Abstract
Background
Asthma is an inflammatory condition often punctuated by episodic symptomatic worsening, and accordingly, individuals with asthma may have waxing and waning adherence to controller therapy.
Objective
To measure changes in inhaled corticosteroid (ICS) adherence over time, and to estimate the effect of this changing pattern of use on asthma exacerbations.
Methods
ICS adherence was estimated from electronic prescription and fill information for 298 participants in the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE). For each individual we calculated a moving average of ICS adherence for each day of follow-up. Asthma exacerbations were defined as the need for oral corticosteroids, an asthma-related emergency department visit, or an asthma-related hospitalization. Proportional hazard models were used to assess the relationship between ICS medication adherence and asthma exacerbations.
Results
Adherence to ICS medications began to increase prior to the first asthma exacerbation and continued afterward. Adherence was associated with a reduction in exacerbations, but was only statistically significant among individuals whose adherence was >75% of the prescribed dose (hazard ratio [HR] 0.61; 95% confidence interval [CI] 0.41–0.90) when compared with individuals whose adherence was ≤25%. This pattern was largely confined to individuals whose asthma was not well controlled initially. An estimated 24% of asthma exacerbations were attributable to ICS medication non-adherence.
Conclusions
Inhaled corticosteroid adherence varies in the time period leading up to and following an asthma exacerbation, and non-adherence likely contributes to a large number of these exacerbations. High levels of adherence are likely required to prevent these events. [ClinicalTrials.gov number NCT01142947]
Keywords: medication adherence, inhaled corticosteroids, asthma, patient compliance, asthma exacerbations
INTRODUCTION
Although inhaled corticosteroid (ICS) treatment is widely considered to be the cornerstone therapy for the control of asthma symptoms,(1) we and others have consistently documented poor adherence to this class of medications.(2–5) Despite the well documented benefit of inhaled steroid use in mitigating asthma complications and exacerbations,(6–8) the methodology of quantifying the relationship between ICS adherence and these asthma-related outcomes poses particular challenges. First, asthma exacerbations resulting in oral steroid use, an emergency room visit, or hospitalization can be infrequent; for example, in an earlier study we found that 45%, 25%, and 9% of adults experienced these events over a two year period.(9) Therefore, ICS adherence needs to be estimated for a relatively large number of patients in order to detect an association with exacerbation. Next, as asthma can be an episodic condition, so too can asthma medication use, especially with recent studies suggesting a potential benefit from as needed ICS treatment.(10) Therefore, assessing the relationship between ICS use and outcomes related to poor control may demonstrate a reverse causation bias, such that controller use may appear to be associated with exacerbations. At the other extreme, we have also shown that people who never fill their asthma controller medications (i.e., primary non-adherent patients), and thus have a measured adherence of 0%, may have less severe disease and hence less inclination to ever use their medication.(11)
In an earlier study, we looked at the cross-sectional relationship between adherence and asthma outcomes.(9) However, given the episodic nature of asthma, we hypothesized that ICS adherence changes with time. Therefore, the purpose of this study was to measure changes in adherence over time and to estimate the effect of ICS adherence on asthma exacerbations (i.e., burst therapy with oral corticosteroids, an asthma-related emergency room visit, or an asthma-related hospitalization), accounting for changing patterns of ICS use. Also novel to this analysis, we adjusted for contemporaneous measures of underlying asthma severity, such as changes in short-acting beta-agonist use,(12) so as to better estimate the relationship between ICS adherence and severe asthma exacerbations.
METHODS
Patient population
This study was approved by the institutional review board of Henry Ford Health System and was consistent with its Health Insurance Portability and Accountability Act policy. All adult study participants signed written consent, and study participants less than age 18 years signed a written assent with written consent signed by a legal guardian. Participants were part of the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity (SAPPHIRE), a prospective asthma cohort study, which has been described in detail elsewhere.(13;14) Potentially eligible participants were first identified though health system electronic records and met the following criteria: age 12–56 years, a recorded clinical diagnosis of asthma, and no recorded diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Individuals meeting these criteria were invited to undergo a screening examination as part of the intake evaluation for the parent pharmacogenomics study, SAPPHIRE (ClinicalTrials.gov number NCT01142947). The current analysis includes individuals who confirmed that they had a physician diagnosis of asthma at this initial visit for the SAPPHIRE study (henceforward referred to as the ‘initial visit’) and who had both medical and pharmacy coverage through an affiliated health maintenance organization (the latter criterion allowed us to identify medication use and clinical outcomes retrospectively and prospectively). In addition, as we were estimating the contribution of non-adherence to asthma exacerbation, we included only persons who had at least one ICS prescription fill (i.e., SAPPHIRE participants with at least one non-zero measurement of inhaled corticosteroid adherence during the study period).
