Abstract
Background
Food allergy is a common and increasing health concern in westernized countries. No effective treatment is available and accidental ingestion can be life threatening. Food allergy herbal formula-2 (FAHF-2) blocks peanut anaphylaxis in a murine model of peanut-induced anaphylaxis. It was found to be safe, and well tolerated in an acute phase I study of food allergic patients.
Objective
To assess the safety of FAHF-2 in an extended phase I clinical trial and determine potential effects on peripheral blood basophils from food allergic patients.
Methods
Patients in an open-label study received 3.3 grams (6 tablets) of FAHF-2 three times a day for 6 months. Vital signs, physical examinations, laboratory data, pulmonary function tests and electrocardiographic data were acquired at baseline and at 2 month intervals. During the course of the study, basophil activation and basophil and eosinophil numbers were evaluated using CCR3/ CD63 staining and flow cytometry.
Results
Of eighteen patients enrolled, 14 completed the study. No significant drug-associated differences in laboratory parameters, pulmonary function studies, or electrocardiographic findings before and after treatment were found. There was a significant reduction (p<.010) in basophil CD63 expression in response to ex vivo stimulation at month 6. There was also a trend towards a reduction of eosinophil and basophil numbers after treatment.
Conclusion
FAHF-2 was safe, well-tolerated, and had an inhibitory effects on basophils in an extended phase I clinical study. A controlled phase II study is warranted.
Clinical Implications
FAHF-2 was safe, well-tolerated and inhibited basophils numbers and activation in a 6 month clinical trial for food allergic patients. FAHF-2 may provide a safe immunotherapeutic option for food allergic patients.
Capsule Summary
FAHF-2 was safe and well-tolerated in a six-month phase-I open label clinical trial for food allergy patients. Immunological beneficial effects of FAHF-2 were decreased basophil numbers and inhibition of activation.
Keywords: Food allergy, FAHF-2, Basophil activation
INTRODUCTION
Food-induced allergic reactions affect approximately 5% of young children and 3–4% of adults (1). To date, the treatment for food allergy involves avoidance of the triggering food. (2) However, accidental ingestions of the allergic food substances can happen and can lead to potentially life-threatening anaphylaxis. In addition, food allergies also negatively impact the quality of life of the patient. Development of safe and effective interventions for food allergy is a priority.
Traditional Chinese medicine (TCM) has a long human use history in China and other Asian countries, such as Korea and Japan, for treating and preventing disease, and is part of main stream medicine in these countries. TCM is beginning to play a role in the United States (US). Acupuncture needles have been approved by the US Food and Drug Administration (FDA) as a medical device. Traditional Chinese herbal medicines are viewed as dietary supplements and the cost of these preparations is not covered by insurance. However, this situation may change in the future if herbal medicines are shown to be effective. The US FDA provides guidance for investigating botanical drug products, including complex formulas containing several herbs, focusing on efficacy, safety and consistency.(3) Several publications including ours indicate that TCM has potential for treating asthma,(4–9) food allergy, (10–13) managing allergic rhinitis (14) and improving the quality of life of atopic dermatitis patients. (15) Therefore some TCM remedies may become botanical prescription drugs via clinical investigation. Food Allergy Herbal Formula-2 (FAHF-2), derived from Wu Mei Wan, used to treat parasite infection and food allergy-like symptoms (16) is an extract of nine herbs. We previously showed that FAHF-2 completely protects against peanut-induced anaphylactic symptoms in the peanut allergic murine model,(10;11) and that this protection persists for at least 6 months post-therapy following a single 7 wk course of treatment.(12) Clinical protection was associated with reduction of Th2 cytokines and serum IgE levels and increased levels of IFN-γ and IgG2a. Furthermore, we recently showed that FAHF-2 reduced basophil and mast cell numbers and mast cell activation, independent from FAHF-2 mediated suppression of IgE and enhancement of IFN-γ.(12;13) Thus, FAHF-2 has immunotherapeutic effects on both T/B lymphocytes and effector cells such as mast cells and basophils. Previous animal studies showed that FAHF-2 has a high ‘margin of safety’ because mice fed 24 times the effective dose of FAHF-2 showed no mortality or morbidity(11). This preclinical study provided the rationale for a clinical trial of FAHF-2 aimed at developing a botanical drug for treating food allergies. We received approval on an IND for FAHF-2 from the US FDA and completed an acute, one week, randomized double-blinded placebo controlled, dose escalation phase I trial in subjects with peanut and/or tree nut, fish and shellfish allergies. In that acute phase I study, 18 patients received one of three doses of FAHF-2 or placebo: 2.2g (4 tablets), 3.3g (6 tablets), 6.6g (12 tablets) 3 times a day for 7 days. The results showed that FAHF-2 was safe and well-tolerated over a one week course of treatment and that in vitro treatment of patient peripheral blood mononuclear cells (PBMCs) with FAHF-2 reduced IL-5, and increased IFN-γ and IL-10 secretion. (17)
Basophils comprise less than 1% of leukocytes, but are critical to allergic reactions, as are tissue mast cells. CCR3 is a well established basophil marker by flow cytometry.(18) CD63 expression, a marker of basophil activation, (19) correlates with release of histamine from intracytoplasmic granules and is triggered by IgE and allergen cross-linking of FcεRI receptors. The basophil activation test (BAT) is a flow cytometry-based assay that detects basophil CD63 expression, requires the use of very small quantities of blood, and does not require isolation of cells. The real time flow cytometry read-out makes this assay a convenient tool for longitudinal clinical studies. (20–23) A recent study by Jones et al. found that basophil activation was significantly reduced by 4–6 months of oral immunotherapy (OIT) (20) and that inhibition of basophils, but not IgE correlated with clinical protection.
