involved in pollen induced allergic inflammation, the present study was conducted using a well-characterized murine model of allergic conjunctivitis induced by SRW pollen in BALB/c, Tlr4 deficient and MyD88 knockout mice, as well as a murine topical ocular surface challenge model and a culture model of primary human corneal epithelial cells exposed to an aqueous extract of defatted SRW pollen.
METHODS
Reagents
Ambrosia artemisiifolia short ragweed (SRW) pollen and the lyophilized aqueous extract of defatted short ragweed pollen were purchased from Greer Lab (Lenoir, NC). The amount of endotoxin in these SRW preparations were 0.00038±0.00026 EU/μg protein, as measured by ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ). Imject Alum from Pierce Biotechnology (Rockford, IL). lipopolysaccharide (LPS) from Escherichia coli were from Sigma-Aldrich (St. Louis, MO).
Animals
The animal research protocol was approved by the Center for Comparative Medicine at Baylor College of Medicine. All animals used in this study were maintained in specific pathogen-free conditions in microisolator cages and were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. BALB/c and BALB/c based homozygote Tlr4 deficient (C.C3-Tlr4Lps-d/J) mice aged at 6–8 weeks of both genders were purchased from the Jackson Laboratory (Bar Harbor, ME). The age- and gender-matched mice were used for each experiment. The heterozygous MyD88 (myeloid differentiation primary response gene 88) knockout mice in C57BL/6 background were kindly provided by Dr. Shizuo Akira (Research Institute for Microbial Disease, Osaka University, Japan) through Dr. Eric Pearlman (Department of Ophthalmology and Visual Sciences, Case Western Reserve University, Cleveland, Ohio).18 The genotyping was performed by polymerase chain reaction (PCR) of the tail DNA with three specific primers (MyD88F: TGGCATGCCTCCATCATAGTTAACC; MyD88R: GTCAGAAACAACCACCACCATGC; MyD88R-Neo: ATCGCCTTCTATCGCCTTCTTGACG using a previous described method.19 The age- and gender-matched MyD88+/+ littermates of the MyD88−/− mice grown to 8–10 week old were used for experiments.
Murine model of experimental allergic conjunctivitis (EAC) induced by SRW pollen
The mouse EAC model was induced using previously reported methods20,21 with modification.8 In brief, mice were immunized with 50μg of SRW pollen (Greer Lab, Lenoir, NC) in 5 mg Imject Alum (Pierce Biotechnology, Rockford, IL) by footpad injection on day 0. Allergic conjunctivitis was induced by given topical applications of 1.5 mg SRW pollen suspended in 10 μl phosphate buffered saline (PBS), pH 7.2, into each eye once a day from days 10 to 12. PBS eyedrop treated SRW-sensitized and untreated mice were used as controls. Animals were examined clinically for signs of immediate hypersensitivity responses 20 minutes after each topical challenge with SRW pollen. A clinical scoring scheme described by Magone and colleagues20 was used to evaluate chemosis, conjunctival redness, lid edema, and tearing. On day 13, 24 hours after the last SRW challenge, the corneal epithelium, conjunctiva, cervical lymph nodes and whole eyes were harvested for gene expression assays and histopathological studies. Four mice per group were used in each experiment, and the same experiments were repeated 4 times.
Murine model for TSLP induction by ocular surface topical challenge with aqueous extract of defatted SRW pollen (SRWe)
Mice were topically instilled with SRWe (Greer Lab) at 150μg/5μl/eye or 5μl PBS as controls for 4–24 hours. SRWe was applied without or with pre-instilled rat anti-mouse TLR4 antibody (1μg/5μl/eye, eBioscience) or its isotype rat IgG2a. In Tlr4-d, MyD88+/+ and MyD88−/− mice, lipopolysaccharide (LPS) from Escherichia coli (Sigma-Aldrich, St. Louis, MO) at 5μg/5μl/eye was used as a positive control to SRWe treatment. Tissue specimens including corneal epithelium and conjunctival tissues were collected at different time points and stored at −80°C before used for TSLP mRNA and protein assays. Four mice/group was used in each experiment, which was repeated 4 times.
In vitro culture models for TSLP induction by primary human corneal epithelial cells exposed to SRWe
Fresh human corneoscleral tissues (<72 hours after death) from donors aged 19–67 years without allergic conditionswere obtained from the Lions Eye Bank of Texas (Houston, TX). Human corneal epithelial cells (HCECs) were cultured in 12-well plates using explants from corneal limbal rims in a supplemented hormonal epidermal medium (SHEM) containing 5%FBS using our previous methods.22,23 Confluent corneal epithelial cultures were switched to serum-free SHEM and treated for different time periods (4–48 hours) with different concentration of SRWe (0.1–50 μg/ml). SRWe at 10μg/ml was applied without or with pre-incubated mouse anti-human TLR4 antibody (10μg/ml, eBioscience), its isotype mouse IgG2a k, or quinazoline, an NF-kB Activation Inhibitor (10μM, EMD Biosciences). Each experiment was repeated four times.