The initial visit as part of the SAPPHIRE study also included pulmonary function testing which followed 2005 ATS/ERS recommendations.(15;16) Forced expiratory volume at one second (FEV1) was measured in liters, and the percent of predicted FEV1 was estimated using the reference equations of Hankinson et al.(17) Bronchodilator response was assessed after administering albuterol sulfate hydrofluoroalkane. A 360μg dose of albuterol was delivered from a standard metered dose inhaler (MDI) using an AeroChamber Plus® Z STAT spacer (Monahan Medical Corp., Plattsburgh, NY). Bronchodilator reversibility was calculated as the percent change in FEV1 pre- and post-albuterol administration.(15)
Calculation of ICS adherence
We defined ICS adherence as the percent of the prescribed dose taken by an individual. Inhaled corticosteroid adherence was estimated by linking electronic prescription data with pharmacy claims data. We have previously shown that these data are nearly complete for our covered population with very few prescriptions being filled through other providers.(11;15) In order to estimate the length of time each filled ICS canister would last, we linked dosage information from electronic prescription data to information on canister size as gleaned from National Drug Code information recorded at the time of the prescription fill. Together these data were used to calculate a days’ supply for each ICS fill. As we and others have done previously,(2;18;19) adherence was estimated as the cumulative days’ supply divided by the number of days of observation (i.e., a moving 6-month observation period for the current study). These calculations also accounted for (i.e., prorated) prescription fills that partially overlapped with the beginning and end of each observation period and incorporated when a medication was discontinued by a physician. As patient medication use could change over time, we calculated a moving 6-month average of medication use for each day of study follow-up. That is, for each day of follow-up past the initial SAPPHIRE visit we calculated the proportion of ICS medication taken as prescribed for the preceding 6-month period. We chose the 6-month window of exposure based on our prior experience showing that this time interval provides stable estimates of use when derived from electronic data sources (i.e., electronic prescriptions and pharmacy claims); we have also shown that estimates using this time window are not only associated with asthma but also with outcomes for another disease condition.(20)
Statistical analysis
The primary outcome was the composite of the following events: an asthma-related hospitalization, an asthma-related emergency department (ED) visit, or the use of oral corticosteroids. As we performed a time-to-event analysis, the primary analysis included follow-up to the occurrence any of the aforementioned events. The events included in our composite measure of serious asthma exacerbations are also consistent with recent efforts to standardized the definition of asthma exacerbations for research studies and clinical practice.(21) Secondary outcomes analysis included follow-up to each of these event types in isolation (i.e., only asthma-related hospitalizations, only asthma-related emergency department (ED) visit, or only burst oral corticosteroid use).
Cox proportional hazard models were used to assess the relationship between ICS medication adherence and the time to asthma-related outcomes. These time-to-event models estimate a hazard ratio [HR] for each covariate, which is similar to a relative risk but specific to regression models using survival analysis. As events could happen multiple times for a given individual, we accounted for the intensity of repeated measures (i.e., clustering of events per individual) using the methods described by Andersen and Gill.(22) Moreover, events occurring simultaneously were handled via the method described by Breslow.(23) Individuals were censored if and when they disenrolled from the health plan or at the end of observation on November 1, 2010. As mentioned above, adherence measures were updated for each day of follow-up (i.e., entered into the regression models as time-updated covariates) and each day’s measure approximated the proportion of the prescribed ICS dose taken over the preceding 6-month period per individual. Therefore, adherence was a continuous measure for which we estimated the risk reduction (for an asthma exacerbation) per 0.25 increase in this proportion (i.e., a 25% increase in adherence). Regression models adjusted for patients’ age, sex, and race-ethnicity. To account for underlying disease severity and control, we included FEV1 (in liters) and the degree of bronchodilator reversibility (as a percent change in FEV1) taken at the time of the initial visit, and a history of asthma exacerbations (i.e., oral steroid use, asthma related emergency department visits, and asthma related hospitalizations) in the baseline period. The baseline period was defined as the 6–12 months prior to the initial visit and was chosen so as not to overlap with ICS adherence measured at the time of the initial visit (i.e., the 6-month window of ICS use measured from 6 months prior to the initial visit to the time of the initial visit). The regression models also included separate indicator variables for whether patients were using a long-acting beta-agonist or ‘other’ asthma controller medications (i.e., anti-leukotrienes, mast cell stabilizers, immunomodulatory medications, or theophylline derivatives) at the time of their initial visit. Since we previously showed that prospective measures of short-acting beta-agonist (SABA) use predict future asthma exacerbations,(12) we also included time-updated measures of SABA use in our regression models as a proxy for changing disease severity. Similar to the measure of ICS adherence, SABA use was calculated as the total number of doses dispensed over the preceding 6 months (i.e., contemporaneous with the measurement of ICS adherence). Separate variables were created for SABA nebulizer and SABA MDI use based on our earlier published findings regarding their predictive import.(12) Therefore, we had estimates of SABA use for each day of follow-up, and these measures were included in the regression models as time-updated covariates.
