Abstract
Ecstasy use has been associated with neurotoxicity and neurocognitive impairment in a variety of domains, including prospective memory (ProM), which involves the delayed execution of a previously encoded intention in response to a specific cue. The present study adopted the multiprocess theory of ProM to evaluate the hypothesis that ecstasy users would evidence differentially impaired ProM on longer versus shorter ongoing task delays. Ecstasy (n = 31) users, high-risk alcohol users (n = 21) and healthy nonusers (n = 31) completed the short (2-min) and long (15-min) delay ProM scales of the Memory for Intentions Screening Test. Results showed a significant group by ProM delay interaction, such that ecstasy users performed comparably to the comparison groups on short-delay trials, but were impaired on long-delay ProM, particularly for time-based cues. Among the ecstasy users, long-delay ProM was positively associated with risky decision-making, but not with retrospective memory or other aspects of executive functions. These findings suggest that ecstasy users may be particularly susceptible to deficits in strategic target monitoring and maintenance of cue-intention pairings over longer ProM delays. Findings are discussed in the context of their potential everyday functioning (e.g., academic, vocational) and treatment implications for ecstasy users.
Keywords: Prospective memory, ecstasy, substance abuse, episodic memory, executive functions, time perception, cognition
Introduction
Human and animal studies have established that the commonly-used psychoactive drug ecstasy (primary component 3,4-methylenedioxymethamphetamine, or MDMA) has adverse effects on several different neurotransmitter systems, most prominently serotonin (e.g., Buchert et al, 2004; Rudnick & Wall, 1992). Neurotoxic effects of MDMA, which may involve oxidative stress, excitotoxicity, and/or mitochondrial dysfunction (Yammamoto et al., 2010), have been associated with lasting effects on serotonergic innervations (Hatzidimitriou et al, 1999). Nuclear neuroimaging studies have found evidence of reduced 5-HT reuptake transporters across a variety of brain regions within the serotonergic system, including the temporal cortex (e.g., Buchert et al., 2006) and frontostriatal loops (e.g., McCann et al., 1998). A variety of structural and functional neuroimaging studies have similarly reported abnormal findings associated with ecstasy use (e.g., smaller volumes and reduced N-acetyleaspartate), particularly in the hippocampus and prefrontal cortex (e.g., Obrocki et al, 1999; see Cowan, 2007 for a review).
Meta-analytic studies have consistently reported mild-to-moderate deficits across a number of neurocognitive domains (e.g., Nulsen et al, 2010; Kalechstein et al, 2007; Zakzanis, 2007), including processing speed, attention, working memory and executive functions. Arguably, the most prominent and reliable cognitive domain affected among ecstasy users is verbal memory (e.g., Zakzanis, 2007), for which effect sizes generally fall in the moderate-to-large range (e.g., Laws & Kokkalis, 2007). The profile of learning and memory deficits suggest that ecstasy users have particular difficulty with encoding of new material, with relatively spared consolidation and perhaps mild impairment in retrieval (e.g., Ward et al., 2006). The primary deficit in initial acquisition apparent in ecstasy users may be driven by limited use of spontaneous self-initiated strategic processes (Brown et al., 2010), interference effects (Ward et al., 2006), and task complexity (Brown et al., 2010), which has led some researchers to hypothesize a possible frontostriatal pathophysiology.
Although the effects of ecstasy on retrospective memory (RM; i.e., recall of events from the past) are relatively well established, our understanding of its impact on prospective memory (ProM) is only beginning to emerge. ProM, or “remembering to remember” involves the execution of a previously encoded intention in response to a specific cue, with a well-established distinction between response cues that are based on events (EB; e.g., passing along a message when you next encounter a friend) versus time (TB; e.g., attending an appointment at 2:00). Importantly, ProM has been found to be particularly sensitive to changes in everyday functioning in clinical samples, including instrumental activities of daily living (Woods, Iudicello, et al., 2008), unemployment (Woods, Weber, et al., 2011), and medication adherence (see Zogg et al., in press).
ProM is a higher-level complex cognitive process, and theorists (e.g., Kliegel et al., 2008) have identified a range of critical component processes, including encoding of the intention and cue, and retention of the intention-cue pairing over the course of a delay interval during which an ongoing task diverts attentional resources from the intention. During this period, relatively automatic and/or strategic monitoring of the environment for the appropriate circumstance to enact the intention (i.e., cue detection) may occur. Once the cue is detected, successful ProM performance also requires retrieval of the previously encoded intention from RM, which is then executed, and the outcome evaluated for accuracy. Given that ProM processes require not just adequate encoding and consolidation of intention-cue pairs, but also coordinated and efficient deployment of a variety of executive functions (e.g., McDaniel, Glisky, Rubin, et al, 1999) for successful execution, it is unsurprising that studies using a variety of methodologies are consistent in linking ProM to a distributed neural network involving prefrontal (viz., Brodmann’s area 10), temporal, and inferior parietal lobe structures (Burgess et al., in press).
Evaluation of ProM function amongst ecstasy users has consistently found evidence of elevated self-reported ProM failures in daily life as compared to non-users (e.g., Rodgers et al, 2001, Montgomery et al, 2007). A small, but growing research also supports the presence of objective ProM deficits amongst ecstasy users (e.g., Zakzanis et al, 2003; cf. Montgomery et al., 2010). For example, Rendell et al. (2007) found global impairment across both time- and event-based ProM cues after controlling for marijuana, psychopathology and sleepiness, which was associated with greater frequency of ecstasy use. Hadjiefthyvoulou et al. controlled for a wider variety of co-occurring substance use and also found TB and EB ProM deficits in both experimental (2010) and clinical measures (2011). Finally, Bedi and Redman (2008a) reported a significantly lower score on a behavioral action-cued ProM task in their ecstasy-using group (although they dismissed this finding as Type I error). As concerns the neural substrates of ecstasy-associated ProM deficits, Ramaekers et al. (2009) observed a relative reduction in task-associated deactivation (relative to placebo) in the inferior parietal lobule and basal ganglia corresponding to ProM task failures amongst experienced ecstasy users on an EB ProM task following acute administration of a single 75 mg MDMA dose.
