Abstract
Background
Brain and spinal cord white matter can support extensive axonal growth. This growth is generally constrained to an orientation that is parallel to the longitudinal axis of the fiber tract. This constraint is presumably due to permissive and non-permissive substrates that are interleaved with each other and oriented in parallel within the tract.
Results
Embryonic chick sympathetic neurons were cultured on cryostat sections of rat brain and the orientation of neurite growth on white matter was assessed. To determine if haptotaxis is sufficient to guide parallel neurite growth, neurons were cultured under conditions designed to interfere with interactions between growing neurites and factors that act as biochemical contact guidance cues but not interactions with haptotactic cues. Under these conditions, neurites extending on white matter were not exclusively oriented in parallel to the fiber tract, suggesting that biochemical cues are involved. To assess the role of myelin in guiding parallel neurite growth, neurons were cultured on myelin-deficient corpus callosum. These neurons also extended neurites that were not constrained to a parallel orientation. Moreover, preincubation with NGF and treatment with cAMP analogs, manipulations that attenuate overall myelin-mediated inhibition of neurite growth, also led to a reduced parallel orientation of neurite growth.
Conclusions
The present studies suggest that some of the relevant factors that constrain axonal growth on white matter are not haptotactic in nature and appear to be partly mediated by factors that are associated with myelin and may involve myelin-associated "inhibitors".
Background
It previously was believed that axonal growth within white matter was not possible. This belief was based on the well-documented failure of injured axons to regenerate within the central nervous system (CNS) [1] and reinforced by studies showing that neurons attach poorly to white matter in vitro [2,3,4,5,6,7,8,9]. These studies, in part, supported the hypothesis that CNS myelin contains axon-growth inhibiting molecules. Additional investigations identified myelin-associated molecules, including Nogo (formerly NI-35/250), myelin-associated glycoprotein (MAG), and chondroitin sulfate proteoglycans, that inhibit neurite growth [10,11,12,13,14,15,16,17,18,19].
Early studies, in which transplanted embryonic neurons extended parallel axons within white matter, appeared to be inconsistent with this hypothesis [20,21,22,23,24,25,26,27,28,29]. However, successful growth was attributed to the possibility that embryonic neurons may not express receptors for myelin-associated inhibitors [26,28,29,30]. Recent studies, however, demonstrated that white matter can support extensive parallel axonal growth from transplanted adult neurons [31,32]. Recent tissue section culture experiments also demonstrated that white matter can support parallel neurite growth [33].
Given the growing evidence that white matter can support axonal growth, we sought to identify the properties that mediate its parallel orientation. Physical edges and contours (haptotactic cues) can guide axonal growth independently of biochemical composition [34]. Physical edges arranged in parallel within white matter, such as astroglial processes and axons, could theoretically guide parallel neurite growth. Alternatively, biochemical cues may guide parallel growth. Cryostat sections of rat brain were manipulated to deactivate biochemical guidance cues while preserving haptotactic cues and were then used as substrata for cultured neurons. These manipulations included prior fixation or mounting on polyornithine-coated culture dishes and, in both cases, non-parallel neurite growth occurred on white matter suggesting that biochemical cues are required for parallel growth.
Additional experiments assessed the contribution of myelin to the parallel orientation of neurites. The orientation of neurites on myelin-deficient corpus callosum was assessed. Also, neurons were cultured with cAMP analogs or preincubated with nerve growth factor (NGF), treatments known to attenuate the overall inhibitory effects of myelin [35,36]. Neurites extending on myelin-deficient corpus callosum or from neurons that were preincubated with NGF or treated with cAMP analogs were significantly less parallel. These results suggest that myelin contributes to the parallel orientation of neurite growth on white matter and that this effect may be mediated by its overall neurite-inhibitory properties.
Results
Neurite growth on the corpus callosum
As previously reported [33], neurites extending on the corpus callosum near the midline and medial to the cingulum were mostly limited to orientations that were in parallel with the longitudinal axis of the fiber tract. Neurites also extended on more lateral portions of the corpus callosum but these neurites extended in all directions, including perpendicular orientations. Neurites on lateral portions of the fiber tract could also be commonly observed extending from white matter onto gray matter, whereas those medial to the cingulum were generally confined to the fiber tract. As it is difficult to assess the orientation of the underlying corpus callosum laterally, analysis of neurite orientiation relative to the underlying tract was restricted to portions of the corpus callosum medial to the cingulum.
Neurons attached in varying densities under all conditions but neurite orientation was not dramatically affected by neuronal density. Neurite orientation is qualitatively best demonstrated by high density cultures. Consequently, the photomicrographs presented in the following results are generally of higher density cultures. However, since it is difficult to follow a single neurite from soma to growth cone in the case of high neuronal densities, the quantitative results presented herein were derived from all assessible neurons from cases where low rates of attachment occurred.
Haptotaxis appears insufficient to mediate the parallel orientation of neurites
In order to deactivate biochemical guidance cues while preserving haptotactic cues that may guide parallel neurite growth, brain sections were fixed prior to use as substrata for cultured neurons. After 39 hours in culture, many neurons had attached to the corpus callosum of both fixed and unfixed tissue sections and extended short neurites freely on these fiber tracts. On the corpus callosum in unfixed sections, the neurites were oriented mostly in parallel with the longitudinal axis of the tract, as has been previously shown [33]. In contrast, on fixed sections, neurite growth occurred in all directions on the corpus callosum. In fact, neurite growth often occurred in directions perpendicular to the tract.
After 3 days in culture, neurites continued to extend freely on both fixed and unfixed sections. On the unfixed corpus callosum, scattered neurons continued to extend neurites parallel to the tract, forming dense plexi of neurites attached firmly to the underlying substrate. On the fixed corpus callosum, however, many of the neurons had migrated together forming small clusters that extended neurites in all directions across the fiber tract. Many of these neurites grew in close association, appearing to use each other as a substrate. While the growth cones of these neurites remained firmly attached to the underlying substrate, the proximal segments often detached from the substrate and straightened under the tension applied by the outward migrating growth cone. After one week in culture dense parallel plexi of firmly-attached neurites were found on the unfixed corpus callosum (Fig. 1A). Neurites extending on the fixed corpus callosum (Fig. 1B) formed a web of loosely attached neurites radiating in all directions across the fiber tract.