To assess for a potential threshold effect in the relationship between ICS adherence and outcomes, we categorized adherence (i.e., the proportion of dose taken) as follows: 0–25% [referent], 26–50%, 51–75%, and 75%–100. These categorical variables were included as time-updated dummy variables in the regression equations. We stratified the analysis for asthma control status based on participant responses to the Asthma Control Test (ACT). Baseline ACT scores ≤19 were considered uncontrolled asthma and scores >19 were considered controlled asthma.(24) These regression models adjusted for all other covariates previously discussed.
Lastly, we estimated the proportion of serious asthma exacerbations (i.e., the combined primary outcome) attributable to ICS medication non-adherence. We estimated the rate of events with perfect adherence by generating a hazard for each person in analysis with ICS adherence set to 100%. This estimation included all other individual risk factors weighted by the model parameter estimates for that risk factor and for that individual. The number of avoidable events due to non-adherence was the difference between the observed number of events and the sum of the calculated hazards for the analytic population. We divided this value by the sum of the observed events in the analytic population and the number of events in individuals without ICS use (i.e., conservatively assuming that these events are immutable) to estimated the proportion of exacerbations attributable to ICS non-adherence. Restated, we estimated the total proportion of asthma exacerbations which could have been avoided through improved ICS adherence in the entire study population by first estimating the number of events which could have prevented in those taking an ICS and second by incorporating the number of events in those not taking ICS medication in the denominator (this latter number was conservatively assumed to be fixed).
We accepted a type-I error rate threshold of 5% (i.e., P-value<0.05) for determining statistical significance. Analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC).(25)
RESULTS
The characteristics of the 298 study participants with asthma from the SAPPHIRE cohort are shown in Table 1. Mean age was 34.5 years (± 15.7 years, standard deviation [SD]) and 68.5% were female. Almost all patients (98.3%) were of African American race-ethnicity by self-report. At the initial screening visit, approximately half of the participants (48.7%) reported asthma that was not controlled based on their ACT score. Mean adherence to ICS medication was 26.3% at the time of the initial visit.
Table 1.
Characteristics of SAPPHIRE study participants (N = 298)*
| Variable | |
|---|---|
| Age (in years) – mean ± SD | 35.4 ± 15.7 |
| Female sex – no. (%) | 204 (68.5) |
| Race-ethnicity – no. (%) | |
| African American | 293 (98.3) |
| White | 3 (1.0) |
| Other | 2 (0.7) |
| Asthma Control Test score† | |
| Score ≤19 – no. (%) | 145 (48.7) |
| Score >19 – no. (%) | 153 (51.3) |
| Pulmonary function‡ | |
| FEV1 (in liters) – mean ± SD | 2.44 ± 0.76 |
| Percent of predicted FEV1 (%) – mean ± SD | 87.1 ± 19.9 |
| FEV1 bronchodilator reversibility (% change in FEV1) – mean ± SD§ | 1.08 ± 1.38 |
| Oral steroid use in baseline period – no. (%)§ | 79 (26.5) |
| Asthma-related emergency department visit in baseline period – no. (%)|| | 20 (6.7) |
| Asthma-related hospitalization in baseline period – no. (%)§ | 3 (1.0) |
| Use of an additional controller medication at the time of the initial visit – no (%)¶ | |
| Long-acting beta-agonist** | 151 (50.7) |
| Other controller medication†† | 50 (16.8) |
| Inhaled corticosteroid adherence at the time of the initial visit (%) – mean ± SD‡‡ | 26.3 ± 25.4 |
SAPPHIRE denotes the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity; SD, standard deviation; and FEV1, the forced expiratory volume at one second.