Although the limited research published to date clearly supports an adverse effect of ecstasy on ProM, the specific cognitive mechanisms of the observed deficit have not been widely explored beyond those associated with TB and EB cues, which appear to be comparably affected (Hadjiefthyvoulou et al., 2011; Rendell et al., 2007). This line of investigation is particularly important due to the complex and multifaceted nature of ProM as outlined above. One relatively understudied factor affecting ProM performance is the length of the ongoing task delay; that is, the period of time between intention formation and execution, during which the participant may monitor the environment for retrieval cues (e.g., McDaniel & Einstein, 2000), but is otherwise is engaged in a distracter task in order to minimize overt rehearsal. Findings have been consistent in supporting longer response delays being associated with poorer recall in the retrospective episodic memory literature (e.g., Wixted & Ebbeson, 1991). The highly influential multi-process theory would predict a similar pattern in ProM, positing that executive processes essential to monitoring and cue detection are limited and therefore easily taxed as delay length increases (McDaniel & Einstein, 2000). Supporting this supposition, longer delay-related declines have been primarily found in the ProM literature as strategic demands are increased. For example, Martin and colleagues observed that increases in ongoing task delays (which presumably increase strategic demands) resulted in poorer ProM performance, while increasing length of filler tasks (which allow for greater opportunity for rehearsal) marginally improved ProM, but only under conditions of short ongoing delay (Martin, Brown & Hicks, 2011). Further, nonfocal ProM cues -- which place strong demands on strategic and monitoring resources -- produce more consistent declines in longer-delay conditions versus more automatic focal cues (Einstein et al, 2005).
These experimental findings would suggest that deficits affecting the ability to efficiently utilize strategic resources could result in differentially impaired performance for longer versus shorter delay ProM tasks amongst ecstasy users and other clinical populations susceptible to executive and memory dysfunction (Zakzanis, 2007). Initial studies (Heffernan, Jarvis, Rodgers, et al., 2001; Heffernan, Ling & Scholey, 2001) using self-report measures found that ecstasy users reported generalized ProM impairments across scales assessing both short- and long-term delays. Interestingly, Rodgers and colleagues found that ecstasy use was a significant predictor of long delay, but not short delay ProM complaints. Of note, however, such findings have not been consistently seen (i.e., Montgomery et al., 2007) and we are unaware of any experimental, performance-based investigations of delay interval on ProM in ecstasy users. Nevertheless, a recent study by Hadjiefthyvoulou et al (2010) hints that delay interval might be particularly relevant in ecstasy-related ProM deficits. The authors noted in passing that ecstasy users displayed more pronounced deficits on a time-based habitual ProM task (i.e., report on sleepiness level every 20 minutes) during the latter half of the experiment (Cohen’s d = 1.16) as compared with the first half (Cohen’s d = .70). In light of the evidence reviewed above, the present study aimed to systematically evaluate the effect of task interval on ProM performance amongst ecstasy users, controlling for the potential confounding effects of co-occuring substance use, mood, and lifestyle factors as required. Given the above literature, multiprocess theory would predict that ecstasy users may be particularly vulnerable to deficits in strategic target monitoring and maintenance of cue-intention pairings as ProM delay length increases. Accordingly, it is hypothesized that ecstasy users will have differentially impaired ProM complaints and performance on longer (as compared with shorter) ongoing task delays relative to non-ecstasy-using comparison groups, and that long delay performance will be associated with executive dysfunction.
Method
Participants
Eighty-three participants were selected from 291 individuals recruited for a larger study of substance use and neuropsychological function. Recruitment took place via posters placed around the University of Western Australia campus, student email and webpages, social networking sites (e.g., Facebook), and word of mouth. General inclusion criteria were age 18–30 years and English as a first language. Exclusion criteria were any history of significant medical or psychiatric history (e.g., traumatic brain injury with loss of consciousness > 5 minutes, seizure disorder, Attention-Deficit/Hyperactivity Disorder, learning disabilities). Individuals with severe mental illness (e.g., bipolar or schizophrenia) were excluded; however, individuals with depression and anxiety were allowed in all groups in order to better represent actual substance using populations. Additionally, participants who had used any substance (other than alcohol) in the previous three days, or had consumed alcohol at binge levels (> 4 units; e.g., Naimi, Brewer, Makdad et al, 2003) the previous day were excluded to ensure that any effects seen were not due to the acute effects of any substance. The primary group of interest was the 31 individuals from the larger sample who had used ecstasy 10 or more times in their life (XTC; 38.7% male). We then matched this XTC group to two comparison groups from the larger sample, giving weight to demographic factors, including education, gender, ethnicity, and age: 1) high risk alcohol users (ethanol, or ETOH; n = 21, 42.9% male) and, 2) healthy adults (HA; n = 31, 35.5% male). To be included in the ETOH group, individuals must have scored ≥15 on the Alcohol Use Disorders Identification Test (AUDIT), thus reflecting a high likelihood of problematic drinking (e.g., Babor, Higgins-Biddle et al., 2001), and have reported no lifetime use of ecstasy. The HA group was selected on the basis of no lifetime use of ecstasy, AUDIT scores < 8 (consistent with low risk drinking), minimal use of cannabis (i.e., < 10 lifetime uses), and no lifetime use of any other illicit substances.
With regard to substance and alcohol use history, both substance using groups produced mean AUDIT scores in the elevated range, with the ETOH group producing higher scores than the XTC group (p < .05). The mean score on the Drug Abuse Screening Test-20 (DAST-20) for the XTC group was significantly higher versus the ETOH and HA groups (ps < .05), with 67.7% of the XTC group scoring above cutoffs suggested for identification of problematic substance abuse (e.g., Cocco & Carey, 1998). The XTC group reported a median of 30 lifetime doses of ecstasy (mean = 56.5, SD = 61.1), with relatively modest use of other substances (e.g., median estimated lifetime units of cannabis = 15). The ETOH group reported limited use of cannabis and benzodiazapines, whilst use of cocaine and amphetamine was rare.