Total RNA extraction, reverse transcription (RT) and quantitative real-time PCR (qPCR)
Total RNA was isolated from mouse tissue specimens and human cells using a Qiagen RNeasyR Micro kit according to manufacturer’s protocol, and quantified by NanoDrop® ND-1000 Spectrophotometer and stored at −80°C. The first strand cDNA was synthesized by RT from 1μg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described.24,25 Real-time PCR was performed with specific primers and probes using TaqMan® gene expression assays and TaqMan® master mix (Applied Biosystems, Foster City, CA) in Mx3005P QPCR System (Stratagene). TaqMan® Gene Expression Assay IDs for murine GAPDH, TSLP, TSLPR, OX40L, CD11c, OX40, CD4, IL-4, IL-5, IL-13, and IFNγ were Mm99999915, Mm00498739, Mm00497362, Mm00442039, Mm00498698, Mm00445259, Mm00437214, Mm00442754, Mm0099999063, Mm00434204, and Mm00801778_m1, respectively. The results were analyzed by the comparative threshold cycle (Ct) method26 and normalized by GAPDH as previously reported.25,27
Enzyme-linked immunosorbent assay (ELISA)
ELISA MAX™ Deluxe kits for human and mouse TSLP were from BioLegend (San Diego, CA). The TSLP protein levels in the cell lysates from mouse ocular specimens, and in the supernatants from HCECs were measured according to the manufacturer’s protocols, similar to our previous report.15
Immunohistochemical and immunofluorescent staining
The eyes and lids of mice in each group were excised, embedded in optimal cutting temperature (OCT) compound (VWR, Suwanee, GA), and flash-frozen in liquid nitrogen. Sagittal 8μm cryosections from mouse globes were cut with a cryostat (HM 500; Micron, Waldorf, Germany), and stored at −80°C before use. Immunohistochemical and immunofluorescent staining was performed as previously described.28,29 The primary antibodies used for this study included: rat anti-mouse CD4, hamster anti-mouse CD11c and rat anti-mouse IL-4 from BD Pharmingen (San Jose, CA); rat anti-mouse OX40L and rat anti-mouse/human IL-5 from Biolegend (San Diego, CA); rabbit anti-human TSLP from ProSci Incorporated (Poway, CA); goat anti-mouse TSLPR and OX40 from Santa Cruz Biotechnology (Santa Cruz, CA); and rabbit anti-human IL-13 from ABD Seratec (Raleigh, NC).
Statistical analysis
Student’s t-test was used to compare differences between two groups. One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post-hoc test. P values <0.05 were considered statistically significant.
RESULTS
TSLP/OX40L/OX40 signaling in a Th2-dominant EAC murine model induced by SRW pollen
To explore the role of TSLP and its signaling molecules in pollen-triggered allergic inflammation, EAC model was induced in BALB/c mice sensitized and topically challenged by SRW pollen (SRW mice), with PBS-treated (PBS mice) and untreated mice as control groups. Repeated topical challenges with SRW allergen generated typical signs mimic to human allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, and frequent scratching of the eye lids. These signs and scratching behavior were detected within 20 minutes and persisted for at least 8 hours after SRW challenge. Some mice developed signs that persisted for 24 hours.
Evaluated by RT-qPCR, TSLP mRNA expression was significantly upregulated in the corneal (1.95±0.22 fold) and conjunctival (2.53±0.58 fold) epithelia (all P<0.05) from SRW mice compared with the PBS alone and untreated control groups (Fig 1A, 1B). Immunohistochemical staining confirmed the increase of TSLP production in the SRW-challenged eyes. TSLP immunoreactivity was absent or weak in the ocular surfaces of PBS mice and untreated groups. The corneal and conjunctival epithelia in SRW mice displayed much stronger TSLP staining throughout entire epithelium, especially in the superficial epithelial layers of the conjunctiva (Fig 3A). These data indicate that TSLP mRNA expression and protein production by ocular surface epithelia increased in this SRW-induced EAC model.