Includes all participants from SAPPHIRE with at least one non-zero measurement of inhaled corticosteroid adherence during the study period.
Asthma control was assessed at the initial screening visit. An Asthma Control Test score ≤19 implies uncontrolled asthma and a score >19 implies controlled asthma.
Pulmonary function was measured during the initial screening visit at the time of enrollment.
Bronchodilator reversibility was measured as the percent change in FEV1 between the initial measurement and following administration of a 360μg dose of albuterol sulfate hydrofluoroalkane.
Baseline oral steroid use, asthma-related emergency department visits, and asthma-related hospitalizations were measured prior to inhaled corticosteroid adherence measurements (i.e., 6–12 months prior to the initial screening visit).
Initial visit denotes the time of the first examination for the SAPPHIRE study
Number of individuals using an inhaled corticosteroid who were concomitantly using an inhaled long-acting beta-agonist medication.
Number of individuals using an inhaled corticosteroid who were concomitantly using one of the following additional asthma controller medications: an anti-leukotriene, a mast cell stabilizer, an immunomodulatory agent (i.e., omalizumab), or a theophylline derivative medication.
Inhaled corticosteroid adherence at the time of the initial evaluation represents the average proportion of prescribed ICS medication taken per individual for time period starting 6 months prior to the initial visit to the time of the initial visit. The value presented represents the average across all study individuals.
The total duration of patient follow-up was 581.6 patient-years, or an average duration of follow-up per study participant of 1.95 years (± 0.93 SD). During follow-up, 40.6% had one or more treatments with an oral corticosteroid, 23.2% had an asthma-related emergency room visit, and 4.0% had an asthma-related hospitalization; 46.3% of participants had at least one of these events for a total of 435 asthma exacerbations. Among the 138 individuals with ≥1 asthma exacerbation the median time between events was 84 days (minimum time, 0 days; maximum time, 1,028 days).
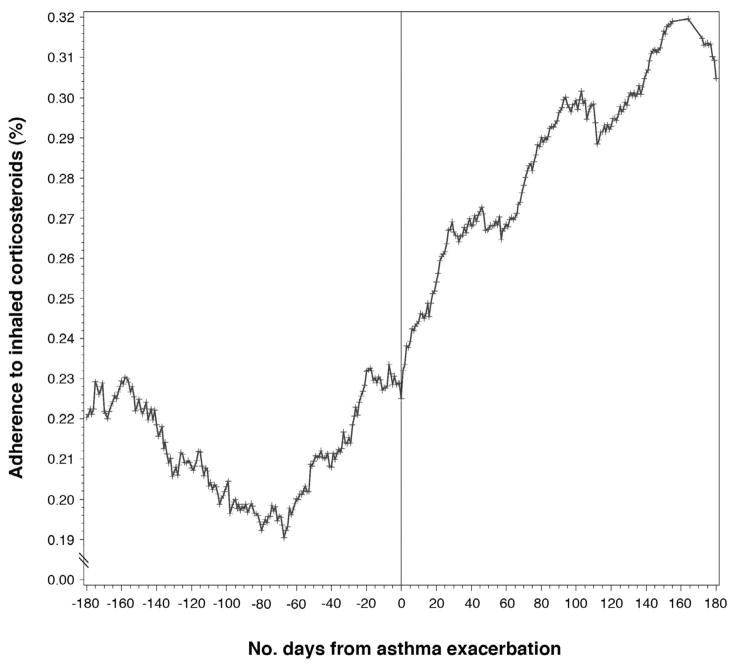
Figure 1 demonstrates the variation in adherence with respect to the first asthma-exacerbation (i.e., burst therapy with oral corticosteroids, an asthma-related ED visit, or an asthma-related hospitalization). The first events among study participants were aligned to demonstrate the changes in adherence preceding and following the exacerbation. As can be seen, ICS use began to increase prior to the exacerbation and continued following the event.
Figure 1.
Change in corticosteroid adherence over time with respect to the first asthma exacerbation in SAPPHIRE study participants. The first asthma exacerbation (i.e., burst oral steroid use, asthma-related emergency room visit, or asthma-related hospitalization) is aligned at time zero. Average adherence for the 180 days preceding and following the exacerbation are shown.