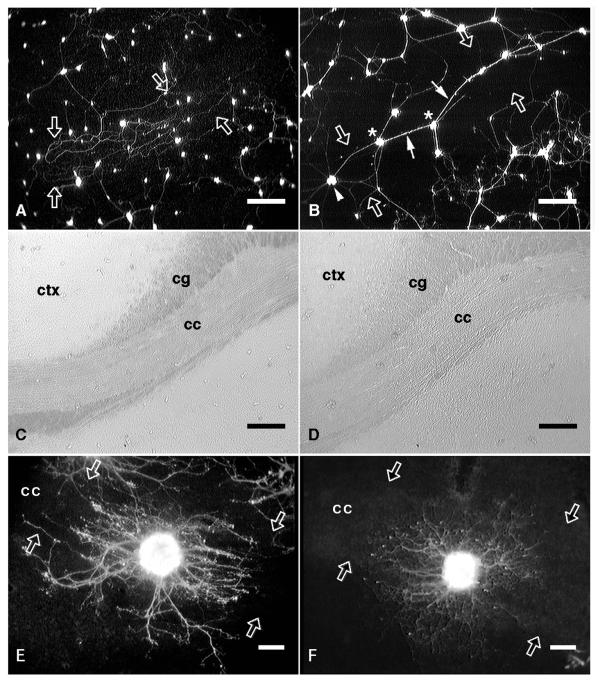
Figure 1.
Neurites extending on fixed and unfixed corpus callosum or on unfixed corpus callosum treated with polyornithine. A, Fluorescein-labelled neurons cultured for one week on unfixed corpus callosum (edges of the fiber tract are indicated by open arrows). The neurites extending from these neurons were mostly unfasciculated and generally extended in parallel with the longitudinal axis of the fiber tract. B, Neurons cultured for one week on fixed corpus callosum. These neurons were mostly clustered (white asterisks) and many of the neurites extending on the fixed fiber tract were fasciculated (white arrows). From one neuron cluster (white arrowhead), unfasciculated neurites radiated outward in all directions over the fixed fiber tract. C, D, Phase-contrast micrographs of the underlying anatomy of panels A and B, respectively. E, A fluorescein-labelled explant attached on the corpus callosum (cc; edges are indicated by open arrows) in an unfixed tissue section that had been mounted on an untreated culture dish. The neurite outgrowth halo (after 6 days in culture) was oriented in parallel with the underlying fiber tract. F, An explant attached on the corpus callosum in an unfixed tissue section that had been mounted on a polyornithine-treated culture dish. The neurite outgrowth halo (after 3 days in culture) extended radially with no parallel orientation. cc, corpus callosum; ctx, neocortex; cg, cingulum. Scale bars: A, B, C, D, 200 μm; E, F, 100 μm.
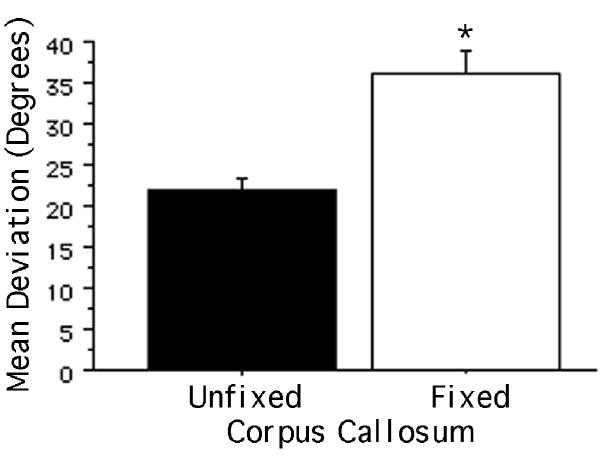
Quantification of neurite orientation showed a significant difference between those extending on fixed and unfixed corpus callosum. Since the results were essentially the same regardless of the length of time that the neurons were cultured, Figure 2 shows the mean orientations for neurites assessed after culture durations of 39 hours to 7 days. To assure that these values reflected neurites in contact with the underlying tissue section rather than neurites growing on each other, only the orientations of neurites that extended in isolation from other neurites were assessed. In addition to a significantly greater (p < 0.0001) mean angular deviation from an orientation that is parallel to the longitudinal axis of the tract, the variance in the deviation on fixed corpus callosum was significantly greater than that on unfixed corpus callosum [F(100,289) = 1.541; p < 0.01]. This is consistent with the interpretation that neurites are free to grow in a wider range of orientations on fixed white matter.
Figure 2.
Orientation of neurites extending on fixed and unfixed corpus callosum. The y-axis represents the mean angular deviation (degrees) from an orientation parallel to the longitudinal axis of the corpus callosum (a mean deviation of 0º indicates parallel neurite growth; a mean deviation of 90º indicates perpendicular neurite growth). The mean deviation of neurites extending on fixed corpus callosum was significantly greater than that of neurites extending on unfixed corpus callosum, indicating that the former were significantly less parallel in their orientation. Bars indicate mean + SEM (*p < 0.0001).
As documented previously [33], explants of sympathetic ganglia attached to corpus callosum in unfixed sections mounted on untreated culture dishes exhibited neurite outgrowth halos that were oriented in parallel with the underlying tract (Fig. 1E). However, when sections were mounted on polyornithine-coated culture dishes, a manipulation also designed to mask chemical cues but not haptotactic interactions between growing neurites and the underlying substrate, neurite outgrowth was found to radiate in all orientations without preference for the longitudinal axis of the fiber tract (Fig. 1F).