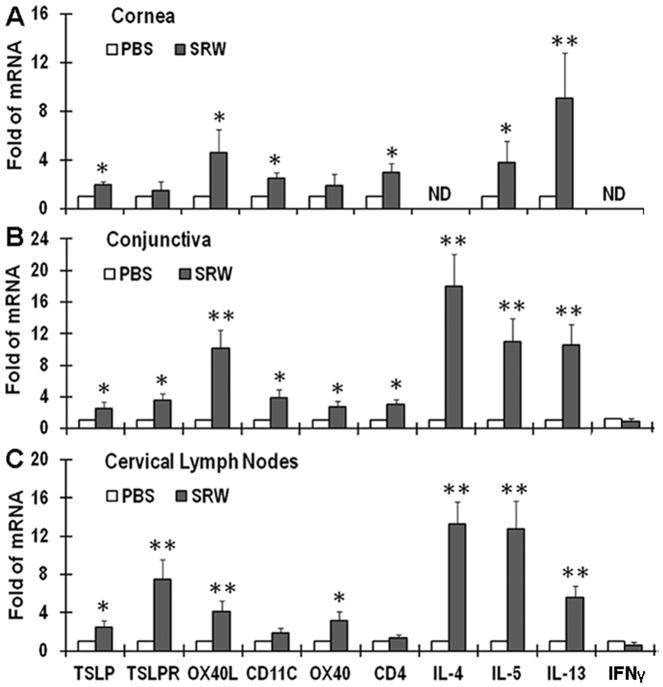
Fig 1.
The stimulated expression of TSLP signaling molecules and Th2 cytokines in SRW pollen-induced EAC in BALB/c mice. The mRNA expression of TSLP, its downstream signals in dendritic (TSLPR, OX40L, & CD11c) and T cells (OX40 & CD4), as well as Th1 (IFNγ) and Th2 cytokines (IL-4, IL-5 and IL-13) by corneal epithelium (A), conjunctiva (B) and cervical lymph nodes (CLN) (C) were evaluated by RT-qPCR in EAC BALB/c mice, with PBS-treated mice as controls. Results shown are the Mean ± SD of four independent experiments. *P<0.05, **P<0.01; n=4. ND: not detectable.
Fig 3.
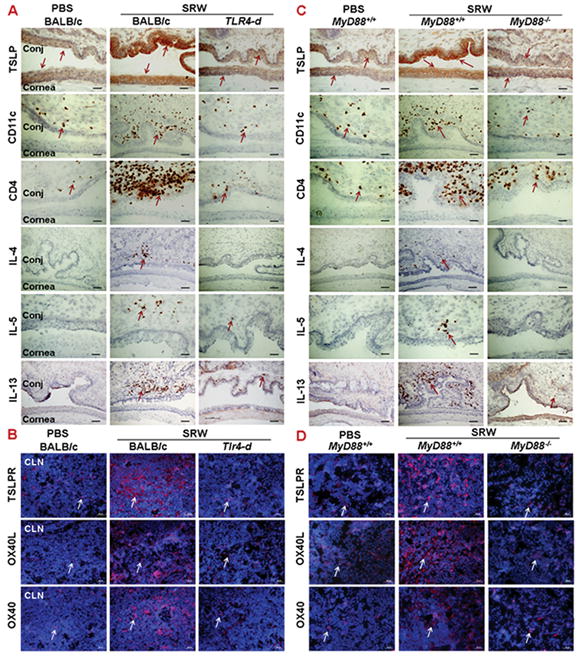
The stimulated production of TSLP signaling proteins and Th2-dominant inflammation in SRW-induced EAC model requires TLR4 and MyD88. Top panel showed immunohistochemical staining of TSLP signaling molecules and Th2 cytokines on cornea and conjunctiva (Conj) of wild type and Tlr4 deficient BALB/c mice (A), and C57BL/6 based wild type MyD88+/+ and MyD88−/− mice (C), challenged by SRW pollen, with PBS-treated mice as controls. B, D: immunofluorescent staining of TSLP activated signals, TSLPR, OX40L and OX40 in CLN of different strains as described above. Bar: 20μm; Arrows: red or red brown positive staining signals.
The accumulation of CD11c positive (CD11c+) dendritic cells (DCs) on the ocular surface was detected in this EAC model by RT-qPCR and immunostaining. As shown in Fig 1A & 1B, the increased mRNA levels of CD11c, TSLP receptor (TSLPR) and OX40 ligand (OX40L) were observed in the ocular surface, especially in conjunctival tissues, where their transcripts increased to 4.16, 3.67, or 10.19 fold, respectively (P< 0.05, 0.05 or <0.01), from SRW-challenged mice, compared with PBS mice. The large amount of CD11c+ DCs was accumulated in the SRW-challenged ocular surface, primarily in the stroma subjacent to conjunctival epithelia (Fig 3A). These results suggest that in SRW-induced EAC, ocular surface was infiltrated with TSLP activated DCs that express TSLPR and produce OX40L.