The increased ICS use around the time of an exacerbation may result in a counterfactual relationship between ICS adherence and the risk of an exacerbation. This is demonstrated in Table 2 where the unadjusted relationship between ICS adherence and asthma exacerbations shows an increased risk (HR 1.12; 95% confidence interval [CI] 1.04–1.20). However, adjusting for other markers of concomitant asthma severity (e.g., concurrent short-acting beta-agonist use) and historic asthma severity (i.e., prior history of oral steroid use, asthma-related ED visit, or asthma related hospitalization) resulted in a consistent protective relationship between ICS adherence and asthma exacerbation (see Table E1 in on the online supplement for the parameter estimates for the other covariates). Every 25% increase in ICS adherence was associated with an 11% decreased risk in the composite measure of asthma exacerbation (HR 0.89, 95% CI 0.81–0.97, P-value 0.009).
Table 2.
Unadjusted and adjusted relationship between inhaled corticosteroid medication adherence and asthma exacerbations*
| Outcome | Adherence to inhaled corticosteroids† | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | aHR (95% CI)‡ | P-value | |
| Primary outcome | ||||
| Combined asthma exacerbations* | 1.12 (1.04–1.20) | 0.002 | 0.89 (0.81–0.97) | 0.009 |
| Secondary outcomes | ||||
| Oral corticosteroid use | 1.12 (1.03–1.22) | 0.006 | 0.90 (0.80–1.00) | 0.043 |
| Asthma-related emergency department visit | 1.06 (0.92–1.22) | 0.428 | 0.87 (0.73–1.03) | 0.114 |
| Asthma-related hospitalization | 1.37 (1.03–1.81) | 0.029 | 0.99 (0.65–1.51) | 0.971 |
HR denotes hazard ratio; CI, confidence interval; and aHR, adjusted hazard ratio.
An asthma exacerbation was considered to be an event requiring the initiation of oral corticosteroids, an asthma-related emergency department visit, or an asthma-related hospitalization. These events combined comprised the primary study outcome.
Hazard ratios for adherence represent the estimated effect for a 25% improvement in inhaled corticosteroid adherence.
Adjusted for patient age, sex, race-ethnicity, concomitant short acting beta-agonist use (i.e., separately adjusting for metered dose inhaler and nebulizer use), percent of predicted forced expiratory volume at one second (FEV1) at the time of the initial visit, the degree of bronchodilator reversibility in the FEV1 at the time of the initial visit, historic exacerbations during the baseline period (i.e., separate variables for oral steroid use, asthma-related emergency department visits, and asthma-related hospitalizations), use of a long-acting beta-agonist as an ICS combination inhaler at the time of the initial visit, and the use of other asthma controller medication at the time of the initial visit (i.e., anti-leukotrienes, mast cell stabilizers, immunomodulatory medication, or theophylline derivatives).
We also examined whether the relationship between the level of adherence and asthma exacerbations was linear. As can be seen, the largest and only statistically significant reduction in the risk of exacerbation was seen among individuals whose ICS adherence exceeded 75% of prescribed (Figure 2A). However, after stratifying our sample by asthma control status at baseline (i.e., an ACT score ≤19 for uncontrolled and >19 for controlled), we found that the benefit of adherence was largely confined to individuals whose asthma was not controlled at baseline. In this latter group, the protected effect of ICS controller therapy was again largely confined to those with an adherence >75% (HR 0.59, 95% CI 0.37–0.95, P-value 0.03) (Figure 2B). Although, increasing ICS adherence also appeared protective among individuals whose reported asthma was controlled at baseline, these findings did not reach statistical significance and there was no obvious threshold effect (Figure 2C). These data may also be found in the online supplement Table E2.
Figure 2.