Demographic and other essential descriptive data are presented in Table 1. The study groups did not differ in gender composition, estimated premorbid intellectual function, self-reported distress, ethnic background, or education level (all ps > .10). The XTC group was marginally older than the ETOH and HA groups (p < .05) and reported greater levels of sleepiness than the HA group at trend levels (p = .052). Of note, however, age and sleepiness level were not significantly related to the prospective memory measures (all rs < .20, all ps > .10). In addition, the scope of the age range (that is, 18–30 years) was very limited and mean group age differences (less than two years) are not clinically significant in this age group. Further, inclusion of age (and/or sleepiness) as a covariate in the statistical models produced comparable results. Therefore, given the relatively small sample sizes, and for simplicity of interpretation, results without covariates are presented below.
Table 1.
Demographic, Psychiatric, and Substance Use Characteristics of the Study Groups.
| Variable | HA (n = 31) | ETOH (n = 21) | XTC (n = 31) | p-value |
|---|---|---|---|---|
| Age (years) | 19.7 (1.6) | 19.5 (2.1) | 21.4 (3.3) | .010 |
| Education (years) | 13.0 (1.3 | 12.7 (0.9) | 13.4 (1.5) | .166 |
| Est. VIQ | 112.6 (8.1) | 113.9 (7.8) | 109.9 (9.7) | .225 |
| Gender (% male) | 35.5% | 42.9% | 38.7% | .866 |
| Ethnicity (% White) | 83.9% | 85.7% | 96.8% | .172 |
| DASS 21 Total | 13.0 (9.4) | 16.5 (12.1) | 13.4 (8.3) | .420 |
| Epworth Sleepiness Scale | 5.4 (3.0) | 7.7 (4.3) | 7.1 (3.5) | .052 |
| DAST-20 | 0.5 (1.0) | 1.8 (2.2) | 6.0 (3.2) | <.001 |
| Ecstasy Use | ||||
| Lifetime doses | ---- | ---- | 30 (20, 70) | ---- |
| Age at first use (years) | ---- | ---- | 17 (17, 18) | ---- |
| Recency (% used in past mo) | ---- | ---- | 32.3% | ---- |
| Frequency (% > 1/mo) | ---- | ---- | 22.6% | ---- |
| Duration (% > 6 months) | 77.4% | |||
| Alcohol Use | ||||
| AUDIT | 3.8 (2.4) | 19.8 (4.4) | 15.0 (5.3) | <.001 |
| Lifetime drinks (% > 100 units) | ---- | 61.9% | 87.1% | .100 |
| Age at first use | ---- | 14.8 (1.6) | 13.9 (1.4) | .034 |
| Recency (% used in past mo) | ---- | 95.2% | 100% | .220 |
| Frequency (% > 1/week) | ---- | 71.4% | 71.0% | .722 |
| Duration (% > 6 months) | 47.6% | 64.5% | .226 | |
| Other substance use* | ||||
| Cannabis | ---- | 2 (0, 5) | 15 (6, 150) | <.001 |
| Benzodiazapines | ---- | 6 (2, 10) | 15 (1, 30) | .565 |
| Cocaine | ---- | 0 (0, 0) | 8 (2, 40) | <.001 |
| Amphetamine | ---- | 0 (0, 0) | 2 (0, 30) | <.001 |
Note. HA = healthy adults. ETOH = High Risk Alcohol Users. XTC = Ecstasy Users. Est. VIQ = estimated premorbid verbal IQ derived from the Wechsler Test of Adult Reading. DASS 21 = Depression Anxiety Stress Scales – 21 item version. DAST = Drug Abuse Screening Test – 20-item version. AUDIT = Alcohol Use Disorders Identification Test.
Estimated units of lifetime use.
Procedure
Participants were administered the research version of Memory for Intentions Screening Test (MIST; Raskin et al., 2010), which is a standardized measure of prospective memory that demonstrates evidence of reliability (e.g., Woods, Moran, Dawson, Carey, & Grant, 2008) and construct validity (e.g., Raskin, 2009). The MIST is a 30-minute laboratory-based measure of ProM that includes four 2-min and four 15-min trials, during which the participant is engaged in an ongoing distracter task (i.e., a standardized word search). Responses are coded from 0–2 for each item, such that scale total scores range from 0–8, with higher scores reflective of better performance (see Woods et al., 2008 for more details). The 2- and 15-min trials are balanced on time- and event-based cues and action versus verbal responses. All cues are nonfocal. For the present study, 4 of the 8 items were slightly modified in order to change items that required subjects to write down participant identifiable information (due to ethical considerations), or items with limited relevance to young adults. Specifically, the item “When I hand you a red pen, sign your name on your paper” (an EB task with 15 min delay), was changed to “When I hand you a red pen, write down the current month on your paper”; “When I hand you a postcard, self-address it” (EB, 15 min), was changed to “When I hand you a postcard, write down the name of city and country that we are located in on it”; “When I hand you a request for records form, write your doctors’ names on it” (EB, 2 min), was changed to “When I hand you a request for records form, write down the name of any hospital on it”; and “In 15 minutes, use that paper to write the number of medications you are currently taking” (TB, 15 min) was changed to “In 15 minutes, use that paper to write down your age”.
Self-reported prospective and memory complaints were assessed with the Long-term and Short term PM scales of the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith et al. 2000). The PRMQ is a 16-item, self-report inventory that measures the frequency with which perceived memory difficulties occur in everyday life on a 5-point Likert-type scale that ranges from 1 (“never”) to 5 (“very often”). The PRMQ includes eight ProM complaints, which are separated into four Short-term items, half of which are self-cued (e.g., “Do you decide to do something in a few minutes’ time and then forget to do it?”) and half environmentally-cued (e.g., “Do you intend to take something with you before leaving a room or going out, but minutes later leave it behind, even though it’s there in front of you?”), and four Long-term items, also comprising self-cued (“Do you forget appointments if you are not prompted by someone else or by a reminder such as a calendar or diary?”) and environmentally-cued (e.g., “Do you forget to buy something you planned to buy, like a birthday card, even when you see the shop?”) ProM complaints.