Myelin may contribute to the parallel orientation of neurites
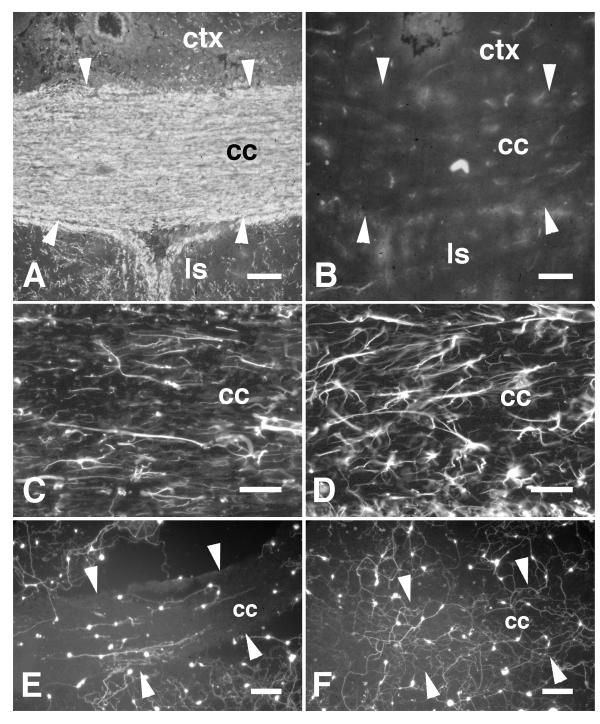
The corpus callosum of myelin-deficient (md) rats was difficult to distinguish from gray matter when viewed using phase-contrast optics (data not shown) and was clearly less myelinated when labelled using Rip immunohistochemistry (Fig. 3A,B) and Luxol Fast Blue histochemistry (data not shown). GFAP-immunohistochemistry showed hypertrophy of astroglia within the md fiber tracts compared with wild-type (Wt) tracts, as has been previously reported [37]. However, the orientation of GFAP-immunoreactivity was mostly in parallel with the longitudinal axis of both md and Wt fiber tracts (Fig. 3C,D).
Figure 3.
Neurites extending on myelin-deficient corpus callosum. A, The corpus callosum (cc) of a wild-type rat (21 days old) is shown labelled by the myelin-specific antibody Rip. The contrast in myelin content between the corpus callosum and gray matter such as the neocortex (ctx) and lateral septal nuclei (ls) is evident. B, The corpus callosum (cc; edges of the tract are indicated by white arrowheads) of an age-matched myelin-deficient mutant rat labelled with the Rip antibody. In comparison with the wild-type animal, little Rip-immunoreactivity could be detected in the corpus callosum and there was little contrast between gray and white matter indicating the absence of myelin. C, An adjacent section showing the corpus callosum of the wild-type rat labelled by GFAP-immunohistochemistry showing mostly parallel orientations to the astroglial processes. D, An adjacent section showing the corpus callosum of the myelin-deficient rat labelled by GFAP-immunohistochemistry. The astrocytes appeared hypertrophic but are mostly oriented in parallel with the fiber tract. E, Fluorescein-labelled neurons cultured on a section through the wild-type corpus callosum. Neurites extending on white matter were oriented in parallel with the tract. Neurites extending on gray matter rarely extended across borders with white matter. F, Neurons cultured on a section through the myelin-deficient corpus callosum. The neurites were unoriented on white matter and frequently extended across borders between white and gray matter. Scale bars: A, B, 100 μm; C, D, 50 μm; E, F, 150 μm.
To determine whether the absence of myelin affects the rate and orientation of neurite growth, neurons were cultured on sections of md forebrain and Wt forebrain matched for age (P21) and genetic background (Wistar). Neurite length and orientation were assessed after 7 days in culture. The orientation of neurite growth on Wt corpus callosum (Fig. 3E) was clearly more parallel compared with that on md corpus callosum (Fig. 3F). In addition, the density of neuronal attachment and neurite growth appeared to be greater on md corpus callosum and neurites extending on gray matter were less inhibited at borders with the md corpus callosum (Fig. 3F).
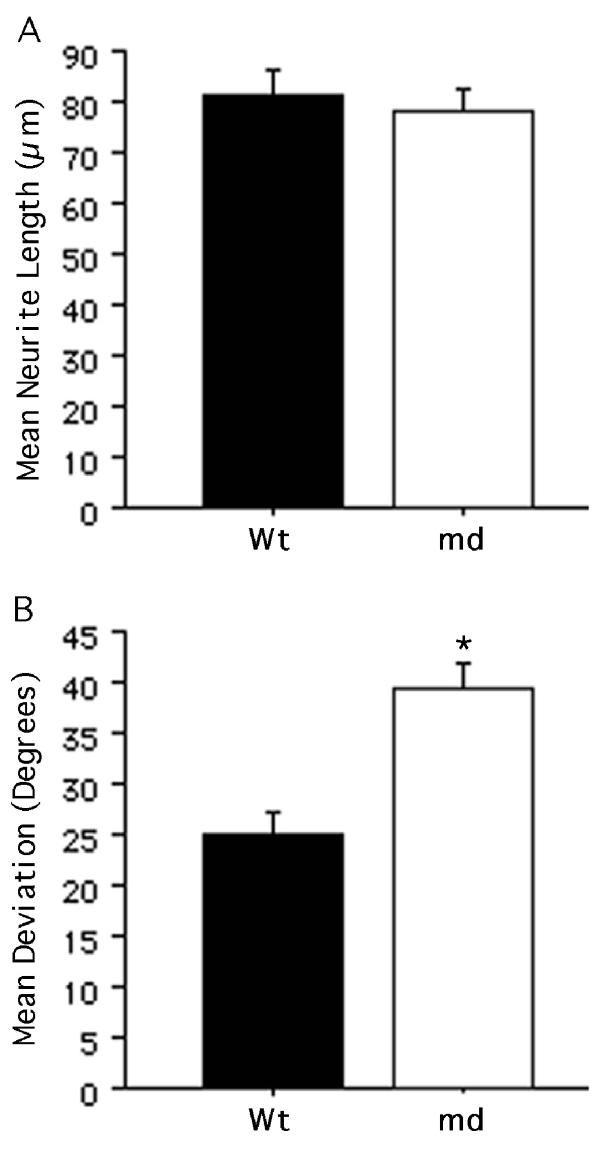
A quantitative comparison of neurites on md and Wt corpus callosum showed that they did not differ in length (Fig. 4A; p = 0.63). However, a comparison of the mean deviation from an orientation parallel to the longitudinal axis of the fiber tract showed that neurites on md corpus callosum were significantly less parallel compared with those on Wt corpus callosum (Fig. 4B; p < 0.0001). Moreover, the variance in the orientation on md corpus callosum was significantly greater than that on Wt corpus callosum [F(114,131) = 1.452; p < 0.05], consistent with the interpretation that neurites are free to grow in a wider range of orientations on the md fiber tract. Interestingly, neurite growth on P21 Wt corpus callosum appeared less constrained to a parallel orientation than that normally seen on white matter in sections of adult Wt brain (compare Figure 4BWt with Figure 5B and 6A,B controls).