A Th2-dominant inflammatory response was clearly observed on ocular surface in SRW-challenged mice. T lymphocyte infiltration was evidenced by the markedly increased CD4 mRNA level (Fig 1, A–B) and immunopositive (CD4+) cells in the ocular surface, especially in the conjunctiva from SRW mice (Fig. 3A), compared with PBS mice. These CD4+ T cells appear to be in Th2 lineage because the transcripts of three key Th2 cytokines, IL-4, IL-5 and IL-13, were expressed at significantly higher levels, while Th1 cytokine IFNγ mRNA was at lower levels in corneal (P<0.05 or <0.01) and conjunctival (all P<0.01) tissues of SRW mice than the PBS controls (Fig 1A, 1B). Immunostaining data confirmed that the conjunctival stroma was largely infiltrated by IL-4, IL-5 and IL-13-producing Th2 cells (Fig. 3A).
To confirm the presence of TSLP signaling in SRW-induced EAC mice, the ocular surface draining cervical lymph nodes (CLN) were collected for evaluation. Compared with PBS controls, mRNA levels of TSLPR and OX40L were significantly stimulated to 7.47 and 4.07 fold respectively (both P<0.01), while CD11c expression slightly increased in CLN from SRW mice, indicating CD11c+ DCs were markedly activated by ocular epithelia-derived TSLP. Interestingly, the mRNA levels of OX40 (3.39 fold, P<0.05) and Th2 cytokines, IL-4, IL-5 and IL-13 (13.24, 12.78 and 5.54 fold, respectively, all P<0.01), significantly increased while IFNγ mRNA was lower and CD4 expression did not change in the CLN from SRW mice, indicating naive CD4+ T cells were largely differentiated to Th2 cells primed by OX40L produced by TSLP-activated DCs.4 The increased number of TSLPR+, OX40L+ or OX40+ cells in the draining CLN of SRW mice was confirmed by dramatically increased cell membrane and cytoplasmic immunoreactivity of these 3 signaling proteins in CLNs (Fig. 3B). All these results suggest a critical role of TSLP/OX40L/OX40 signaling in Th2-dominant EAC induced by SRW pollen.
SRW pollen induced TSLP-triggered allergic inflammation via a TLR4-dependent innate immune response
TLR-mediated TSLP induction has been recognized.16,17,30 We have demonstrated that TSLP was largely induced by specific TLR ligands in human corneal epithelial cells.15 MyD88 is a universal adapter protein necessary for response to all TLRs except TLR3.31,32 Tlr4-deficient (Tlr4-d, C.C3-Tlr4Lps-d/J) and MyD88 knockout (MyD88−/−) mice have been used to identify TLR4 mediated signaling.33,34 To explore whether SRW pollen stimulates TSLP through TLR4-dependent innate response, we sensitized and topically challenged the Tlr4-d and MyD88−/− mice with SRW pollen.
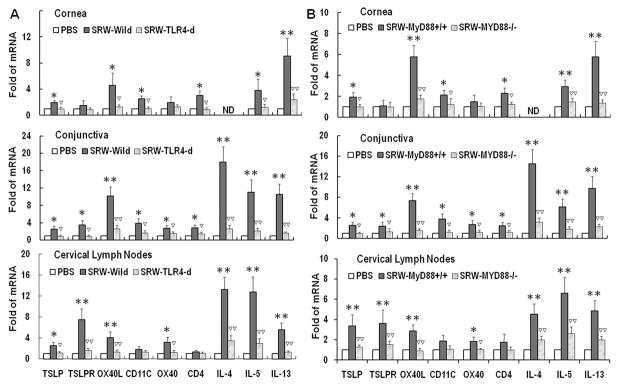
Compared with wild-type BALB/c mice, the ocular allergic signs, stimulated TSLP/OX40L/OX40 signaling and Th2-dominant inflammatory response by ocular mucosa, especially conjunctival tissues, were dramatically reduced or eliminated in BALB/c based Tlr4-d mice. As shown in Fig 2A, the mRNA levels of TSLP, OX40L, OX40 and Th2 cytokines (IL-4, IL-5 and IL-13) were significantly stimulated in cornea, conjunctiva and draining CLN from wild-type BALB/c, but not in those from Tlr4-d mice. The immunostaining results confirmed that SRW pollen did not stimulate TSLP and its downstream molecules or a Th2 response in ocular mucosal tissues (Fig. 3A) and draining CLN (Fig. 3B) of Tlr4-d mice. These findings suggest that TLR4-dependent TSLP signaling was involved in the SRW pollen induced allergic inflammation.
Fig 2.