Since peanut, tree nut, fish, and shellfish allergies are typically lifelong, chronic treatment is essential. Long term safety of human FAHF-2 consumption has not been assessed. To further ensure the safety and tolerability of this formulation prior to phase II study, we conducted an open label, single dose, 6 month extension of the phase I study. Although the primary outcome of this study was to evaluate the 6 month safety and tolerability of FAHF-2, based on substantial animal study results, and the fact that this is a 6 month study, we hypothesized that FAHF-2 may have induced some immunotherapeutic effects on basophils.
METHODS
Study patients
Food allergic individuals ages 12 through 45 years of age with a convincing history of allergy to peanut (PN), tree nut (TN), fish or shellfish as documented by a positive skin test (mean wheal diameter >3 mm greater than the mean of saline control) and/or food allergen-specific IgE level (PN, TN, fish or shellfish specific IgE > 0.7 kUA/L) were eligible for the study. Females of childbearing potential were included but had to be sexually inactive or using effective birth control measures, as deemed appropriate by the investigator, for the duration of the study.
Exclusion criteria included acute infection, history of systemic diseases, abnormal hepatic, bone marrow or renal function, clinically significant abnormal electrocardiogram, current uncontrolled moderate to severe asthma with FEV1 <80% predicted, drug or alcohol abuse, pregnancy or lactation, and participation in another research protocol within the previous 30 days.
This study was approved by the Mount Sinai Medical School Institutional Review Board. Written informed consent was obtained prior to enrollment.
Study Design
The initial evaluation consisted of a thorough medical history and physical examination, vital signs, skin prick testing and food-specific IgE testing, baseline pulmonary function tests, electrocardiogram, urinalysis, and routine laboratory blood tests (complete blood count, serum chemistries, renal function, liver function tests, and pregnancy test for female participants).
After initial screening, subjects were started on FAHF-2 (3.3 grams - 6 tablets) three times a day for 6 months. Subjects continued food allergen avoidance for the duration of the study, and were asked to refrain from other herbal medication use. Subjects were seen every eight weeks and telephoned every two weeks by study investigators to reinforce and confirm medication compliance and assess potential adverse events (AEs). Subjects were instructed to complete a symptom diary during participation in the trial. At each study visit, the interim medical history was reviewed and physical exam, spirometry, electrocardiogram and laboratory studies were repeated.
Study Medication
FAHF-2 was in the form of tablets (0.5g/tablet) produced by Xiyuan Chinese Medicine Research and Pharmaceutical Manufacturer, China. The same batch of FAHF-2 that was used in the acute phase I study (17) was used for this open-label study. The quality of raw herbs, manufacturing process and quality control of the final FAHF-2 products were established according to FDA guidance under the botanical drug title (Chemical, Manufacturing, and Control Data [21 CFR 312.23(a) (7)]) as published previously. (17)
We generated the HPLC fingerprint of FAHF-2 as a means of standardizing the FAHF-2 product (17) and to monitor its consistency and shelf-life. On line repository Figure E1 shows the consistency of HPLC fingerprints obtained July 2007 (acute phase I study period, Figure E1A) and July 2008 (current study period, Figure E1B) carried-out on the same batch of tablets. The HPLC methods were described previously. (17)
Study procedures
Skin prick testing
Prior to enrollment and at the end of the 6 months treatment phase, titrated skin prick tests (SPTs) were performed in duplicate with serial 10-fold dilutions [1:20 to 1:200,000] of stock PN, or individual TN, fish and/or shellfish extract [Greer Laboratories; Lenoir, NC]. Negative controls [phenol-saline solution] and positive controls [1 mg/ml histamine base] were also included. SPTs were performed by pricking through a drop of extract with a bifurcated needle. A mean wheal diameter of >3 mm than the negative control was considered a positive response.
Allergen-specific IgE measurements
At each study visit, specific IgE to peanut, treenut, fish, and/or shellfish was detected using the ImmunoCAP® (Phadia, Uppsala, Sweden).
Safety Monitoring
Patients were monitored for potential AEs throughout the study based on our AE criteria table (17). AE criteria were adapted from the World Health Organization (WHO) Recommendations for Grading of Acute and Subacute Toxicity (24) with the following modifications; Grade 1 AEs under the WHO grading were considered as Grade 3 AEs in our study, making our criteria for AEs much more stringent. This grading system was approved by the Food and Drug Administration for our phase I clinical trial. According to the study protocol, any AE of grade 3 in an FAHF-2-treated subject would result in immediate discontinuation of the study medication in that subject.