Figure 4.
Neurite growth on myelin-deficient corpus callosum. A, The mean neurite length on myelin-deficient (md) corpus callosum was not significantly different from that on wild-type (Wt) corpus callosum. B, The mean angular deviation of neurites from an orientation parallel to the longitudinal axis of the underlying corpus callosum was significantly greater on the md tract compared with that on the Wt tract. All bars indicate mean + SEM (*p < 0.0001).
Figure 5.
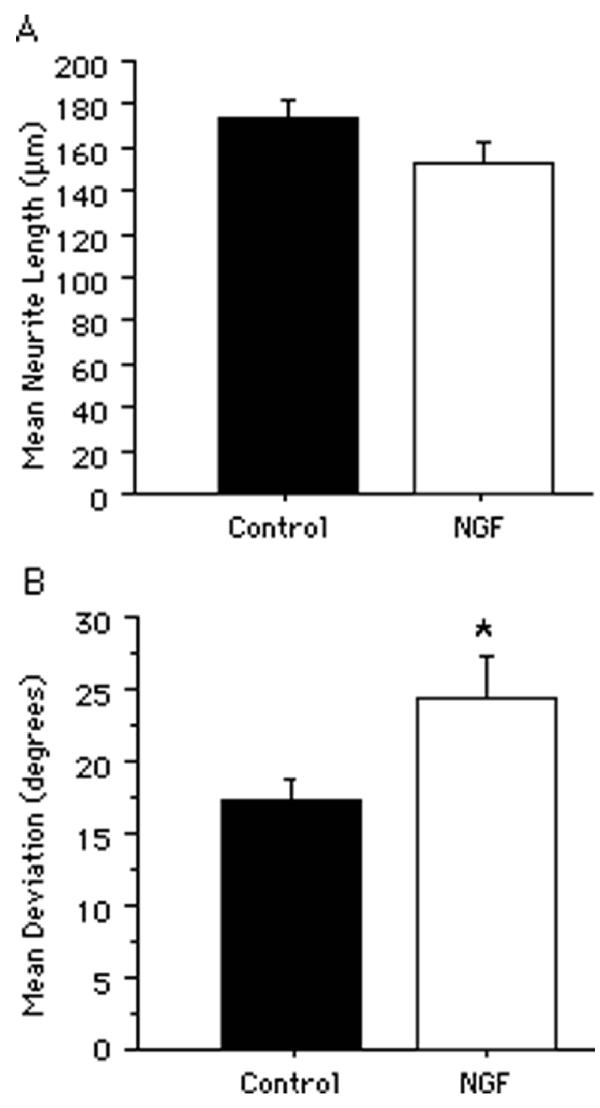
Neurite growth from neurons that had been preincubated with NGF. A, The mean neurite length on the corpus callosum from neurons that had been preincubated with 200 ng/ml nerve growth factor (NGF) did not significantly differ from that of neurons that had been preincubated in culture medium only. B, The mean angular deviation of neurites from an orientation parallel to the longitudinal axis of the underlying corpus callosum was significantly greater from NGF-pretreated neurons compared with those preincubated in culture medium only. All bars indicate mean + SEM (*p < 0.05).
Figure 6.
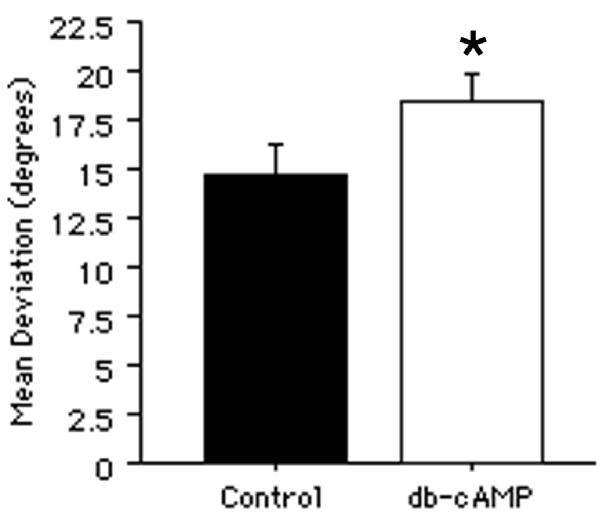
The effects of cAMP analogs on neurite orientation. A, The mean angular deviation of neurites from an orientation parallel to the longitudinal axis of the underlying corpus callosum was significantly greater from neurons treated with cAMP analogs (db-cAMP; 1 mM) compared with those treated with culture medium only. All bars indicate mean + SEM (*p < 0.05).
Myelin-associated inhibitors may contribute to parallel neurite growth
Preincubation of neurons with high concentrations of neurotrophins for 5 to 24 hours blocks the neurite-growth inhibitory effects of myelin [35]. Therefore, neurons were preincubated with nerve growth factor (NGF; 200 ng/ml) for 21 hours prior to plating on sections of adult forebrain. Neurite length and orientation were assessed after 3 days of culture.
No difference in neurite length was detectable between neurons that had been preincubated with NGF and control neurons that had been preincubated for the same length of time in culture medium without NGF (Fig. 5A; p = 0.10). However, the mean deviation of neurites from an orientation parallel to the longitudinal axis of the corpus callosum from neurons that had been preincubated with NGF was significantly greater than that of control neurons (Fig. 5B; p < 0.05). Moreover, the variance in neurite orientation from neurons that had been preincubated with NGF was significantly greater than that of control neurons [F(74, 131) = 1.805; p < 0.01]. The NGF was biologically active as it substantially increased neurite growth from sympathetic explants cultured on polyornithine-coated dishes (data not shown).
Treatment with cAMP analogs has also been shown to block the inhibitory effects of myelin on neurite growth [35,36]. Therefore, neurons were cultured on sections of adult forebrain in the presence of dibutyryl-cAMP (db-cAMP; 1 mM) and the effects on neurite growth were assessed after 3 to 7 days in culture. Interestingly, the length of neurites was significantly decreased by db-cAMP (data not shown). Assessment of neurite orientation showed a significant increase in deviation from a parallel orientation on the corpus callosum compared with medium-treated control cultures (Fig. 6A; p < 0.05). An increased variance in neurite orientation in db-cAMP treated cultures also occurred [F(153, 119) = 1.318; p < 0.05].