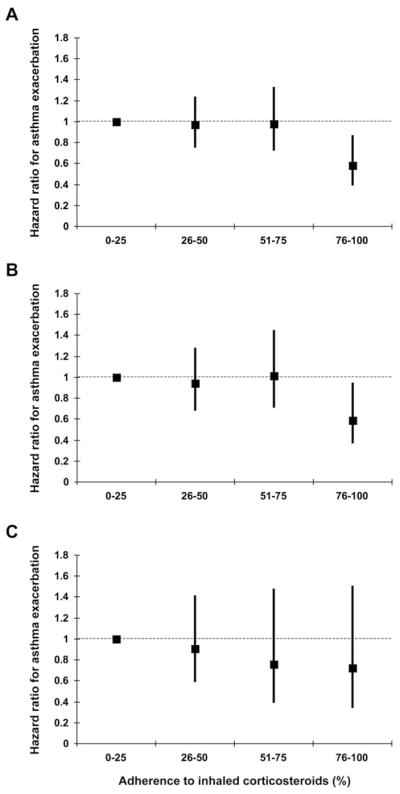
Relationship between level of inhaled corticosteroid (ICS) adherence (i.e., the percent of prescribed ICS medication taken) and the likelihood of an asthma exacerbation (i.e., burst oral steroid use, asthma-related emergency room visit, or asthma-related hospitalization). The relationship between adherence and outcomes is shown for all study participants (A), for those whose asthma was uncontrolled at the initial visit (B), and for those whose asthma was controlled at the initial visit (C). Participants with an ICS adherence of 0–25% are the referent group against which the other adherence categories are compared. Effect estimates are adjusted for all covariates included in Table 2 and in Model 5 from Table E1.
Using the model parameter estimates (Table E1), we estimated that there would have been 124 asthma exacerbations (i.e., the combined outcome) in our analytic population per year were patients to have used their ICS medication as prescribed (i.e., perfect adherence). One hundred ninety-six events were observed in this time period for this group, and an additional 99 events were noted for individuals without ICS exposure. Therefore, we estimate that 24.4% (i.e., [196−124]/[196+99]) of all asthma exacerbations in our study population could have been avoided through improved ICS adherence. Of these 295 events, 176 (59.7%) were oral steroid fills, 107 (36.3%) were emergency department visits, and 12 (4.1%) were hospitalizations.
DISCUSSION
The effectiveness of inhaled corticosteroids to reduce asthma exacerbations has been well described,(26;27) as has the relationship between ICS non-adherence and increased exacerbations.(9;28–30) and the lack of persistence in ICS use over time.(31;32) However, to our knowledge this is the first study to quantify the likely effect of this varying use on severe asthma events. Moreover, our composite measure of severe asthma exacerbations was consistent with recent recommendations to standardize the measurement of these events,(21) and hence our findings may be more easily generalized when compared with earlier studies.
Not surprisingly, we found that medication use as assessed through pharmacy claims increased in the time surrounding an asthma exacerbation. This observation has relevance when assessing the relationship between medication use and outcomes, such that one may observe a positive relationship between ICS adherence and asthma exacerbations if underlying severity is not adequately accounted for in the analysis. We have previously shown short-acting beta-agonist rescue medication use to be a predictor of an impending asthma event,(12) and again have observed that accounting for this use at least in part corrects underlying variation in disease severity,(9) as does accounting for a past history of asthma exacerbations.
We also report the somewhat surprising suggestion of a non-linear reduction in asthma exacerbations with increasing ICS use. In particular, ICS adherences rates >75% of the prescribed dose appeared be a threshold above which asthma exacerbations were significantly reduced. This finding suggests that the seemingly arbitrary thresholds between 70–80% often used to describe the level above which a patient is considered adherent may actually have clinical relevance,(33–35) and is consistent with at least one other study finding increased asthma control among individuals with >80% ICS adherence.(36) However, this cut point may differ by condition, disease severity, and medication class, implying that its predictive validity also needs to be established for other clinical scenarios.(37)
Our findings do appear to conflict with a recent clinical trial suggesting that intermittent dosing of inhaled corticosteroids results in similar exacerbation rates when compared with ICS continuous dosing.(10) However, this study was restricted to individuals with mild persistent asthma, whereas our study was not. Although we did not categorize individuals according to severity as outlined in National Asthma Education and Prevention Program guidelines,(1) we do show that the largest benefit of ICS use was seen in individuals deemed to be uncontrolled based on Asthma Control Test score, and in these individuals only adherence levels >75% were associated with a lower rate of exacerbations. In the trial, adherence was assessed by counting unused inhaler doses. As study participants may dump medication in an attempt to appear compliant,(38) this method of assessing adherence may overestimate actual use and diminish the observed effect of continuous ICS therapy. It is possible that we were underpowered to detect the association between ICS adherence and exacerbations among those who were well controlled at baseline. However, as this group was actually larger than those with an ACT score ≤19, the relationship if present is likely smaller than that reported here.