Basophil Activation Test (BAT)
Basophil activation assays were performed using the Flow 2 CAST kit (Alpco Diagnostics, NH, US). BAT was performed at 0 month (pre-treatment) and at three time-points: 2, 4 and 6 months of treatment. Crude peanut, pecan, hazelnut and cashew extracts prepared using the same protocol previously described (25) were used as specific antigens for ex vivo stimulation. Since the study subjects were allergic to peanut, tree nuts, fish and/or shellfish, the allergen was chosen based on the following criteria: If a subject was allergic to peanut and tree nuts, and possibly other foods, the cells were stimulated only with peanut allergen extract (CPE). If a subject was allergic to tree nuts and other foods but not peanut, the cells were stimulated with one specific tree nut allergen. In this BAT assay, a stimulation buffer containing IL-3 was used as the negative control. Antigen and a highly specific monoclonal antibody (mAb) recognizing the high affinity IgE binding receptor (FcεRI) was used as a positive control and N-formyl-methionyl-leucyl-phenylalanine (fMLP) as non-specific positive control, according to the manufacturer’s instruction. Briefly, 100μl of stimulation buffer containing IL-3, heparin and calcium was added to six aliquots of 50μl of heparinized blood. 50μl of either stimulation buffer, anti-FcεRI, N-formyl-methionyl-leucyl-phenylalanine (fMLP) or allergen (200ng/mL, 200pg/mL and 0.2pg/mL) was added to each aliquot. 20μl of staining reagent containing anti-CCR3-fluorescein isothiocynate and anti-CD63-phycoerythrin labeled monoclonal antibodies was added. The tubes were then incubated at 37°C in a water bath in the dark for 15mins. The red blood cells were lysed and cells were resuspended in wash buffer and acquired on a LSR-II flow cytometer (BD Biosciences, CA). Basophils and eosinophils were gated as CCR3+ cells and segregated on the basis of Side Scatter. CCR3+ cells with low Side Scatter were considered basophils, and among these the CD63+ cells were termed “activated basophils”. A total of 50,000–100,000 leukocytes were acquired and ≥300 basophils were used for analysis by FlowJo software, version 5.4 (Tree Star, Inc, Ashland, Ore).
Statistical Analysis
Of the patients who completed this 6 month long-term, open-label extension safety trial, 86% were the same subjects that were enrolled in the initial acute Phase I trial. All subjects received FAHF-2. Formal statistical methods were not used to calculate the sample size. The number of subjects was chosen to minimize risk by not exposing a large number of subjects to treatment, but still provide a sample size sufficient to gain impressions of chronic safety and possible preliminary immunological effects.
The ex vivo immunological data were analyzed as follows: A mixed model analyses was performed to evaluate effect of time on the outcomes examined in the context of allergen specific basophil activity (three doses of allergen stimulation, stimulation buffer, anti-FcεRI, fMLP). For the initial analysis we assumed the correlation between times was the same (compound symmetry) since this leads to a more robust analysis in which outliers cannot have an undue effect. If this analysis showed any trend towards significance, we carefully evaluated more complex models, in particular the unstructured model which makes no assumptions concerning the pattern of correlations. If there was a difference between the four time points, pairwise comparisons were performed to compare months 2, 4 and 6 to baseline. A Bonferroni correction was used for multiple testing. Wilcoxon signed rank test (nonparametric) was performed to evaluate the difference between percent basophils and eosinophils before and after 6 months of FAHF-2 treatment. A p value <0.05 was considered to be statistically significant. These statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute, Cary, NC) ; in particular PROC GENMOD was used for the repeated measurements analysis and PROC UNIVARIATE for the changes in basophils and eosinophils.
RESULTS
Subject characteristics
Eighteen subjects were enrolled in this study. Four patients withdrew; one due to pregnancy and 2 for difficulty with compliance related to time commitment, high number of tablets required daily, and 1 with transient abdominal complaints, without vomiting or diarrhea. Fourteen patients completed the 6 month treatment. Baseline characteristics of study subjects are shown in Table 1. The median age of the patients was 16 years (range, 12–27 years) and 67% were males. Peanut allergy often co-exists with other food allergies; 77% of peanut allergic patients were also allergic to at least one tree nut with or without shellfish/fish allergies, 17% were allergic to a tree nut only, and 11% to tree nut and shellfish/fish. No patient was allergic only to peanut. In this cohort, 95% of study subjects exhibited other allergic diseases including asthma (11%), allergic rhinitis (6%), asthma and allergic rhinitis (22%), asthma and atopic dermatitis (22%), allergic rhinitis and atopic dermatitis (6%), asthma, allergic rhinitis and atopic dermatitis (28%) and no other allergies (5%). Allergen specific IgE levels were 25 kUA/L (5–50) (median (IQR), 100 kUA/L was the upper limit) and total IgE levels were 360 kIU/L (190–794), median (IQR).
Table 1.