We also administered clinical tests of executive functions and retrospective memory in order to examine the neurocognitive correlates of PM impairment in the XTC using participants. Numerous authors have underscored the multifactorial nature of executive functions (e.g., Miyake, 2000); therefore, measures assessing separable components of executive functions, including complex/divided attention, working memory, word generation, and decision-making, were administered. Complex/divided attention was assessed with the Trailmaking Test Part B (Reitan & Wolfson, 1985) and Stroop Neuropsychological Screening Test (Trennery, 1989). Working memory was assessed with the Letter Number Sequencing and Digit Span-Backwards subtests of the Wechsler Adult Intelligence Test – III (WAIS-III; The Psychological Corporation, 1997). Word generation was assessed with the Controlled Oral Word Association (CFL version; Benton et al 1994), Animal Naming (Piatt, Fields, Paolo, et al, 1999a), and Action Fluency Tests (Piatt, Fields, Paolo, et al, 1999b). Decision-making was assessed using the Iowa Gambling Task (Bechara, 2007), and the Balloon Analogue Risk Task (Lejuez, Read, Kahler, et al., 2002). Retrospective memory was assessed with the delayed free recall trials of the Rey Auditory Verbal Learning Test (Schmidt, 1996) and the Rey Complex Figure Test (Meyer & Meyer, 1995). Raw scores were converted to population-based z-scores and averaged across domains using standard, published methods (e.g., Iudicello, Weber, Grant, et al., 2011).
Ecstasy, alcohol and other drug (e.g., cannabis, cocaine, amphetamine, etc) use information (total lifetime units, frequency and duration of use, most recent use, and age at first use), as well as self-reported history of treatment or diagnosis of psychiatric and medical conditions (depression, anxiety, bipolar disorder, schizophrenia, ADHD, learning disorders, seizure disorder, traumatic brain injury, other neurologically relevant conditions) was collected by computer assisted interview. In addition, substance related problem behavior was assessed collected using the AUDIT and Drug Abuse Screening Test-20 (Skinner, 2001). Mood and emotional distress was assessed using the Depression, Anxiety and Stress Scale – 21 (DASS-21; Lovibond & Lovibond, 1995), self-reported daytime sleepiness was measured with the Epworth Sleepiness Scale (Johns, 1991), and estimated premorbid intelligence was assessed by the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001). Participants were reimbursed $35 AUD for time and travel expenses. The study procedures were approved by the Human Ethics Research Office at the University of Western Australia.
Results
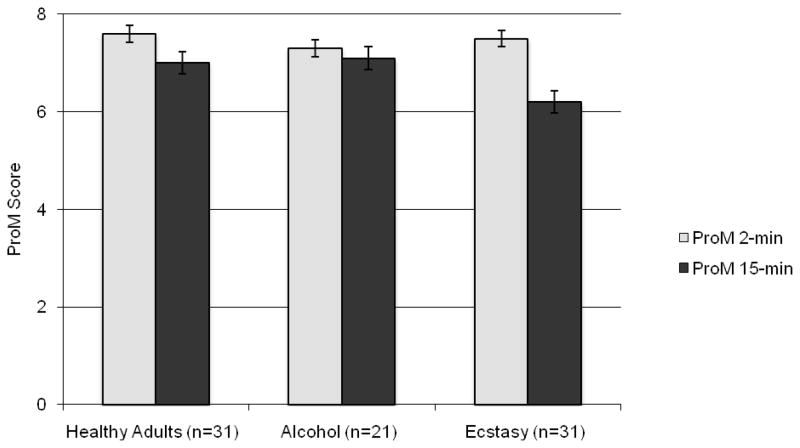
A repeated measures mixed-model ANOVA1 with substance use (i.e., XTC, ETOH, and HA) as the between-subjects variable and delay interval (i.e., 2-min vs. 15-min) as the within-subjects factor revealed a trend-level main effect of substance group [F (2,80) = 2.91, p = .06, ηp2 = .07)], and a significant main effect of delay interval [F (1,80) = 20.6, p < .001, ηp2 = .21]. However, interpretation of these main effects was tempered by a significant interaction between substance group and delay interval [F (2,80) = 4.02, p < .05, ηp2= .09]. As shown in Figure 1, follow-up one-way ANOVAs revealed no effect of group on the 2-min delay PM tasks [F (2,80) = .67, p > .10, ηp2= .02], but a significant between-subjects effect on the 15-min delay PM tasks [F (2,80) = 4.89, p < .05, ηp2 = .11]. Planned comparisons indicated that the XTC group performed significantly more poorly than the ETOH (p = .011, Cohen’s d = .70) and HA (p = .008, Cohen’s d = .67) groups. The HA and ETOH groups did not differ (p = .890). In order to assess the potential confounding influence of co-occuring substance use on PM performance in the above findings, correlations between 15-min PM task performance and other substances (i.e., marijuana, amphetamine, etc.) use within the XTC group were conducted (NB. due to limited substance use in the ETOH group, use of a covariate approach was not appropriate). No substances were significantly related to the 15-min scale (all rs < .25, all ps > .10).
Figure 1.
Bar chart displaying the interaction between prospective memory (ProM) delay interval (2- vs. 15-min) and ecstasy use in the study groups.
In order to determine if the XTC effect on the 15-min delay PM tasks varied by cue type (i.e., time- vs. event-based cues), separate one-way ANOVAs were conducted on the 2-item TB and EB tasks within the 15-min scale. Results revealed that groups differed on TB 15-min PM [F (2,80) = 4.88, p < .05, ηp2= .11], but not EB 15-min tasks [F (2,80) = .89, p > .10, ηp2 = .02], with the XTC group producing lower mean TB scores than the ETOH (p = .021, Cohen’s d = .59) and HA (p = .005, Cohen’s d = .71) groups, which did not differ (p = .801). Self-reported ProM complaints did not differ across groups for Short- (XTC group M = 11.42, SD = 2.17; ETOH M = 12.38, SD = 2.60; HA M = 10.97, SD = 3.06) or long (XTC M = 10.52, SD = 2.28; ETOH M = 11.71, SD = 2.83; HA M = 10.16, SD = 2.92) delays (all ps > .10). MIST 2- and 15-minute scales were not significantly related to Short- or Long-Delay PRMQ scores (all ps > .10).
With regard to the relationship between PM performance and ecstasy use variables, longer duration of use was associated with poorer performance on the 15-min scale (Spearman’s ρ = −.33, p = 0.066) at trend level, and the relationship was stronger for the 15-min TB tasks (ρ = − .50, p = 0.004). However, other ecstasy use variables, including lifetime doses, age at first use, frequency of use, or recency of use, were not related to PM performance (ps > .10). Self-reported Short- and Long-term ProM complaints were not significantly related to any ecstasy use variable (all ps > .10).