Discussion
The contributions of biochemical and haptotactic cues to parallel neurite growth
Several biochemical factors influence axonal growth by either attraction or repulsion and may guide axonal growth by "hemming in" the growth cone to a channel containing permissive molecules surrounded by repulsive molecules [38,39]. For example, this mechanism may explain somite-mediated segmentation of developing motor axons [40,41].
Mechanical (haptotactic) cues can also guide neurites. Early theories proposed that neurites extend in the direction of least resistance (reviewed by Ramón y Cajal [1]). Harrison proposed that haptotaxis guides neurites along fibrin threads in tissue culture [34]. He also noted that developing peripheral nerves grow "through the notches between the muscle plates". "...the nerves follow definite paths, which are preformed...by the configuration of other organs. Grooves...between the more solid embryonic organs seem to be paths of predilection" [34]. Grooves etched into plastic or glass also guide neurites [42,43].
Tissue sections supported neuronal attachment and neurite growth on gray and white matter despite prior fixation. However, neurites did not extend in parallel on fixed corpus callosum. By cross-linking proteins, fixation should interfere with biochemical contact-guidance cues without altering the edges and contours that could act as haptotactic cues. In fact, the histology of the sections was preserved after fixation (compare Figures 1C and 1D) when viewed with phase-contrast optics, which depends on refraction and diffraction, indicating that the edges and contours were not altered. Nevertheless, the resolution afforded by light microscopy is insufficient to rule out the possibility that fixation altered surface contours, thereby contributing to the loss of parallel orientation.
The polyornithine experiments provide additional evidence that haptotaxis is insufficient to guide parallel growth. Whether polyornithine acted through the tissue section and/or reentered solution, secondarily depositing onto the sections, is not clear. Either way, it is unlikely that polyornithine altered the topology of the sections to mask haptotactic cues in the same manner that would have occurred through fixation. Therefore, taken together, the effects of fixation and polyornithine suggest that haptotactic cues are insufficient to guide parallel neurite growth.
It is likely that several biochemical guidance cues exist in white matter including not only repulsive molecules (e.g. Nogo or MAG) but also growth-permissive molecules (e.g. laminin). It is possible that only a subset of them are blocked by either fixation or polyornithine and it is not possible to determine which of these factors have been blocked in our experiments, especially since many of these factors may not be known. Therefore, if parallel neurite growth had been observed on fixed corpus callosum or on corpus callosum in sections mounted on polyornithine, it would not have been possible to interpret the data with regard to the relative contributions of biochemical guidance cues and haptotaxis. However, since the parallel growth constraint was eliminated under these conditions, it is clear that the relevant biochemical cues were blocked. To the extent that these biochemical cues, but not haptotactic cues, were blocked by fixation or mounting on polyornithine, these data suggest that haptotaxis is insufficient to account for the parallel orientation. The fact that the neurite outgrowth was more fasciculated points to the possibility that growth-promoting and/or adhesive factors were also affected by the treatments. Therefore, the loss of parallel growth could be attributed to the loss of either positive or negative cues (or both) that normally serve to align neurite outgrowth.
The contribution of myelin to parallel neurite growth
White matter is replete with parallel-oriented cells that could provide biochemical orienting cues for growing axons, e.g. non-reactive astrocytes [31,32]. Alternatively, given their tendency to fasciculate, axons could provide a permissive parallel substrate.
However, for white matter to have an orienting influence, there must also be a relatively non-permissive substrate that restricts growing axons to a parallel course. Myelin-associated inhibitors are obvious candidates for this role. To test the contribution of myelin, neurons were cultured on sections of myelin-deficient (md) brain. Neurites on md corpus callosum were significantly less parallel than those on wild-type (Wt) corpus callosum. Since deviation from parallelism is restricted to the range of values from 0º to 90º, where 0º indicates strict parallelism and 90º indicates perpendicularity, randomly-oriented neurites would have a mean deviation approaching 45º. In fact, the mean deviation from parallelism on md corpus callosum was 38.9º. These data suggest that myelin contributes to the parallel orientation of axons on white matter. Interestingly, neurites on P21 Wt corpus callosum were less parallel than those on adult Wt corpus callosum, possibly because of incomplete myelination at P21.
Neurite lengths on md and Wt corpus callosum did not differ. The absence of myelin-associated neurite-growth inhibitors might have been expected to increase neurite growth on md corpus callosum. However, myelin-mediated inhibition has mostly been studied under conditions in which the organization of myelin was disrupted [10,11,13,14,15,16,17,18,44,45]. There is little evidence that myelin-associated inhibitors reduce neurite growth when maintained in their normal geometric organization. In fact, neurites extending in parallel on corpus callosum in vitro are comparable in length to those on gray matter [33].
It is possible that demyelination affects other properties that affect neurite orientation. For example, hypertrophy of astrocytes and axonal swellings have been reported in the white matter of these animals [37]. In fact, the axons and astrocytes appear to remain oriented mostly in parallel. Thus, the possibility that alterations in astrocytic morphology, or other changes in the tissue, contribute to the reduced parallel outgrowth cannot be ruled out. However, when these data are taken in combination with the reduced parallel neurite growth from neurons preincubated with NGF or treated with cAMP analogs, conditions known to interfere with the overall inhibitory effects of myelin on neurite growth (see discussion below), there appears to be a preponderance of data suggesting that the loss of myelin is directly responsible for the reduced parallel orientation of neurite growth.
The contribution of myelin-associated inhibitors to parallel neurite growth
To test whether myelin-associated neurite-inhibitors are involved, conditions known to block myelin-associated inhibitory activity were used. Since the parallel growth constraint may be due to the combined action of multiple inhibitors, treatments that are known to attenuate the overall inhibitory properties of myelin were selected. Priming neurons with NGF or treatment with db-cAMP reduced the parallel constraint on growth. Together, these data suggest that myelin-associated neurite-growth inhibitory activity may contribute to the parallel constraint on growth in white matter but do not necessarily implicate specific inhibitors since priming with neurotrophins and treatment with cAMP analogs inactivate the inhibitory activity of myelin as a whole [35,36]. Whether the test neurons used in this study are responding to specific factors that have been previously identified, such as MAG and NoGo, is unknown. It is possible that other myelin-associated inhibitors exist and contribute to parallel neurite orientation [15].