Demographics of the patients enrolled in the 6 month extended phase I clinical study
| Patient | Age (yrs) | Sex | Food Allergies | Other Allergies | Allergen- Specific IgE (kUA/L) [all peanut, where indicated] | Total IgE (kIU/L) | Time since last reaction to allergen |
|---|---|---|---|---|---|---|---|
| 1 | 22 | M | PN, TN, sesame | Asthma | >100 | 1440 | 21 years |
| 2 | 15 | M | PN, TN, sesame, SF, milk | Asthma, AD | 7.59 | 1455 | Never ingested |
| 3 | 18 | M | PN, TN, milk, egg, legumes | Asthma, AR | 4.37 | 284 | 14 yrs |
| 4 | 18 | F | PN, TN, fish | Asthma, AR | >100 | 529 | 17 yrs |
| 5 | 19 | F | PN, TN, sesame | Asthma, AR | 18.4 | 600 | 6 mo |
| 6 | 16 | F | PN, TN, sesame | Asthma, AD | 76.7 | 254 | 1 yr |
| 7 | 23 | M | TN | None | 9.35 (hazelnut) | 106 | Never ingested |
| 8 | 16 | M | PN, TN, soy, legumes | Asthma, AR, AD | 52.2 | 168 | 10 yr |
| 9 | 12 | M | PN, TN | AR, AD | 24.9 | 142 | 7 yr |
| 10 | 15 | M | PN, TN, fish, SF | Asthma, AR, AD | 3.79 | 2877 | 14 yr |
| 11 | 16 | M | PN, TN, seeds, egg | Asthma, AD | 4.43 | 1067 | 15 yr |
| 12 | 16 | M | PN, TN, seeds, egg | Asthma, AR, AD | 28 | 254 | 14 yr |
| 13 | 17 | F | TN, seeds, fish, SF, egg | Asthma, AR, AD | 3.03 (pecan) | 681 | 1 yr |
| 14 | 12 | M | TN | Asthma, AR, AD | 41.4 (cashew) | 831 | 1 yr |
| 15 | 12 | M | TN | Asthma, AR | 3.40 (pecan) | 56 | 4 yr |
| 16 | 17 | F | TN, SF | AR | 25.0 (pecan) | 418 | 12 yr |
| 17 | 12 | M | PN, TN | Asthma | 71.1 | 320 | 10 yr |
| 18 | 27 | F | PN, TN, fish, SF | Asthma, AD | 36.2 | 68 | 3 mo |
Abbrevations: PN = peanut, TN = treenut, SF = shellfish, AD = atopic dermatitis, AR = allergic rhinits
Laboratory testing results
There were no changes in hematology or chemistry laboratory values, pulmonary function studies or electrocardiogram findings obtained at baseline, at 2 months intervals, or after completing 6 months of FAHF-2 treatment (Table 2). There were no changes in SPTs results at baseline or after 6 months of FAHF-2 treatment.
Table 2.
Summary of laboratory results for subjects completing the extended Phase 1 trial (6 months of treatment with FAHF-2)
| Component | FAHF-2 (N=14) | Reference range | |
|---|---|---|---|
| Pre-treatment | Post-treatment | ||
| Glucose (mg/dL) | 72 (16) | 69 (24.8) | 60–120 |
| Sodium (meq/L) | 140 (2) | 140 (1.4) | 135–145 |
| Potassium (meq/L) | 3.9 (0.3) | 3.9 (0.2) | 3.5–5 |
| Chloride (meq/L) | 101 (2) | 101 (2) | 96–108 |
| CO2 (meq/L) | 27.7 (2.0) | 26.6 (2.2) | 22.0–32 |
| Urea (mg/dL) | 14 (2.5) | 13 (3.0) | 11–25 |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.8 (0.2) | 0.4–1.2 |
| SGPT (U/L) | 16 (3.6) | 20 (8.1) | 01–53 |
| SGOT (U/L) | 22 (4.7) | 25 (5.2) | 01–50 |
| WBC (×103/μL) | 6.5 (1.7) | 7.2 (1.7) | 4.5–11 |
| Hemoglobin (g/dL) | 14.0 (1.5) | 14.2 (1.3) | 13.9–16.3 |
| Platelet (×103/μL) | 245 (49) | 213 (77) | 150–450 |
CO2 = Carbon Dioxide, SGPT = Serum Glutamic Pyruvic Transaminase, SGOT = Serum Glutamic Oxaloacetic Transaminase, WBC = White Blood Cell. Data are presented as mean (SD).
Clinical Adverse Events
One patient had an adverse event. This subject had a history of eosinophilic esophagitis (EoE), diagnosed 2 years prior, but was not felt to have active disease and was not on treatment at the time of enrollment. After 5 ½ weeks on FAHF-2 treatment, she contacted the study coordinator to report that she had what she felt to be a recurrence of her EoE. Symptoms included intermittent moderate-severe, non-crampy abdominal pain, food impaction and nausea. She was instructed to discontinue FAHF-2 until she was evaluated by her gastroenterologist. Her gastroenterologist performed an upper endoscopy. Based on the endoscopy results, which showed eosinophilic infiltration in the esophagus, the subject was started on swallowed fluticasone and Prevacid. X-ray revealed significant amount of stool throughout the colon and she was given an enema and later started on Miralax for constipation. The abdominal pain did not recur. She was restarted on FAHF-2 two months later and completed the study with no other abdominal complaints.