Correlational analyses revealed that the 2-min delay interval was not significantly related to retrospective memory or to any executive subdomain (all ps > .10). While the 15-min PM score was not related to the retrospective memory z-score (p > .10), there was a significant correlation with the decision-making z-score (r = .45, p = .014). No other correlation between the 15-min PM score and executive functions reached the level of statistical significance (all ps > .10). Self-rated ProM was not related to any executive or memory domains (all ps > .10).
Discussion
A handful of studies now show that ecstasy users are at increased risk of failures in ProM (e.g., Hadjiefthyvoulou, Fisk, Montgomery, et al, 2010 and 2011; Rendell, 2007), but the potential role of delay interval on the expression of ProM deficits was heretofore largely unknown. The current results support a deficit in ProM functioning amongst ecstasy users for longer (15 min) ongoing task delays, as compared with shorter (2 min) delays, where ecstasy users performed similarly to the high-risk alcohol and normal comparison samples. It is unlikely that these findings are an artifact of demographics, lifestyle factors, or affective distress, as the groups were well matched on these factors. Moreover, within the ecstasy group, use of cannabis and other substances was not related to ProM, whereas longer duration of ecstasy use was negatively associated with performance on long delay ProM, suggesting that these findings were not simply attributable to polysubstance use among the ecstasy users.
The pattern of results found in the present study are consistent with predictions based on the multiprocess theory: specifically, that ProM tasks requiring greater self-initiated strategic resources would be most affected among ecstasy users, who commonly evidence deficits in strategic RM encoding and executive functions (Zakzanis, 2007). In other words, our data suggest that ecstasy users experience difficulty with the complex processes of strategic target monitoring and maintenance of the cue-intention pairing over longer ongoing task delay intervals. This hypothesis is commensurate with recent evidence of poorer ProM performance on longer versus shorter delay task intervals in clinical samples with executive impairment, including HIV infection (Contardo et al., 2009) and traumatic brain injury (Tay et al., 2009). Moreover, Raskin et al. (2011) found that individuals with Parkinson’s disease displayed impairments for both 2- and 15-minute delays on the MIST relative to healthy adults, but that the effect size was notably greater for longer (Cohen’s d = .90) compared with shorter delays (d = .47). Considering the convergence of these clinical conditions on frontostriatal systems and associated executive dysfunction, one might reasonably assert that the observed interaction between ecstasy use and ProM cue delay may be most cogently attributed to strategic dyscontrol of monitoring and cue detection. Indeed, Rendell et al (2007) argued for a potential primary role of executive dysfunction in accounting for the ecstasy-related ProM deficit.
Consistent with this hypothesis, longer response delays produced the greatest effect for TB rather than EB tasks in the current study. This is most likely related to the increase in the executive load of TB ProM; that is, when tasks are equivalent on other relevant variables (e.g., cue focality) ProM for TB cues is thought to place greater demands on executive (i.e., self-initiated strategic monitoring and cue detection) processes than EB ProM, where retrieval is thought to be less effortful and prompted by external cueing (Einstein, McDaniel, Richardson, et al., 1995). The executive processes involved in TB ProM are thought to be resource intensive and differentially vulnerable to the effects of dysfunction of the frontostriatal pathways, for example in persons with Parkinson’s disease (e.g., Raskin et al., 2011). Consistent with this proposition, previous research has supported a link between TB ProM and performance on other measures of executive function, whereas EB ProM is more consistently related to measures of retrospective memory (e.g., Raskin, Woods, et al, 2011; Zogg, Woods, et al, 2011; Costa, Peppe et al, 2009).
Consistent with this conceptual argument, the present results revealed a relationship between long-delay ProM and the executive decision-making domain, which prior research shows is strongly linked to the integrity of prefrontostriatal circuits (e.g., Bechara et al., 2000). In fact, this association was stronger for long-delay tasks with TB cues than it was for EB cues, commensurate with the above-detailed executive demands theorized to be greater for the former versus the latter. We are unaware of other research reporting a specific relationship between ProM and laboratory measures of decision-making. However, Martin and colleagues found that ProM deficits were associated with self-report of a greater number of infection exposure-related risky behaviors (i.e., risky sexual and injection practices) among polysubstance abusers with HIV infection (Martin, Nixon, Pitrak, et al, 2007). They speculated that ProM and decision-making may share common cognitive mechanisms, including anticipation of future goals, but also that whether one engages in risky behaviors is likely influenced in part by the ability to plan, maintain and retrieve risk reduction strategies under the appropriate circumstances. Testing this hypothesis could be accomplished by experimental implementation of ProM strategic interventions (e.g., strategies to increase encoding, such as response visualization, or content-free cuing; Fish et al, 2007) in impaired groups and monitoring objective risk behaviors. Interestingly, long-delay ProM was not associated other executive function domains; however, the observed effect sizes suggest that these null findings may be a function of limited power, particularly in the case of the working memory domain.
Conversely, the current findings could be argued to represent the effects of increased demands on retrospective memory functions for ProM tasks with longer delays. Retrospective memory research has supported a decline in performance with increasing delay length (e.g., Wixted & Ebbeson, 1991), and retention of intention-cue pairs in ProM may similarly decay over time. The veracity of this alternate interpretation would be supported by differences in various aspects of RM in the present results. For example, the MIST includes a recognition trial in which participants are asked to identify all intentions associated with specific cues from distracter items, with poor performance indicative of inadequate encoding or retention of RM. A post hoc analysis revealed that groups did not differ on MIST recognition memory (p > .10), and indeed 90% of the participants in the ecstasy group achieved perfect scores on this scale. It is also possible that a more subtle retrieval deficit (i.e., of the appropriate intention-cue pair from RM) may have contributed to poorer ProM performance; however, we did not observe a significant relationship between the standard clinical RM and long delay ProM in the ecstasy-using group. Accordingly, these data do not support RM deficits as the primary factor driving the observed differential long-delay ProM deficit in ecstasy users, but future studies may wish to examine possible effects of increased RM load and even longer ProM delays to more carefully evaluate this notion. This may be particularly relevant as ProM delays involving hours and days may be associated with fewer strategic and more automatic monitoring processes as compared to the briefer paradigm adopted in this study (e.g., McDaniel & Einstein, 2000).