While we have again shown pharmacy claims-based estimates of medication adherence to be predictors of asthma-related outcomes, we must note that these metrics do not measure the erratic patterns of daily use, nor are they capable of assessing improper inhaler technique, both of which may be highly prevalent.(39;40) Moreover, we could not determine whether some filled medication was dumped or went unused. Nevertheless, we anticipate that this unmeasured deviation would result in underestimating the true effect of adherence to ICS medication on asthma exacerbations, and in this regard our estimates may be conservative. Similarly, as this is an observational study, it is possible that we did not account for other important confounders in our analysis. It is also uncertain whether our finding can be generalized to others, as the patients analyzed were all members of a single health system and were predominantly African American. Excluding and including the few individuals who did not report African American race-ethnicity had no significant effect on our results (data not shown). Moreover, we have recently shown that ICS treatment response is not likely to vary according to the proportion of one’s African and European ancestry,(14) suggesting that the effect of ICS adherence observed here may be generalized to multiple population groups.
The low baseline level of ICS adherence that we measured here (26.3%) is consistent with other large population-based studies by us and others.(2;41) We estimate that approximately 24% of our combined exacerbation outcomes could have been avoided through improved ICS use. At first glance this is lower than our previous estimate of 60% of asthma hospitalizations attributable to ICS non-adherence,(9) yet it is important to note that hospitalizations are a rare event and therefore our current estimates represent a much larger number of events avoided. Our estimates are also conservative in that they assume that events in individuals without ICS exposure are fixed; however, we know from the work of others that even individuals with severe asthma can be under-prescribed ICS medication and therefore may benefit from treatment.(42)
In summary, we now are able to demonstrate the relationship between ICS adherence and asthma exacerbations in a manner that accounts for the changing patterns of inhaler use over time. Not surprisingly, ICS adherence appears to be protective for these events. While these findings are consistent with the results of clinical trials involving the use of ICS medication for asthma,(27) even these trials do not account for varying degrees of ICS use by participants. In this manner we were able to ascertain that the primary treatment benefit was experienced by those with relatively high degrees of use (>75% of the prescribed dose) over a 6-month period. While these population-level estimates accounted for multiple proxies of disease severity, the exact strength and duration of ICS treatment needed for an individual patient will vary. Nevertheless, developing and refining these measures of medication use from automated data sources (i.e., electronic prescription information, claims data, and the electronic medical record) has a number of exciting potentials. These include improvement in patient care through the identification of individuals at high risk for poor outcomes and even application in the areas of pharmacoepidemiology and pharmacogenomics where accurate assessments of drug exposure are needed.(13;43)
Acknowledgments
This work was supported by grants from the Fund for Henry Ford Hospital, the American Asthma Foundation, and the National Institute of Allergy and Infectious Diseases (R01AI079139, R01AI061774), the National Heart Lung and Blood Institute (NHLBI) (R01HL079055), and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK064695), National Institutes of Health (NIH) to Dr. Williams. Dr. Burchard receives funding through the NHLBI, NIH(R01HL078885, R01HL088133) and the Flight Attendant Medical Research Institute. Dr. Kumar also receives funding through the NHLBI, NIH (K23HL093023-01). These funding agencies did not have a role in the study design, analysis, drafting of the manuscript, or revision of the manuscript.
We would like to sincerely thank all those on the SAPPHIRE staff whose help made this study possible, especially Janis Campbell, John Gaggin, Gloria Karungi, Lynn DiBartolomeo, Kim Sadlocha, and Mao Yang. We would also like to thank John Grybas for his technical assistance on this manuscript.