Reduced allergen specific basophil activity in response to ex vivo antigen challenge after FAHF-2 treatment
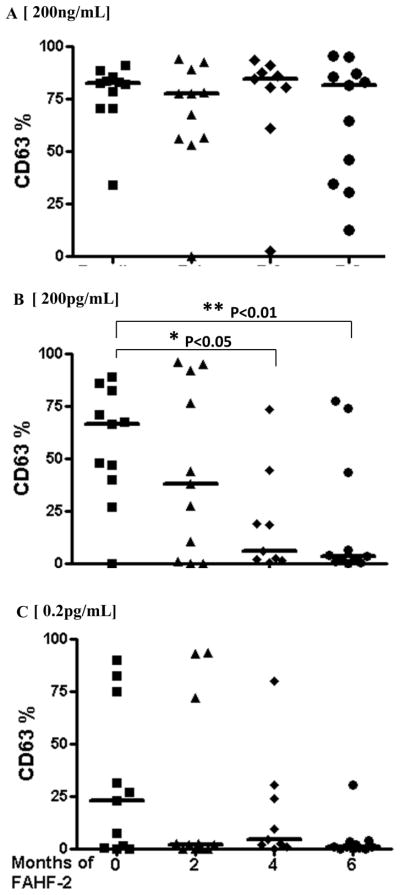
Basophil activity assays of peripheral blood samples from 11 patients were completed at all time points. Basophils were identified on the basis of CCR3+ staining and distinguished from eosinophils by low Side Scatter (On line Repository Figure E2). Activated basophils were defined as the percentage of CD63+ CCR3+cells. After correcting for multiple testing, there was a significant reduction (p<.010) in the percentage of CD63+ basophils in blood from patients after 6 months of FAHF-2 treatment following 200 pg/mL allergen stimulation (Figure 1B), as compared to the baseline levels. Although p<.02 for the non-adjusted difference at month 4, after adjustment with Bonferroni test for multiple testing, there was a significant reduction (p<.05). The data following the low (0.2pg/ml, Figure 1C), and high doses (200 ng/ml; Figure 1A) of allergen stimulation tended to be bunched near 0 or 100% respectively, and were not conducive to a parametric statistical analysis (highly non-normal with outliers that would remain even after transformation).
Fig 1. Suppression of allergen stimulated basophil activation by FAHF-2 in a six month phase-I clinical study.
Patients’ blood pre-treatment (0 month) and at consecutive 2 month time-points during a six month clinical phase-I study for FAHF-2 was stimulated with increasing doses of allergen in the presence of stimulation buffer. Percent CD63 for A) 200ng/mL, B) 200pg/mL and C) 0.2pg/mL of allergen are shown.*, p<0.05; **, p<0.01. Each symbol represents individual patient and bar is the median from each group.
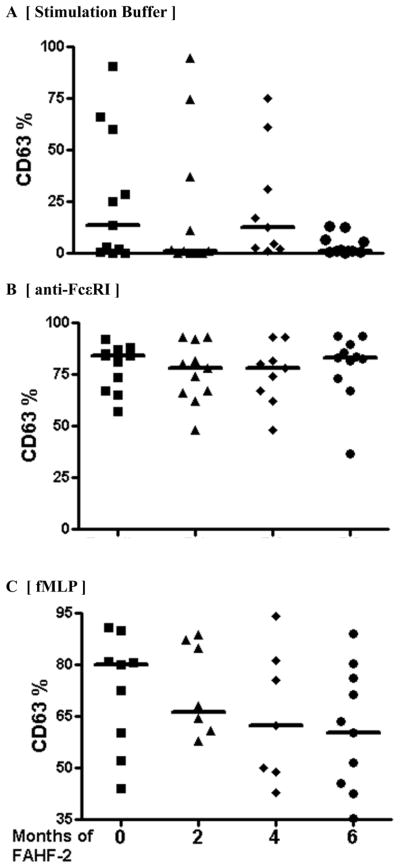
Based on the information from the Alpco Diagnostics, maker of the Flow 2 CAST kit, and the literature, (26–28)basophils from some subjects, termed non-responders, in BAT assays do not react to the positive control stimulation (< 5% activation). The opposite phenomena is basophil hyper-releaseability in which subjects’ basophils respond to negative control stimulation (>5% activation). In this study we found high baseline percentages of CD63+ cells in IL-3-containing stimulation buffer (median 13% CD63+ basophils (0–60%). There were no non-responders. Basophils from all subjects’ responded to anti-FcεRI and fMLP positive stimulation, and these responses were not different at baseline or following treatment (Figure 2). Blood samples from healthy individuals (n=8) contained <2% CD63+ basophils when stimulated with allergen or IL-3 containing stimulation buffer and responded to anti-FcεRI and fMLP positive stimulation (Data not shown).
Fig 2. Effect of FAHF-2 on IL-3, anti-FcεRI and fMLP stimulated basophils in a six month phase-I clinical trial.
Patients’ blood pre-treatment (0 month) and at consecutive 2 month time-points during a six month clinical phase-I study for FAHF-2 was stimulated in the presence of A) stimulation buffer alone, B) stimulation buffer + anti-FcεRI and C) stimulation buffer + fMLP.