Contrary to our study hypotheses, we did not observe any effect of delay interval on self-reported ProM; in fact, the ecstasy group endorsed similar ProM complaints as both the high-risk alcohol and healthy comparison groups. This was unexpected and runs in contrast to findings from a host of previous studies that have shown that ecstasy users reliably report experiencing elevated ProM failures in their daily lives (e.g., Heffernan, Ling, & Scholey, 2001; Rodgers et al., 2001; Rodgers et al., 2003). However, these prior studies did not reliably consider the potentially confounding effects of affective distress, which can be observed at least to a modest degree in ecstasy users (e.g., Sumnall & Cole, 2005). For example, Bedi and Redman found that anxiety and depression confounded self-reported memory complaints amongst ecstasy users, perhaps due to the effects of mood-related negative self-appraisals (2008b). The ecstasy users in the current study were not significantly distressed (i.e., 94% of the ecstasy participants scored in the normal range on the DASS-21 Depression scale, and all elevated profiles were only mild in severity). Furthermore, at the group level, the ecstasy users did not differ from the high-risk alcohol or healthy comparison groups in overall affective distress. It is likely, therefore, that low levels of distress and group equivalence at least partly explain the absence of self-reported ProM findings in the current study.
Questions remain as to the extent to which ecstasy use patterns (e.g., quantity, frequency, duration, age at onset, etc.) relate to ProM. Although a prior meta-analysis found that greater lifetime quantity of ecstasy use is associated with worse cognitive outcomes (Zakzanis et al., 2007), this relationship has not been reliably observed in the ProM literature. In the current study, only duration of ecstasy use was significantly associated with performance on long delay ProM. Prior studies of ProM amongst ecstasy users have sometimes (e.g., Rendell, 2007), but not always (Hadjiefthyvoulou et al; 2010) found frequency of ecstasy to be predictive of ProM impairment. This level of inconsistency in directly linking cognition to substance use patterns is by no means unique to ecstasy (e.g., Scott et al., 2007), and likely reflects the poor reliability of self-report and semi-structured interview techniques for gathering and coding historical use variables across substances. Moreover, the amount and nature of information collected specific to participant ecstasy use patterns is highly variable across the literature, thereby making clear conclusions about what aspects of ecstasy use habits are most relevant to neurotoxicity difficult to ascertain in naturalistic studies (cf. controlled experiments; e.g., Ramaekers et al. [2009]).
Limitations of the current study are similar to those found in many studies of neurocognition among substance abusers (e.g., limited sample sizes, polysubstance use, use of self-report methodology to collect substance use information, and relatively short periods of abstinence from alcohol and other substances). In addition, our sample of ecstasy users had a relatively low level of lifetime use (median 30 units). It is notable, however, that we were still able to detect an effect of ecstasy relative to high risk alcohol users and healthy comparisons on ProM, which is consistent with other studies that have observed neurocognitive deficits in similarly lower-frequency ecstasy samples (e.g., Yip & Lee, 2005). Further, the present study collected extensive information about use of co-occurring substances and used a comparative group with similar alcohol consumption patterns. The pattern of results and lack of relationship found between other substance use variables and ProM would support that the delay-related deficits found are likely related to ecstasy use, and not simply an artifact of polysubstance use alone. However, the use of a non-ecstasy using comparison group matched on both alcohol and marijuana use variables would be desirable in future studies. Of note, the present results may also have been influenced by the relative differences in difficulty between short and long delay tasks (Chapman & Chapman, 1973). That is, we cannot rule out the possibility that the observed group by delay effects were due to the relatively greater difficulty of long delay tasks. Finally, the design of our study is limited by the use of overlapping delay (i.e., 2- and 15-min) and cue type (i.e., time- and event-based) trials embedded in a clinical measure, which future investigations may rectify by using a parametric design and randomized, separate trials of psychometrically comparable tasks across systematically manipulated delay intervals.
The increasing importance of improving the ecological validity of neuropsychological evaluations has been noted (e.g., Chaytor, Schmitter-Edgecombe & Burr, 2006), and ProM has displayed promise as a predictor of functional status. For example, previous research has found ProM to be a unique predictor of declines in general instrumental activities of daily living (e.g., Woods et al., 2008), unemployment (Woods et al, 2011), and daily living skills (e.g., Twamley et al., 2008) amongst some clinical samples. The association between ProM and ecstasy has potential implications for academic, employment and higher level functioning, as well as treatment of ecstasy users. ProM impairments may be especially relevant in the population of young adults, as significant ProM-related problems may arise in this important transitional period, such as failure to attend classes and failing to complete important academic or job-related tasks. With regard to the potential treatment implications of these data, users do not commonly present for treatment identifying a problem with ecstasy abuse (El-Mallakh & Abraham, 2007). Instead it is more common for individuals with a history of ecstasy use to report other substances (alcohol, marijuana, cocaine) as primary substances of abuse (Maxwell & Spence, 2005). However, the evidence for ProM deficits amongst ecstasy users, regardless of identified primary substance, would suggest that it is important for treatment providers to consider the potential effects of memory difficulties. For example, difficulties in learning and remembering coping strategies or completion of homework tasks may interfere with cognitive behavioral interventions. Additionally, use of serotonin selective reuptake inhibitors (El-Mallakh & Abraham, 2007) and anticholinesterase inhibitors (Jovanovski & Zakzanis, 2003), has been raised as a potential treatment for ecstasy abuse and ProM has demonstrated unique predictive ability for medication adherence (Zogg et al, in press). Further research to determine whether ProM deficits translate into functional decrements (e.g., social and occupational function, quality of life) amongst ecstasy users is needed.
Acknowledgments
This research was supported by a 2008 Research Development Scheme Award from the University of Western Australia to MW and National Institute of Mental Health R01-MH73419 to SPW. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. Aspects of these data were presented at the 2011 Mid-Year Meeting of the International Neuropsychological Society in Auckland, New Zealand. The authors extend their gratitude to Dr. Sarah Raskin for providing us with the MIST and to students (Helen Shield, Simone Fernandez, Amy Hamilton, Gillian Wakeford, Dianna Bradley, and Whitney Swope) for their assistance with data collection and processing.