Do myelin-associated inhibitors influence the orientation of neurite growth in vivo?
MAG is reportedly expressed only at the interface between axons and their myelin sheaths [12]. If so, MAG is not accessible to axons extending within myelinated tracts without disruption of the myelin sheaths. Nogo, in contrast, is expressed on the outer surface of myelin sheaths [46] and is, therefore, accessible to axons extending within uninjured fiber tracts. In fact, infusion of the Nogo-inactivating IN-1 Fab fragment into the vermis of the rat cerebellum induced sprouting of uninjured Purkinje cell axons suggesting that Nogo normally constrains the growth and orientation of axons [47]. A role of Nogo in restricting the orientation of neurite growth is further supported by studies showing that IN-1-sensitive inhibitors expressed in the already-myelinated sensory tracts of the rat spinal cord appeared to restrict the position of developing corticospinal axons [48].
A limitation of tissue section culture is that it accounts for only two of the three dimensions normally present in vivo. However, the intent of this study was to examine the role of the parallel array of tissue elements in regulating axonal growth and the two-dimensional sections can be viewed as a sampling of this three-dimensional parallel geometry. It also cannot be ruled out that by sectioning the fiber tract, as is done in tissue section culture, that intracellular molecules may be exposed artifactually. The striking similarity to the parallel axonal growth within white matter observed from transplanted neurons in vivo [20,21,22,23,26,28,29,31,32], however, makes it unlikely that the pattern of neurite growth in tissue section culture is entirely due to artifactual unmasking of normally sequestered molecules or to a limitation in the available degrees of freedom. The correspondence between the orientation of neurite growth in this assay and that observed in vivo indicates that tissue section culture models the relevant tissue properties and geometry.
Conclusions
These data suggest that the orienting influence of white matter geometry on neurite outgrowth involves myelin and, possibly, its associated neurite-growth inhibitory activity. The term "inhibitor" refers to the effects of myelin on neurites in studies that did not consider its normal geometric organization within white matter. In fact, close to the site of injury where its geometry is disrupted, myelin may indeed be an obstacle to regeneration (see Pettigrew et al., companion paper http://www.biomedcentral.com/1471-2202/2/8). It is now clear, however, that the presence alone of undisrupted myelin is insufficient to prevent regeneration [31,32]. While the direct effect of myelin on growing axons in vitro is inhibitory but does little to block axonal growth within intact tracts in vivo, myelin may nevertheless play an important role in regulating axonal growth within intact tracts. Myelin may encourage directed, long-distance growth by restricting it to a parallel orientation. For example, in the spinal cord, where successful regeneration ultimately depends on axon extension in an appropriate direction over long distances, factors that promote parallel axonal growth, and discourage non-parallel growth, should enhance long-distance regeneration. In fact, several studies have been succesful in promoting axonal regeneration across sites of spinal cord or optic nerve injury by blocking the myelin-associated inhibitor Nogo [16,45,49]. However, these axons often fail to reattain a parallel orientation within the distal stump. These axons "followed an irregular course" and "branching was seen quite frequently". The "inhibitory" activity of myelin may actually serve to promote axonal growth in an intact fiber tract (such as the distal stump of the injured spinal cord) by restricting this growth to an orientation that is parallel to the fiber tract while discouraging local meandering and collateral sprouting.
Materials and Methods
Preparation of substrata
Adult Sprague-Dawley rats (maintained in the University of Cincinnati vivarium in accordance with the NIH guide for the care of research animals) were deeply anesthetized using 4 ml/kg i.p. pentobarbital sodium solution (50 mg/ml; Abbott Laboratories, North Chicago, IL) and decapitated. The brains were rapidly removed and frozen at -80ºC. In other cases, myelin-deficient (md) and wild-type (Wt) control brains (Wistar) supplied by Dr. Judith Grinspan (Dept. of Research Neurology, Children's Hospital, Philadelphia, PA) were used (age = 21 days). Brains were cut using a cryostat and four to sixteen micron thick coronal sections were thaw-mounted onto either untreated or 0.05% poly-DL-ornithine (Sigma, St. Louis, MO; Catalog No. P-8638) coated 35 mm diameter plastic culture dishes (Fisher Scientific, Springfield, NJ; Catalog No. 1008) and kept at -20ºC until plating with cells 2 to 4 hours later. In some cases, after mounting onto untreated culture dishes, the sections were post-fixed with 10% formalin for 30 to 45 minutes at 4ºC and rinsed three times with Ham's F12 medium (Sigma). Control sections were treated for the same length of time at the same temperature with Ham's F12 medium rather than formalin.
Tissue culture
Lumbar sympathetic chain ganglia were dissected from embryonic day 10 Leghorn chicken embryos (Spafas, Inc., Boston, MA) in Ham's F12 medium. In some cases, the sympathetic chain ganglia were further dissected into explants (area = 3,000 to 45,000 μm2) using a Bard-Parker scalpel fitted with a No. 10 blade. These explants were seeded either onto 0.05% poly-DL-ornithine coated 35 mm diameter plastic culture dishes or onto the prepared tissue sections. In other cases, the sympathetic chain ganglia were incubated with 0.25% trypsin (Sigma) for 20 minutes at 37ºC. Trypsinization was subsequently blocked by exposure to 100% heat-inactivated fetal bovine serum (FBS; Harlan Bioproducts for Science, Inc., Indianapolis, IN) for 5 minutes and the tissue was washed 2 times with serum-free Ham's F12 medium. The tissue was then dissociated by gentle trituration using flamed Pasteur pipets (Fisher; Catalog No. 13-678-6A) and the cell suspension was seeded onto the prepared tissue sections. All cultures were grown in serum-free Neurobasal™ medium (2 ml per dish) supplemented with B27 (Gibco BRL, Grand Island, NY; 50:1 v/v), 0.5 mM L-glutamine (Sigma) and 100 units/ml penicillin-streptomycin (Gibco; Catalog No. 15140-015). Cultures were grown for 39 hours to 7 days in a humidified environment at 37ºC and 6% CO2.