Reduction in percent basophils and eosinophils after FAHF-2 treatment
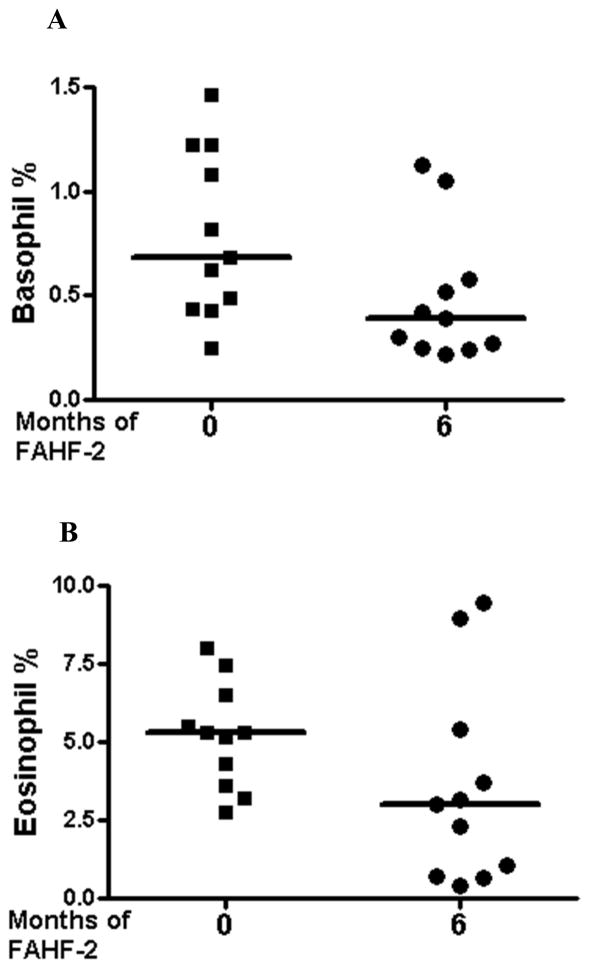
Based on the gating strategy according to the manufacturing instruction (Flow 2 CAST kit, Alpco Diagnostics, NH, USA), we also analyzed the changes of the percentage of peripheral blood basophils and eosinophils between baseline and after 6 months of FAHF-2 treatment. There was a trend associated with borderline statistical significance of reduction in the percentage of basophils (p=.067, Figure 3A) and eosinophils (p=.08, Figure 3B) after 6 months of FAHF-2 treatment. Basophils decreased from a median of 0.68% to 0.39% (Figure 3A), and eosinophils from 5.3% to 3% (Figure 3B).
Fig 3. Effect of FAHF-2 treatment on basophil and eosinophil percent.
Patients’ blood at baseline and after six months of clinical phase-I study for FAHF-2 was stimulated in the presence of stimulation buffer alone or stimulation buffer with anti-FcεRI, fMLP and allergen dilutions. A) Basophil percentage and B) eosinophil percentage are shown. The symbol indicates individual patient and the bar shows median value. A p value of < 0.05 was considered significant.
DISCUSSION
Complementary and alternative medicine treatments are increasingly used in Western countries because of their reputed effectiveness, low cost and favorable safety profiles, and because conventional therapies are not completely satisfactory for allergy. Studies have suggested the utility of Chinese herbal medicine for the treatment of a variety of allergic diseases, including allergic rhinitis and asthma.(8;9;29) We developed a 9-herb formula that is highly effective at protecting peanut-allergic mice from peanut-induced anaphylaxis, and has a high safety profile and long duration of action. Our group is the first to obtain an US FDA IND approval to investigate a botanical product to treat food allergy, and we recently published the results of the acute phase I safety trial, indicating that short-term use of FAHF-2 is safe and well-tolerated. (17) In this communication, we demonstrate that all laboratory parameters, pulmonary function studies, and electrocardiograms of patients treated with FAHF-2 for six months were within the normal range. No patient reported a food allergic reaction during the course of the trial. These results demonstrate that long-term treatment with FAHF-2 was safe and well tolerated by food allergic patients, and FAHF-2 itself does not cause any adverse reactions. This was the expected outcome based on the long term human use history of the constituent herbs and substantial preclinical animal studies. These extended safety results are also consistent with the acute phase I study.
BAT has been used along with other conventional allergy tests in the diagnosis of peanut allergy. (20;27;30) Jones et al. found that basophil activity was significantly reduced by 4–6 months of oral immunotherapy (OIT). (20) FAHF-2 treatment of peanut allergic mice produced significant suppression of mast cell and basophil numbers and activation.(13) This finding prompted us to investigate FAHF-2 treatment effects on basophil activity in this 6 months safety trial using CD63 as a basophil activation marker. We measured basophil activation in response to varying doses of antigen after 6 months of FAHF-2 treatment compared to that at baseline. Basophil activation in response to 200 pg/mL allergen was significantly reduced after 4 and to 6 months of treatment and a reduced trend was observed at 0.2pg/mL allergen dose. In this study, optimal concentration of antigen stimulation was 200pg/ml. At higher (200 ng/ml), and lower (0.2pg/ ml) doses, the data of basophil activation tended to be bunched near 0 or 100% respectively. In the study by Jones et al,(20) quantities of allergen used for basophil activation assay were between 0.1–10 μg/ml. A recent study by Kim et al.(31) used 1ng-1μg/ml of allergen for BAT. The differences in concentrations of peanut allergen used for testing basophil activation in different studies might be due to differences in sensitivity of cells from different populations, different in vitro stimulation times, or differences between the commercial kits used. Therefore it is important to optimize the concentrations of antigens for basophil activation assay. The study did not include an observational untreated food allergic group in which basophil activation was monitored for 6 months. However, Jones et al. (20) reported that mere avoidance of peanut for 6 months does not result in reduced basophil activation responses. Furthermore most of the patients in our study have avoided the allergic food substances for several years before enrolling in the study (Table 1) and still had a high baseline level of basophil activation responses upon ex vivo allergen stimulation. This suggests that the observed reduction in the basophil activation response was due to FAHF-2 treatment. We also found basophil hyper-releaseability [high spontaneous basophil histamine release to negative control stimulation in those subjects]. Although this study was not designed as a controlled study, to confirm the accuracy of BAT, we performed BAT on samples from normal subjects. Less than 2% basophils were CD63+ in response to allergen stimulation. There was no basophil activation following negative control stimulation (stimulation buffer), but similar responses to positive stimulation controls (anti-FcεRI) and (fMLP), suggesting basophil hyper-releaseability was specific to basophils from food allergic subjects as has been reported previously.(32–35) Vonakis et al.(28)demonstrated that cultured peripheral blood basophils from chronic idiopathic urticaria patients spontaneously degranulated, and that basophils from responders among these patients contained significantly increased splenocytes tyrosine kinase (Syk) and decreased SHIP-2 as compared to non responders and normals. In addition, Kepley et al. (36) detected less Syk in basophils of nonresponders. Thus the, balance between Syk and SHIP-2 levels plays an important role in spontaneous histamine release. Song et al.(13) showed that FAHF-2 persistent protection in a murine model of peanut allergy is associated with reduced basophil and mast cell numbers, as well as prevention of IgE-mediated mast cell activation. Three compounds from a purified fraction of FAHF-2 inhibited RBL-2H3 cell degranulation via suppressing Syk phosphorylation.(13) Therefore, the decrease in basophil activation after stimulation buffer only, at 6 months may be because of suppression of Syk levels by FAHF-2. However, this possibility requires further investigation at the molecular level.