Footnotes
MIST data did not meet assumptions for parametric approaches due to a significant negative skew. As there is no nonparametric equivalent, and ANOVA-based approaches are robust to violations of normality given adequate sample sizes (e.g., Howell, 2005), the mixed-model ANOVA remained the most appropriate approach. However, results using Wilcoxon Ranked Sum Tests produced identical results.
References
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. World Health Organisation: Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- Bechara A. Iowa Gambling Task Professional Manual. Lutz, FL: Psychological Assessment Resources; 2007. [Google Scholar]
- Bechara A, Damasio H, Damasio A. Emotion, decision-making, and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bedi G, Redman J. Ecstasy use and higher-level cognitive functions: weak effects of ecstasy after control for potential confounds. Psychological Medicine. 2008a;38:1319–1330. doi: 10.1017/S0033291708002730. [DOI] [PubMed] [Google Scholar]
- Bedi G, Redman J. Metamemory in recreational ecstasy polydrug users: what do self-reports of memory failures mean? Journal of Psychopharmacology. 2008b;22:872–881. doi: 10.1177/0269881107083811. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1994. [Google Scholar]
- Brown J, McKone E, Ward J. Deficits of long-term memory in ecstasy users are related to cognitive complexity of the task. Psychopharmacology. 2010;209:51–67. doi: 10.1007/s00213-009-1766-2. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, Schulze O, Schmidt U, Clausen M. A voxel-based PET investigation of the long-term effects of “Ecstasy” consumption on brain serotonin transporters. American Journal of Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Petersen K, Wilke F, Obrocki J, Nebeling B, Wartberg L, Zapletova P, Clausen M. Reversibility of ecstasy-induced reduction in serotonin transporter availability in polydrug ecstasy users. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:188–199. doi: 10.1007/s00259-005-1850-8. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia. doi: 10.1016/j.neuropsychologia.2011.02.014. in press. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1973;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychology Review. 2003;13:181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychological Assessment. 1998;10:408–414. [Google Scholar]
- Contardo C, Black AC, Beauvais J, Dieckhaus K, Rosen MI. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives of Clinical Neuropsychology. 2009;24:547–554. doi: 10.1093/arclin/acp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Peppe A, Caltagirone C, Carlesimo GA. Prospective memory impairment in individuals with Parkinson’s disease. Neuropsychology. 2008;22:283–292. doi: 10.1037/0894-4105.22.3.283. [DOI] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology. 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Einstein G, McDaniel M, Thomas R, Mayfield S, Shank H, Morrisette N, et al. Multiple processes in prospective memory retrieval: Factors determinining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: Applied. 2005;9:147–162. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Einstein G, McDaniel M, Richardson S, Guynn M, Cunfer A. Aging and prospective memory: Examining the influences of self-initiated retrieval processes. Journal of Experimental Psychology: Learning, Memory and Cognition. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- El-Mallakh R, Abraham H. MDMA (Ecstasy) Annals of Clinical Psychiatry. 2007;19(1):45–52. doi: 10.1080/10401230601163592. [DOI] [PubMed] [Google Scholar]
- Fish J, Evans J, Nimmo M, Martin E, Kersel D, et al. Rehabilitation of executive function following brain injury: “Content-free” cueing improves everyday prospective memory performance. Neuropsychologia. 2007;45:1318–1330. doi: 10.1016/j.neuropsychologia.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Hadjiefthyvoulou F, Fisk J, Montgomery C, Bridges N. Everyday and prospective memory deficits in ecstasy/polydrug users. Journal of Psychopharmacology. 2010;25:453–464. doi: 10.1177/0269881109359101. [DOI] [PubMed] [Google Scholar]
- Hadjiefthyvoulou F, Fisk J, Montgomery C, Bridges N. Prospective memory functioning among ecstasy/polydrug users: evidence from the Cambridge Prospective Memory Test (CAMPROMPT) Psychopharmacology. 2011;215:761–774. doi: 10.1007/s00213-011-2174-y. [DOI] [PubMed] [Google Scholar]
- Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/--)3,4-methylenedioxymethamphetamine seven years previously: Factors influencing abnormal recovery. Journal of Neuroscience. 1999;19:5096–5107. doi: 10.1523/JNEUROSCI.19-12-05096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TM, Ling J, Scholey AB. Subjective ratings of prospective memory deficits in MDMA (‘ecstasy’) users. Human Psychopharmacology: Clinical and Experimental. 2001;16:339–344. doi: 10.1002/hup.290. [DOI] [PubMed] [Google Scholar]
- Heffernan TM, Jarvis H, Rodgers J, Scholey AB, Ling J. Prospective memory, everyday cognitive failure and central executive function in recreational users of Ecstasy. Human Psychopharmacological: Clinical and Experimental. 2001;16:607–612. doi: 10.1002/hup.349. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 5. Belmont, CA: Thomson Wadsworth; 2005. [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP The HIV Neurobehavioral Research Center (HNRC) Group. Misremembering future intention in methamphetamine dependent individuals. The Clinical Neuropsychologist. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness – The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Zakzanis K. Donepezil in a chronic drug user--a potential treatment? Human Psychopharmacology. 2003;18(7):561–4. doi: 10.1002/hup.530. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, Mahoney JJ, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology. 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jager T, Altgasen M, Sum D. Clinical neuropsychology of prospective memory. In: Kliegel M, MecDaniel MA, Einstein GO, editors. Prospective Memory: Cognitive, Neuroscience, Developmental, and Applied Perspectives. New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. pp. 283–308. [Google Scholar]
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Human Psychopharmacoloy: Clinical and Experimental. 2007;22:381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioural measure of risk taking: the balloon analogue risk task (BART) Journal of Experimental Psychology – Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney: Psychology Foundation; 1995. [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, et al. Characteristics of prospective memory deficits in HIV-seropositive substance dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Martin BA, Brown NL, Hicks JL. Ongoing task delays affect prospective memory more powerfully than filler task delays. Canadian Journal of Experimental Psychology – Revue Canadienne de Psychologie Experimentale. 2011;65:48–56. doi: 10.1037/a0022872. [DOI] [PubMed] [Google Scholar]