The stimulated expression of TSLP signaling molecules and Th2 cytokines in SRW-induced EAC model requires TLR4 and MyD88. The mRNA expression of TSLP signaling molecules and Th2 cytokines by corneal epithelium, conjunctiva and CLN in EAC model with wild type and Tlr4 deficient BALB/c mice (A), and with C57BL/6 based MyD88+/+ and MyD88−/− mice (B), sensitized and topically challenged by SRW pollen, with PBS-treated mice as controls. Results shown are the Mean ± SD. *P<0.05, **P<0.01; n=4, compared with PBS controls; ▽P<0.05, ▽▽P<0.01, n=4, compared with wild type mice.
The SRW topical challenges triggered the typical allergic signs and scratching behavior in wild type MyD88+/+ mice, although less severe than BALB/c mice. The expression of TSLP and its signaling molecules, TSLPR, OX40L and OX40, as well as Th2 cytokines IL-4, IL-5 and IL-13 was significantly stimulated in the cornea, conjunctiva and CLN from SRW challenged wild type MyD88+/+ mice at both mRNA (Fig. 2B) and protein levels (Fig 3C, 3D). Clinical allergic signs and stimulated production of TSLP signaling molecules and Th2 cytokines were dramatically reduced or eliminated in SRW challenged MyD88−/− mice as evaluated by RT-qPCR (Fig 2B) and immunostaining (Fig 3C, 3D). These findings suggest that MyD88 pathway is involved in the TLR4-dependent TSLP signaling induced by SRW pollen.
SRWe induced TSLP expression and production by murine ocular surface epithelia through TLR4-dependent innate pathway
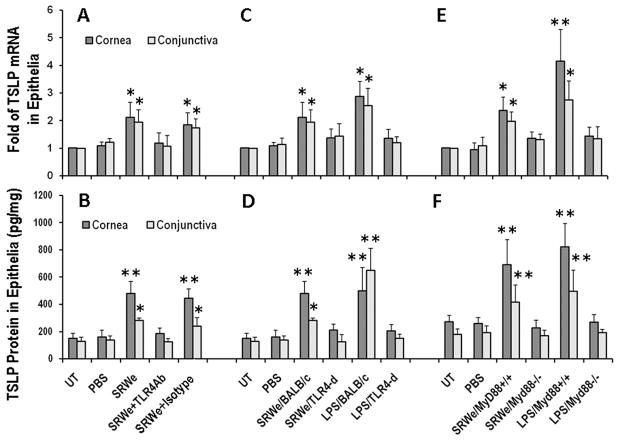
To confirm that SRW pollen directly stimulates TSLP production by ocular mucosal epithelia through TLR4-dependent innate immunity pathway, we created a topical challenge murine model using SRWe at 150μg/5μl/eye for 4–24 hours. TSLP mRNA was induced as early as 4 hours and reached peak levels at 8 hours, and TSLP protein levels increased in 24 hours in ocular epithelia exposed to SRWe. As shown in Fig 4AB, SRWe significantly stimulated TSLP mRNA by 2 fold in corneal and conjunctival epithelia (Both P<0.05), and its protein levels by 3.2 fold (from 150.2±37.6 pg/mg cellular protein to 480.00±89.6 pg/mg) in cornea epithelia and 2.2 fold (from 128.6±29.8 pg/mg to 281.6±19.3 pg/mg) in conjunctiva in BALB/c mice when compared with untreated or PBS-treated controls. The SRWe-stimulated TSLP were significantly blocked at both mRNA and protein levels by a rat anti-mouse TLR4 antibody but not by its isotype rat IgG2a.
Fig 4.
SRWe induces TSLP mRNA (A, C & E) and protein (B, D & F) by murine corneal and conjunctival epithelia through TLR4- and MyD88-dependent pathway. A, B. BALB/c mice were topically instilled with SRWe at 150μg/5μl/eye without or with pre-instilled rat anti-mouse TLR4 antibody (1μg/5μl/eye) or its isotype rat IgG2a. C, D. TSLP induction by topically challenged SRWe or LPS (5μg/5μl/eye) in wild type and Tlr4-d BALB/C mice. E, F. TSLP induction by topically challenge in wild type MyD88+/+ and MyD88−/− mice. Untreated or PBS-treated mice used as controls. Results shown are the Mean ± SD (n=4). *P<0.05, **P<0.01; compared with PBS controls.
Interestingly, the SRWe topical challenge did not increase TSLP mRNA (Fig. 4C) and protein levels (Fig. 4D) in corneal and conjunctival epithelia of Tlr4-d mice. Similarly, LPS (5μg/5μl/eye) stimulated TSLP production in corneal and conjunctival epithelia of BALB/c mice, but not in Tlr4-d mice. Furthermore, we applied this topical challenge model to MyD88 knockout mice and their wild type controls. Interestingly, SRWe promoted TSLP production by ocular epithelia at both mRNA (Fig. 4E) and protein levels (Fig. 4F) only in MyD88+/+ mice, but not in MyD88−/− mice, a similar pattern to that observed following LPS topical challenge. Taken together, these data demonstrated that SRWe directly stimulates TSLP production by ocular mucosal epithelia via a TLR4-dependent innate pathway.