Treatments
Some suspensions of neurons were preincubated at 37ºC with 200 ng/ml nerve growth factor (NGF; Harlan; product code BT-5017) for 21 hours prior to plating. Control suspensions were preincubated in Neurobasal medium for 21 hours at 37ºC. Bioactivity of the NGF was confirmed by culturing explants of sympathetic chain ganglia on polyornithine-coated dishes with or without 25 ng/ml NGF and assessing the extent of neurite outgrowth after 6 days. Some cultures were established with 1 mM dibutyryl-cAMP (db-cAMP; Calbiochem, San Diego, CA; Catalog No. 28745) and were retreated with 1 mM db-cAMP every other day. Control cultures were treated with equal volumes of Neurobasal medium.
Evaluation of neurite growth and substrate anatomy
After 39 hours to 7 days in culture, all media were removed and replaced with Ham's F12 medium. Each dish was treated with 2.5 ng/ml of 5-carboxyfluorescein diacetate, acetoxymethyl ester (vital dye; Molecular Probes, Eugene, OR) for 45 to 90 minutes at 37°C. Subsequently, all media were removed and replaced with fresh Ham's F12 medium. The cultures were then visualized using a Nikon Diaphot fluorescent microscope with a fluorescein filter and a 4x or 10x objective. Substrate histology was visualized using phase-contrast optics or by immunohistochemically labelling glial fibrillary acidic protein (GFAP). In the latter case, the cultures were incubated with a Cy3-conjugated monoclonal antibody raised against porcine GFAP (1:400; Sigma; Product No. C9205) simultaneously with labelling of the neurons with vital dye. GFAP-immunoreactivity was visualized using the same microscope with a rhodamine filter. Photomicrographs were captured with a Nikon 35 mm camera. In other cases, digital images were captured to a Power Macintosh microcomputer with a Data Translation framegrabber card and electronically enhanced to increase contrast.
Quantification of neurite length and orientation
The orientation and spatial extent of the corpus callosum in sections used as substrata were first assessed using phase-contrast or GFAP immunoreactivity. The orientation of the tract was measured by drawing a line parallel to its edges and measuring the angle of this line using NIH Image 1.60 software. The spatial extent of the tract was marked by tracing around the perimeter of the visible tract using the polygon tool. Three subregions of the corpus callosum were analyzed separately: the horizontal segment at the midline, and the two segments oriented at roughly 45° angles from points just lateral to the horizontal midline segment to the dorsal callosal apex immediately ventral to the widest portion of the cingulum. More lateral portions of the corpus callosum were avoided because of the difficulty in assessing the orientation of their composing fibers. Both the orientation of the tract and its spatial extent were recorded by an NIH Image macro program custom-made for this analysis.
This image was then closed and replaced by an image of the same field showing the neurons and their neurites but not the anatomy of the section. Neurite length and orientation were then assessed. A neuron was included for analysis only if its neurite did not form fascicles with other neurites and if it was possible to visually follow the entire length of the neurite. Neurites located on the curved flexures between the previously described 3 segments of the corpus callosum were avoided since it is difficult to assign a fixed orientation to the underlying corpus callosum. Neurite length was assessed by tracing the neurite using the straight-line tool in NIH Image. The net length, i.e. the straight-line distance between the cell body and growth cone, was measured in pixels and then converted to micrometers using a known physical standard. From vector arithmetic we know that this distance is the weighted (by segment length) average of the orientations of each of the segments along the total length of the neurite. The macro program automatically determined whether the neuronal cell body was located within the polygon-delimited spatial extent of the fiber tract and excluded the neuron from the analysis if it was not. Neurite orientation was defined as the angle of the line traced over the neurite. The final measure of orientation for each neurite, the deviation from an orientation parallel to the longitudinal axis of the tract, is the acute angle between the neurite and the longitudinal axis of the tract without regard to the polarity of either. This value ranged from 0° to 90° where 0° indicates a neurite growing in parallel with the longitudinal axis of the underlying tract and 90° indicates a neurite oriented perpendicular to the tract. Analysis of neurite length and orientation were made by an observer without knowledge of which experimental group was being assessed. All statistical comparisons of neurite length were made using two-tailed, unpaired Student's t-tests. Statistical comparisons of neurite orientation were made using one-tailed, unpaired Student's t-tests.
Myelin histochemistry
Sections adjacent to those used as substrata for culture were stained using the Rip immunohistochemical technique for labelling oligodendrocytes and their myelinating processes [50]. Sixteen micron thick sections were mounted on gelatin-coated slides and were post-fixed in 4% paraformaldehyde for 30 minutes at room temperature. The sections were then rinsed with .1 M phosphate-buffered saline (PBS; 3 times, 11 minutes each) and blocked with 20% FBS + 1% Bovine Serum Albumin (Sigma) + 0.1% Triton X-100 (Sigma) in .1 M PBS. The sections were rinsed again and incubated overnight at 4°C with the Rip primary antibody (Developmental Studies Hybridoma Bank, Iowa City, IA; diluted 1:4 in .1 M PBS). The following day, the sections were rinsed again and incubated for 30 minutes at room temperature with FITC-conjugated sheep anti-mouse IgG (Boehringer-Mannheim; diluted 1:100). The sections were again rinsed and cover-slipped using 90% glycerol as a mounting solution and then visualized using a Nikon Microphot microscope with a fluorescein filter and a 4x, 10x or 20x objective. Photomicrographs were captured with a Nikon 35 mm camera.
Other adjacent sections were stained using the Luxol Fast Blue technique [51]. Sixteen micron thick sections were mounted on gelatin-coated slides and were post-fixed in 4% paraformaldehyde for 30 minutes at room temperature. The sections were then dehydrated by immersion in 70% ethanol (3 immersions, 6 minutes each) and then transferred to 95% ethanol for 6 minutes. The sections were then stained with Luxol Fast Blue (in 95% ethanol) for 13 minutes at room temperature, rinsed twice with 100% ethanol and cleared overnight in a third 100% ethanol rinse. Subsequently, the sections were rinsed 3 times in xylene and coverslipped using Permount (Fisher) as a mounting solution.