Ducrest et al. (37) reported that blood basophil percent using CCR3 and CD123 as basophil markers. A more recent study demonstrated robust expression of CCR3 to be a valid single basophil selection marker in flow cytometry (18). The present study analyzed basophil percentage using only the CCR3 marker and discrimination from eosinophils on the basis of Side-Scatter as per manufacturer’s instructions (Alpco Diagnostics). We found a trend of reduction in the percentage of basophils in FAHF-2-treated subjects (p=0.067). There was also a trend towards a reduction of percentage of eosinophils (p=0.08). Food allergy patients often suffer from other allergic conditions such as asthma, allergic rhinitis and atopic eczema. In this study, 95% of subjects also have other allergic conditions. Eosinophils play an important pathological role in these disorders. FAHF-2 may provide additional immunological benefit for food allergy patients with other coexisting allergic conditions. A controlled study is warranted to investigate this possibility.
In our previous acute phase I study, we tested three doses of FAHF-2.(17) Although no dose-limiting side effects were reported in the Phase 1 study, several participants in the high dose group felt that 12 tablets 3-times daily posed a significant burden. Therefore, we used the medium dose in this 6-month open label study, which was equivalent to the half dose used in our previous murine model (11). We did not find a significant reduction in specific-IgE levels (Baseline: 21.65 kUA/L; Post treatment: 20 kUA/L (5–50)] or significant increase in specific-IgG4 levels despite the reduction in basophil activation. Jones et al. (20) found that 4–6 months of peanut OIT reduced basophil activity whereas IgE levels were increased at 6–8 months. Reduction of IgE levels were found after 12–18 months, perhaps due to difficulty in altering memory peanut allergic B cells. This data along with previously published reports suggest an IgE independent suppression of basophil activation, perhaps due to reduced Syk expression (20;36) and that higher dose and longer duration of treatment should be considered for the phase II study design to achieve greater efficacy.
In conclusion FAHF-2 was safe and well-tolerated by food allergic patients in a 6 month extended phase I clinical study. The results are consistent with a previous acute phase I study. FAHF-2 reduced allergen stimulated basophil activation, hyper-releaseability and percentage of circulating basophils. The suppression of basophil activation together with FAHF-2 in vitro effects on PBMCs in a previous study, i.e. increased IL-10 and IFN-γ and reduced IL-5 (17) in peanut-allergic mice, (11;12) suggest FAHF-2 exhibits beneficial immunological effects. A double-blind, placebo-controlled phase II efficacy trial is planned. Studies are also underway to investigate the mechanisms of action of FAHF-2 on basophil responses of food allergy patients.
Acknowledgments
We would like to thank Luda Bardina for her help with allergen extract preparation. This work was partially supported by the Food Allergy Initiative, Winston Wolkoff Integrative Medicine for Allergy and Immunology Foundation, and by National Institutes of Health grant # 1 R01AT001495-01A1 and 2R01AT001495-05A1 awarded to Dr X-M Li.
Abbreviations
- FAHF-2
Food Allergy Herbal Formula-2
- PN
Peanut
- TN
Tree nuts
- CAM
Complementary and Alternative Medicine
- IND
Investigational New Drug
Footnotes
Author disclosure statement related to this study
Hugh A. Sampson MD and Xiu-Min Li MD hold US Patent PCT/US 05/08600 on FAHF-2, and have shares of Herbal Springs, LLC, which acquires and distributes herbal products. The other authors have no competing financial interests to disclose.