SRWe induces TSLP expression and production by human corneal epithelial cells through TLR4 and NF-kB signaling pathways
To explore whether this phenomenon occurs in humans, we investigated TSLP expression in human corneal epithelium. TSLP mRNA was upregulated at 4 hours and its protein was detected at 24 hours in HCECs exposed to SRWe, which is consistent with our previous report.15 TSLP induction at mRNA (Fig. 5A) and protein levels (Fig. 5B) was concentration-dependently stimulated by SRWe in primary HCECs. TSLP protein was barely detectable (4.83±1.60 pg/ml) in the supernatant of normal HCEC cultures. SRWe at 10μg/ml increased the TSLP protein to 48.92±4.23 pg/ml (P<0.01), the levels comparable to that stimulated by 10ng/ml of TNF-α in HCECs.15 Interestingly, the SRWe (10μg/ml)-stimulated TSLP mRNA was significantly blocked by pre-incubation of cells with 10μg/ml of neutralizing monoclonal antibody against human TLR4, but not by its isotype mouse IgG2a k (Fig 5C). Furthermore, SRWe stimulated TSLP expression was also significantly inhibited by quinazoline, a NF-kB Activation Inhibitor (Fig 5C). These findings were confirmed by detection of incresed TSLP protein levels as shown in Fig. 5D. These data demonstrate that SRW induces TSLP production in human corneal epithelial cells through TLR4 and NF-kB innate signaling pathways.
Fig 5.
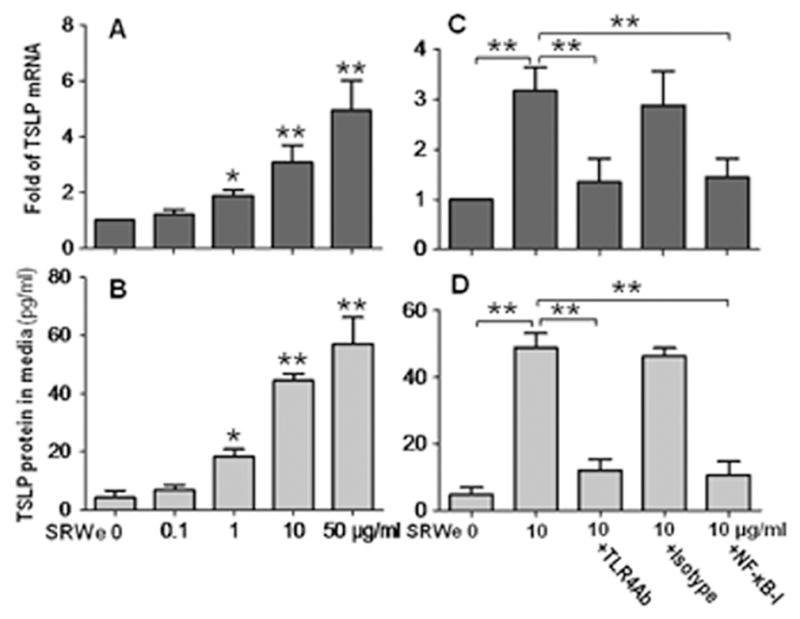
SRWe induces TSLP mRNA and protein by HCECs through TLR4 and NF-κB signaling pathways. A, B. Primary HCECs were treated with 0.1 to 50 μg/ml of SRWe for 4 hours for TSLP mRNA or 48 hours for TSLP protein in the supernatants. C, D. HCECs were pre-incubated with mouse TLR4 antibody (10μg/ml), isotype mouse IgG2a k, or NF-kB Activation Inhibitor quinazoline (NFkB-I, 10μM) for 1 hour before adding 10μg/ml SRWe for 4 hours for TSLP mRNA or 48 hours for TSLP protein in the supernatants. Results shown are the Mean ± SD (n=4). *P<0.05, **P<0.01.
DISCUSSION
Although there have been numerous studies on the development of allergen-induced inflammation, the mechanisms leading to resolution of allergic inflammation remain poorly understood. This represents an important challenge because failure to resolve allergen driven inflammation potentially leads to recurrent or chronic allergic diseases. Pollen is a ubiquitous allergen that affects a large population, and Ambrosia artemisiifolia short ragweed is the most widespread plant in North America. This study uncovered a previously unknown mechanism by which pollen induces TSLP-triggered Th2-dominant inflammation through a TLR4-dependent innate pathway using multiple in vivo and in vitro models.