Abbreviation list
- ICS
Inhaled corticosteroids
- FEV1
Forced expiratory volume at one second
- SAPPHIRE
Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity
- ACT
Asthma Control Test
Footnotes
Clinical implications: Inhaled corticosteroids (ICS) non-adherence is a major contributor to serious asthma exacerbations, and efforts to reduce these events will likely need to achieve high adherence levels in patients with uncontrolled asthma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126(2):225–31. 231. doi: 10.1016/j.jaci.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apter AJ, Boston RC, George M, Norfleet AL, Tenhave T, Coyne JC, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111(6):1219–26. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 4.Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149(2 Pt 2):S69–S76. doi: 10.1164/ajrccm/149.2_Pt_2.S69. [DOI] [PubMed] [Google Scholar]
- 5.Bender B, Wamboldt FS, O’Connor SL, Rand C, Szefler S, Milgrom H, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–21. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 6.Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–92. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- 7.Haahtela T, Jarvinen M, Kava T, Kirviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med. 1994;331(11):700–5. doi: 10.1056/NEJM199409153311103. [DOI] [PubMed] [Google Scholar]
- 8.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 9.Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. The Journal of Allergy and Clinical Immunology. 2004;114(6):1288–93. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352(15):1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 11.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–9. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Paris J, Peterson EL, Wells K, Pladevall M, Burchard EG, Choudhry S, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol. 2008;101(5):482–7. doi: 10.1016/S1081-1206(10)60286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Hu D, Peterson EL, Eng C, Levin AM, Wells K, et al. Dual-specificity phosphatase 1 as a pharmacogenetic modifier of inhaled steroid response among asthmatic patients. J Allergy Clin Immunol. 2010;126(3):618–25. doi: 10.1016/j.jaci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould W, Peterson EL, Karungi G, Zoratti A, Gaggin J, Toma G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol. 2010;126(6):1131–8. doi: 10.1016/j.jaci.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 19.Wells K, Pladevall M, Peterson EL, Campbell J, Wang M, Lanfear DE, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med. 2008;178(12):1194–201. doi: 10.1164/rccm.200808-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib ZA, Tzogias L, Havstad SL, Wells K, Divine G, Lanfear DE, et al. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):437–47. doi: 10.1002/pds.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 22.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. The Annals of Statistics. 1982;10(4):1100–20. [Google Scholar]
- 23.Breslow N. Covariance analysis of censored survival data. Biometrics. 1974;30(1):89–99. [PubMed] [Google Scholar]
- 24.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute Inc. SAS/STAT Users Guide. Cary, NC: SAS Institute Inc; 2008. Version 9.2 ed. [Google Scholar]
- 26.Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–4. doi: 10.1136/thorax.57.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sin DD, Man J, Sharpe H, Gan WQ, Man SF. Pharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysis. JAMA. 2004;292(3):367–76. doi: 10.1001/jama.292.3.367. [DOI] [PubMed] [Google Scholar]
- 28.Stern L, Berman J, Lumry W, Katz L, Wang L, Rosenblatt L, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97(3):402–8. doi: 10.1016/S1081-1206(10)60808-3. [DOI] [PubMed] [Google Scholar]
- 29.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy Clin Immunol. 2008;122(3):490–5. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Milgrom H, Bender B. Nonadherence to asthma treatment and failure of therapy. Curr Opin Pediatr. 1997;9(6):590–5. doi: 10.1097/00008480-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Marceau C, Lemiere C, Berbiche D, Perreault S, Blais L. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol. 2006;118(3):574–81. doi: 10.1016/j.jaci.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Breekveldt-Postma NS, Koerselman J, Erkens JA, van der MT, Lammers JW, Herings RM. Treatment with inhaled corticosteroids in asthma is too often discontinued. Pharmacoepidemiol Drug Saf. 2008;17(4):411–22. doi: 10.1002/pds.1552. [DOI] [PubMed] [Google Scholar]
- 33.Clerisme-Beaty EM, Bartlett SJ, Teague WG, Lima J, Irvin CG, Cohen R, et al. The Madison Avenue effect: How drug presentation style influences adherence and outcome in patients with asthma. J Allergy Clin Immunol. 2011;127(2):406–11. doi: 10.1016/j.jaci.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1810–7. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 35.Cochrane GM. Compliance and outcomes in patients with asthma. Drugs. 1996;52 (Suppl 6):12–9. doi: 10.2165/00003495-199600526-00004. [DOI] [PubMed] [Google Scholar]
- 36.Lasmar L, Camargos P, Champs NS, Fonseca MT, Fontes MJ, Ibiapina C, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64(5):784–9. doi: 10.1111/j.1398-9995.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 37.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW, et al. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146(6):1559–64. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- 39.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117(2):542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 40.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(6 Pt 1):1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 41.Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899–904. doi: 10.1016/j.jaci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Legorreta AP, Christian-Herman J, O’Connor RD, Hasan MM, Evans R, Leung KM. Compliance with National Asthma Management Guidelines and specialty care. Arch Intern Med. 1998;158:457–64. doi: 10.1001/archinte.158.5.457. [DOI] [PubMed] [Google Scholar]
- 43.Wilke RA, Xu H, Denny JC, Roden DM, Krauss RM, McCarty CA, et al. The Emerging Role of Electronic Medical Records in Pharmacogenomics. Clin Pharmacol Ther. 2011 doi: 10.1038/clpt.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]