www.clinicaltrials.gov Identifier: NCT00602160
References
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S116–S125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37(5):651–60. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. Revised. 2004. [Google Scholar]
- 4.Chan CK, Kuo ML, Shen JJ, See LC, Chang HH, Huang JL. Ding Chuan Tang, a Chinese herb decoction, could improve airway hyper-responsiveness in stabilized asthmatic children: a randomized, double-blind clinical trial. Pediatr Allergy Immunol. 2006;17(5):316–22. doi: 10.1111/j.1399-3038.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang TT, Huang CC, Hsu CH. Clinical evaluation of the Chinese herbal medicine formula STA-1 in the treatment of allergic asthma. Phytother Res. 2006;20(5):342–7. doi: 10.1002/ptr.1843. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CH, Lu CM, Chang TT. Efficacy and safety of modified Mai-Men-Dong-Tang for treatment of allergic asthma. Pediatr Allergy Immunol. 2005;16(1):76–81. doi: 10.1111/j.1399-3038.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 7.Kao ST, Wang SD, Wang JY, Yu CK, Lei HY. The effect of Chinese herbal medicine, xiao-qing-long tang (XQLT), on allergen-induced bronchial inflammation in mite-sensitized mice. Allergy. 2000;55(12):1127–33. doi: 10.1034/j.1398-9995.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 8.Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009;123(2):297–306. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma 23. J Allergy Clin Immunol. 2005;116(3):517–24. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–55. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–51. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Qu C, Srivastava K, Yang N, Busse P, Zhao W, et al. Food allergy herbal formula 2 protection against peanut anaphylactic reaction is via inhibition of mast cells and basophils. J Allergy Clin Immunol. 2010;126(6):1208–17. doi: 10.1016/j.jaci.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jindal V, Ge A, Mansky PJ. Safety and efficacy of acupuncture in children: a review of the evidence. J Pediatr Hematol Oncol. 2008;30(6):431–42. doi: 10.1097/MPH.0b013e318165b2cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hon KL, Leung TF, Ng PC, Lam MC, Kam WY, Wong KY, et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2007 doi: 10.1111/j.1365-2133.2007.07941.x. [DOI] [PubMed] [Google Scholar]
- 16.Bensky D, Barolet R. Chinese Herbal Medicine: Formulas & Strategies. Seattle: Eastland Press; 1990. [Google Scholar]
- 17.Wang J, Patil S, Yang N, Ko J, Lee J, Noone S, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula (FAHF-2) in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Ann Allergy Asthma Immunol. 2010;105(1):75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausmann OV, Gentinetta T, Fux M, Ducrest S, Pichler WJ, Dahinden CA. Robust expression of CCR3 as a single basophil selection marker in flow cytometry. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 19.Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007;72(3):196–203. doi: 10.1002/cyto.b.20142. [DOI] [PubMed] [Google Scholar]
- 20.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. 300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008;145(3):193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- 22.Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, et al. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146 (Suppl 1):47–53. doi: 10.1159/000126061. [DOI] [PubMed] [Google Scholar]
- 23.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009;123(4):789–94. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin HR, Simonetti GP, Dubbelman AC, Bokkel Huinink WW, Taal BG, Wigbout G, et al. Toxicity grading systems. A comparison between the WHO scoring system and the Common Toxicity Criteria when used for nausea and vomiting. Ann Oncol. 1994;5(2):113–7. doi: 10.1093/oxfordjournals.annonc.a058760. [DOI] [PubMed] [Google Scholar]
- 25.Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107(6):1077–81. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 26.Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandene L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320(1–2):40–8. doi: 10.1016/j.jim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Ocmant A, Mulier S, Hanssens L, Goldman M, Casimir G, Mascart F, et al. Basophil activation tests for the diagnosis of food allergy in children. Clin Exp Allergy. 2009;39(8):1234–45. doi: 10.1111/j.1365-2222.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- 28.Vonakis BM, Gibbons S, Jr, Sora R, Langdon JM, MacDonald SM. Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J Allergy Clin Immunol. 2001;108(5):822–31. doi: 10.1067/mai.2001.119159. [DOI] [PubMed] [Google Scholar]
- 29.Li XM. Complementary and alternative medicine in pediatric allergic disorders. Curr Opin Allergy Clin Immunol. 2009 doi: 10.1097/ACI.0b013e328329226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moneret-Vautrin DA, Sainte-Laudy J, Kanny G, Fremont S. Human basophil activation measured by CD63 expression and LTC4 release in IgE-mediated food allergy. Ann Allergy Asthma Immunol. 1999;82(1):33–40. doi: 10.1016/S1081-1206(10)62657-9. [DOI] [PubMed] [Google Scholar]
- 31.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–6. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banks JR, Kagey-Sobotka A, Lichtenstein LM, Eggleston PA. Spontaneous histamine release after exposure to hyperosmolar solutions. J Allergy Clin Immunol. 1986;78(1 Pt 1):51–7. doi: 10.1016/0091-6749(86)90114-4. [DOI] [PubMed] [Google Scholar]
- 33.Barg W, Wolanczyk-Medrala A, Obojski A, Wytrychowski K, Panaszek B, Medrala W. Food-dependent exercise-induced anaphylaxis: possible impact of increased basophil histamine releasability in hyperosmolar conditions. J Investig Allergol Clin Immunol. 2008;18(4):312–5. [PubMed] [Google Scholar]
- 34.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321(4):228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 35.Vonakis BM, Gibbons S, Jr, Sora R, Langdon JM, MacDonald SM. Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J Allergy Clin Immunol. 2001;108(5):822–31. doi: 10.1067/mai.2001.119159. [DOI] [PubMed] [Google Scholar]
- 36.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol. 2000;165(10):5913–20. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- 37.Ducrest S, Meier F, Tschopp C, Pavlovic R, Dahinden CA. Flowcytometric analysis of basophil counts in human blood and inaccuracy of hematology analyzers. Allergy. 2005;60(11):1446–50. doi: 10.1111/j.1398-9995.2005.00910.x. [DOI] [PubMed] [Google Scholar]