Traditionally, TLRs recognize conserved microbial components as ligands or agonists. Recent studies have revealed that TLR4 recognizes a wider variety of ligands than previous thought. In addition to its first identified ligand, bacterial LPS, TLR4 was found to recognize certain viral proteins such as the F protein from respiratory syncytial virus and mouse mammary tumor virus. Not limited to pathogen-associated molecular patterns, TLR4 responds to human endogenous structural proteins derived from tissue injury or during inflammation, the damage-associated molecular patterns, such as type III repeat extra domain A of fibronectin, oligosaccharides of hyaluronic acid, human heat-shock protein Hsp60 and Hsp70 (see review35). Interestingly, a few reports have revealed the potential for protein extracts from plants and herbs to activate TLR4, such as taxol, an antitumor agent derived from the Yew plant,36 and aqueous extract of Rhodiola imbricata rhizome, a medicinal plant.37
To identify that SRW pollen serves as a functional TLR4 agonist capable of inducing TSLP triggered Th2-dominant allergic inflammation, firstly we demonstrated the stimulated TSLP/OX40L/OX40 signaling in a well-characterized murine EAC model by SRW pollen.20,21,38 In addition to ocular allergy, SRW pollen and extracts have been widely used for allergic models including asthma, rhinitis, peritonitis and skin allergy, in mouse, guinea-pig, dog and other animal species.1,39,40 However, there is no report showing that SRW pollen triggers TSLP signaling in these allergic models.
Secondly, we demonstrated that TSLP signaling in Th2-dominant inflammation is stimulated by SRW pollen via a TLR4-dependent innate immune response. This novel phenomenon was revealed by strong evidence that all clinical signs of allergic conjunctivitis, TSLP production and its downstream signals, as well as Th2-dominant inflammation were significantly reduced or eliminated in Tlr4-d or MyD88−/− mice when compared with their wild type littermates. Furthermore, we measured the SRW-specific IgE levels in sera from SRW-induced EAC mice in different strains. Interestingly, the results showed that the SRW-specific IgE levels increased significantly in wild type BALB/c mice sensitized by SRW pollen footpad injection, but did not changed in Tlr4-d and MyD88−/− mice with the same treatment (see Online Repository material). These results suggest a possible TLR4-dependent pathway in sensitization stage, but it needs further studies to confirm this finding.
Thirdly, the direct evidence that SRW stimulates TSLP expression and production by ocular surface epithelia via TLR4 innate immune pathways was identified in an in vivo mouse model of SRWe topical challenge, and an in vitro culture model using primary HCECs. We showed that aqueous extract of defatted SRW pollen indeed stimulated TSLP production in a dose-dependent fashion, and its stimulation was largely blocked by exogenous TLR4 antibody or by deletion of Tlr4 or MyD88 genes. In the human culture model, we observed that the SRWe stimulated TSLP expression and production was also blocked by the NF-kB activation inhibitor, quinazoline. This finding is consistent with previous reports,5 including ours.15 This result further supports our hypothesis that SRW serves as a functional TLR4 agonist because NF-kB is a well known signaling pathway of TLRs41,42 including TLR4.43 Further studies are necessary to explore the signaling pathways involved in the NF-kB mediated TLR4 innate immune response induced by SRW.
In conclusion, we have for the first time uncovered a novel phenomenon that short ragweed pollen, serving as a functional TLR4 agonist, induces TSLP/OX40L/OX40 signaling to trigger Th2-dominant allergic inflammation via TLR4-dependent innate immunity pathways. These novel findings shed light on the understanding of innate mucosal epithelial immunity involved in allergic inflammation, and may create new therapeutic targets to cure allergic disease.
Clinical Implications.
Discovery of short ragweed pollen as a functional TLR4 agonist that induces TSLP/OX40L/OX40 signaling to trigger Th2-dominant inflammation creates a new intervention strategy and potential to cure the allergic diseases.
Acknowledgments
Supported by Department of Defense CDMRP PRMRP grant FY06 PR064719 (DQL), National Institutes of Health grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
The authors thank Dr. Marshall Bowes Hamill for his kind support and the Lions Eye Bank of Texas for providing human corneoscleral tissues.