- Maxwell J, Spence R. Profiles of Club Drug Users in Treatment. Substance Use & Misuse. 2005;40:1409–1426. doi: 10.1081/JA-200066968. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of neurotoxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Glisky EL, Rubin SR, Guynn MJ, Routhieaus BC. Prospective memory: a neuropsychological study. Neuropsychology. 1999;13:103–110. doi: 10.1037//0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective remembering: a multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Odessa, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Fisk JE. Everyday memory deficits in ecstasy-polydrug users. Journal of Psychopharmacology. 2007;21:709–717. doi: 10.1177/0269881107077220. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Hatton N, Fisk J, Ogden R, Jansari A. Assessing the functional significance of ecstasy-related memory deficits using a virtual paradigm. Human Psychopharmacology: Clinical and Experimental. 2010;25:318–325. doi: 10.1002/hup.1119. [DOI] [PubMed] [Google Scholar]
- Naimi T, Brewer R, Makdad A, Denny C, Serdula M, Marks J. Binge drinking among US adults. The Journal of the American Medical Association. 2003;289 (1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Nulsen CE, Fox AM, Hammond GR. Differential effects of ecstasy on short-term and working memory: A meta-analysis. Neuropsychology Review. 2010;20:21–32. doi: 10.1007/s11065-009-9124-z. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Buchert R, Vaterlein O, Thomasius R, Beyer W, Schiemann T. Ecstasy – long term effects on the human central nervous system revealed by positron emission tomography. British Journal of Psychiatry. 1999;175:186–188. doi: 10.1192/bjp.175.2.186. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Tröster AI. Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. Journal of Clinical and Experimental Neuropsychology. 1999a;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Neuropsychologia. 1999b;37:1499–1503. doi: 10.1016/s0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Ramaekers JG, Kuypers KPC, Wingen M, Heinecke A, Formisano E. Involvement of inferior parietal lobules in prospective memory impairment during acute MDMA (ecstasy) intoxication: an event-related fMRI study. Neuropsychopharmacology. 2009;34:1641–1648. doi: 10.1038/npp.2008.219. [DOI] [PubMed] [Google Scholar]
- Raskin S, Buckheit C, Sherrod C. MIST Memory for Intentions Test professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2010. [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, Troster AI. A differential deficit in time-versus event-based prospective memory in Parkinson’s disease. Neuropsychology. 2011;25(2):201–209. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin SA. Memory for intentions screening test: psychometric properties and clinical evidence. Brain Impairment. 2009;10:23–33. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rendell P, Gray T, Henry J, Tolan A. Prospective memory impairment in “ecstasy” (MDMA) users. Psychopharmacology. 2007;194:497–504. doi: 10.1007/s00213-007-0859-z. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Buchanan T, Scholey AB, Heffernan TM, Ling J, Parrott A. Patterns of drug use and the influence of gender on self-reports of memory ability in ecstasy users: a web-based study. Journal of Psychopharmacology. 2003;17:389–396. doi: 10.1177/0269881103174016. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Buchanan T, Scholey AB, Heffernan TM, Ling J, Parrott A. Differential effects of ecstasy and cannabis on self-reports of memory ability: a web based study. Human Psychopharmacology: Clinical and Experimental. 2001;16:619–625. doi: 10.1002/hup.345. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of ecstasy [3,4-methylenedioxymethamphetamine (MDMA)] – serotonin transporters are targets for MDMA-induced serotonin release. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory and Verbal Learning Test: A handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Assessment of substance abuse: Drug Abuse Screening Test. In: Carson-DeWitt R, editor. Encyclopedia of drugs, alcohol and addictive behavior. 2. Durham: NC7 Macmillan Reference USA; 2001. [Google Scholar]
- Smith GV, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Sumnall H, Cole J. Self-reported depressive symptomatology in community samples of polysubstance misusers who report Ecstasy use: A meta-analysis. Journal of Psychopharmacology. 2005;19(1):84–92. doi: 10.1177/0269881105048901. [DOI] [PubMed] [Google Scholar]
- Tay SY, Ang BT, Lau XY, Meyyappan A, Collinson SL. Chronic impairment of prospective memory after mild traumatic brain injury. Journal of Neurotrauma. 2010;27:77–83. doi: 10.1089/neu.2009.1074. [DOI] [PubMed] [Google Scholar]
- Trenerry MR. Stroop neuropsychological screening test manual. Odessa FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Twamley EW, Woods SP, Zurhellen CH, Vertinski M, Narvaez JM, Mausbach BT, Patterson TL, Jeste DV. Neuropsychological substrates and everyday functioning implications of prospective memory impairment in schizophrenia. Schizophrenia Research. 2008;106:42–49. doi: 10.1016/j.schres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Hall K, Haslam C. Patterns of memory dysfunction in current and 2-year abstinent MDMA users. Journal of Clininical and Experimental Neuropsychology. 28:306–24. doi: 10.1080/13803390490918174. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Ebbeson EB. On the form of forgetting. Psychological Science. 1991;2:409–415. [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, *Carey CL, Dawson MS, Grant I The HNRC Group. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I The HNRC Group. Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist. 2008;22:864–878. doi: 10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz BM, Twamley EW, Grant I. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56:77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky G. Amphetamine toxicities: classical and emerging mechanisms. Addiction Reviews 2. Annals of the New York Academy of Sciences. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip JTH, Lee TMC. Effect of ecstasy use on neuropsychological function: a study in Hong Kong. Psychopharmacology. 2005;179:620–628. doi: 10.1007/s00213-004-2083-4. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of MDMA use: a quantitative review. Human Psychopharmacology: Clinical and Experimental. 2007;22:427–435. doi: 10.1002/hup.873. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Young DA, Campbell Z. Recreational MDMA (“ecstasy”) users exhibit deficits in episodic prospective memory. Clinical Neuropsychologist. 2003;17:92–93. [Google Scholar]
- Zogg J, Woods SP, Doyle K, *Weber E, Grant I The HNRC Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg J, Woods SP, Sauceda JA, Weibe JM, Simone JS. The role of prospective memory in medication adherence: A critical review of an emerging literature. Journal of Behavioral Medicine. doi: 10.1007/s10865-011-9341-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]