Abbreviations used
- CLN
cervical lymph nodes
- EAC
experimental allergic conjunctivitis
- HCECs
human corneal epithelial cells
- DCs
dendritic cells
- IFNγ
interferon gamma
- MyD88
myeloid differentiation primary response gene 88
- MyD88−/−
MyD88 knockout
- LPS
lipopolysaccharide
- NF-kB
nuclear factor kappa B
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PRRs
pattern-recognition receptors
- RT
reverse transcription
- OX40L
OX40 ligand
- SHEM
supplemented hormonal epidermal medium
- SRW
short ragweed
- SRWe
aqueous extract of defatted short ragweed pollen
- Tlr4-d
Tlr4 gene deficient
- TLR
Toll-like receptor
- TSLP
thymic stromal lymphopoietin
- TSLPR
TSLP receptor
Footnotes
Commercial relationship: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stern ME, Siemasko KF, Niederkorn JY. The Th1/Th2 paradigm in ocular allergy. Curr Opin Allergy Clin Immunol. 2005;5:446–450. doi: 10.1097/01.all.0000182547.60595.64. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY. Immune regulatory mechanisms in allergic conjunctivitis: insights from mouse models. Curr Opin Allergy Clin Immunol. 2008;8:472–476. doi: 10.1097/ACI.0b013e32830edbcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buc M, Dzurilla M, Vrlik M, Bucova M. Immunopathogenesis of bronchial asthma. Arch Immunol Ther Exp (Warsz) 2009;57:331–344. doi: 10.1007/s00005-009-0039-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RW, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 5.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda A, Ebihara N, Yokoi N, Kawasaki S, Tanioka H, Inatomi T, et al. Functional role of thymic stromal lymphopoietin in chronic allergic keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:151–155. doi: 10.1167/iovs.09-4183. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Ma P, de Paiva CS, Cunningham MA, Hwang CS, Pflugfelder SC, et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:3076–3082. doi: 10.1167/iovs.09-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollenberg A, Klein E. Current aspects of innate and adaptive immunity in atopic dermatitis. Clin Rev Allergy Immunol. 2007;33:35–44. doi: 10.1007/s12016-007-0032-9. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma P, Bian F, Wang Z, Zheng X, Chotikavanich S, Pflugfelder SC, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50:2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le TA, Takai T, Vu AT, Kinoshita H, Chen X, Ikeda S, et al. Flagellin Induces the Expression of Thymic Stromal Lymphopoietin in Human Keratinocytes via Toll-Like Receptor 5. Int Arch Allergy Immunol. 2010;155:31–37. doi: 10.1159/000318679. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita H, Takai T, Le TA, Kamijo S, Wang XL, Ushio H, et al. Cytokine milieu modulates release of thymic stromal lymphopoietin from human keratinocytes stimulated with double-stranded RNA. J Allergy Clin Immunol. 2009;123:179–186. doi: 10.1016/j.jaci.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 19.Song XJ, Li D-Q, Farley W, Luo LH, Heuckeroth RO, Milbrandt J, et al. Neurturin-deficient mice develop dry eye and keratoconjunctivitis Sicca. Invest Ophthalmol Vis Sci. 2003;44:4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- 20.Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998;87:75–84. doi: 10.1006/clin.1997.4507. [DOI] [PubMed] [Google Scholar]
- 21.Stern ME, Siemasko K, Gao J, Duong A, Beauregard C, Calder V, et al. Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2005;46:3239–3246. doi: 10.1167/iovs.05-0138. [DOI] [PubMed] [Google Scholar]
- 22.Solomon A, Rosenblatt M, Li D-Q, Monroy D, Ji Z, Lokeshwar BL, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am J Ophthalmol. 2000;130:688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 25.Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 26.Lowe B, Avila HA, Bloom FR, Gleeson M, Kusser W. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal Biochem. 2003;315:95–105. doi: 10.1016/s0003-2697(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 27.de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li D-Q, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li D-Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 32.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6:383–387. [PubMed] [Google Scholar]
- 34.Tsuji RF, Hoshino K, Noro Y, Tsuji NM, Kurokawa T, Masuda T, et al. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin Exp Allergy. 2003;33:249–258. doi: 10.1046/j.1365-2222.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 35.Fasciano S, Li L. Intervention of Toll-like receptor-mediated human innate immunity and inflammation by synthetic compounds and naturally occurring products. Curr Med Chem. 2006;13:1389–1395. doi: 10.2174/092986706776872916. [DOI] [PubMed] [Google Scholar]
- 36.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4. MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 37.Mishra KP, Ganju L, Chanda S, Karan D, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro. Immunobiology. 2009;214:27–31. doi: 10.1016/j.imbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Groneberg DA, Bielory L, Fischer A, Bonini S, Wahn U. Animal models of allergic and inflammatory conjunctivitis. Allergy. 2003;58:1101–1113. doi: 10.1046/j.1398-9995.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 39.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renz H, Saloga J, Bradley KL, Loader JE, Greenstein JL, Larsen G, et al. Specific V beta T cell subsets mediate the immediate hypersensitivity response to ragweed allergen. J Immunol. 1993;151:1907–1917. [PubMed] [Google Scholar]
- 41.Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181–189. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- 42.Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:5623–5629. doi: 10.1167/iovs.09-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]