Acknowledgments
Acknowledgements
This work was supported by the Mayfield Education and Research Foundation. The myelin-deficient brains used in this study and their controls were kindly provided by Dr. Judith B. Grinspan. We also thank Krissy Shockley for her excellent technical assistance and Dr. Linda Levin for her assistance with statistical analysis. A portion of this work has been reported in abstract form at the 1999 and 2000 meetings of the Society for Neuroscience.
Contributor Information
David B Pettigrew, Email: David.Pettigrew@uth.tmc.edu.
Keith A Crutcher, Email: crutchka@email.uc.edu.
References
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System London, UK: Oxford University Press; 1928.
- Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher KA. Tissue sections from the mature rat brain and spinal cord as substrates for neurite outgrowth in vitro: extensive growth on gray matter but little growth on white matter. Exp Neurol. 1989;104:39–54. doi: 10.1016/0014-4886(89)90007-1. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Privitera M. Axonal regeneration on mature human brain tissue sections in culture. Ann Neurol. 1989;26:580–583. doi: 10.1002/ana.410260414. [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Jr, Matthew WD. Identification of a peripheral nerve neurite growth-promoting activity by development and use of an in vitro bioassay. Proc Natl Acad Sci U S A. 1987;84:6934–6938. doi: 10.1073/pnas.84.19.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan D, Berry M, Bedi K, Cohen J. Embryonic optic nerve tissue fails to support neurite outgrowth by central and peripheral neurons in vitro. Eur J Neurosci. 1993;5:809–817. doi: 10.1111/j.1460-9568.1993.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Murakami F. Preferential adhesion of chick central neurons to the gray matter of the central nervous system. Neurosci Lett. 1989;97:69–74. doi: 10.1016/0304-3940(89)90141-9. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Murakami F. Cell attachment to and neurite outgrowth on tissue sections of developing, mature and lesioned brain: the role of inhibitory factor(s) in the CNS white matter. Neurosci Res (N Y) 1990;8:83–99. doi: 10.1016/0168-0102(90)90061-I. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration? Curr Opin Neurobiol. 1995;5:588–595. doi: 10.1016/0959-4388(95)80063-8. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Niederöst BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J Neurosci. 1999;19:8979–8989. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Bandtlow CE, Nicholls J. Developmental expression of myelin-associated neurite growth inhibitors correlates with the loss of regeneration after spinal cord lesions in the opposum. Soc Neurosci Abstr. 1993;19:283–219. [Google Scholar]
- Davies SJ, Field PM, Raisman G. Long fibre growth by axons of embryonic mouse hippocampal neurons microtransplanted into the adult rat fimbria. Eur J Neurosci. 1993;5:95–106. doi: 10.1111/j.1460-9568.1993.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Field PM, Raisman G. Long interfascicular axon growth from embryonic neurons transplanted into adult myelinated tracts. J Neurosci. 1994;14:1596–1612. doi: 10.1523/JNEUROSCI.14-03-01596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C, Bygdeman M, Olson L, Strömberg I. Human fetal neocortical tissue grafted to rat brain cavities survives, leads to reciprocal nerve fiber growth, and accumulates host IgG. J Comp Neurol. 1994;340:337–348. doi: 10.1002/cne.903400305. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Lesauter J, Silver R. Fiber outgrowth from anterior hypothalamic and cortical xenografts in the third ventricle. J Comp Neurol. 1998;391:133–145. doi: 10.1002/(SICI)1096-9861(19980202)391:1<133::AID-CNE11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tønder N, Sørensen T, Zimmer J. Grafting of fetal CA3 neurons to excitotoxic, axon-sparing lesions of the hippocampal CA3 area in adult rats. Prog Brain Res. 1990;83:391–409. doi: 10.1016/s0079-6123(08)61264-9. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Simerly RB, Isacson O, Swanson LW, Björklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum-III. Efferent projecting graft neurons and their relation to host afferents within the grafts. Neuroscience. 1989;30:313–330. doi: 10.1016/0306-4522(89)90256-X. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Brundin P, Gustavii B, Lindvall O, Björklund A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990;347:556–558. doi: 10.1038/347556a0. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Clarke DJ, Bolam JP, Björklund A. Fetal striatal neurons grafted into the ibotenate lesioned adult striatum: efferent projections and synaptic contacts in the host globus pallidus. Neuroscience. 1990;37:301–315. doi: 10.1016/0306-4522(90)90401-O. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Lagenaur CF, Lund RD, Björklund A. Efferent projections to the host brain from intrastriatal mouse-to-rat grafts: time course and tissue-type specificity as revealed by a mouse specific neuronal marker. Eur J Neurosci. 1991;3:86–101. doi: 10.1111/j.1460-9568.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Brundin P, Sauer H, Lindvall O, Björklund A. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats. J Comp Neurol. 1992;323:475–494. doi: 10.1002/cne.903230403. [DOI] [PubMed] [Google Scholar]
- Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J Neurosci. 1995;15:2057–2062. doi: 10.1523/JNEUROSCI.15-03-02057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew DB, Crutcher KA. White matter of the CNS supports or inhibits neurite outgrowth in vitro depending on geometry. J Neurosci. 1999;19:8358–8366. doi: 10.1523/JNEUROSCI.19-19-08358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Bregman BS, Hoffman PN, Filbin MT. cAMP effects in the improved regeneration of dorsal root ganglion neurons after a conditioning lesion. Soc Neurosci Abstr. 2000;26:230–238. [Google Scholar]
- Dentinger MP, Barron KD, Csiza CK. Ultrastructure of the central nervous system in a myelin deficient rat. J Neurocytol. 1982;11:671–691. doi: 10.1007/BF01262431. [DOI] [PubMed] [Google Scholar]
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Segmentation in the vertebrate nervous system. Nature. 1984;310:786–789. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Tosney KW. Contact-mediated mechanisms of motor axon segmentation. J Neurosci. 1993;13:3773–3792. doi: 10.1523/JNEUROSCI.13-09-03773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Dev Biol. 1982;93:13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Rajnicek AM, Drever JS, Nangle MR, McCaig CD. Hierarchy of directional cues in CNS growth cone guidance: substratum shape versus a weak electric field. Soc Neurosci Abstr. 1999;25:711–718. [Google Scholar]
- Cadelli D, Schwab ME. Regeneration of lesioned septohippocampal acetylcholinesterase-positive axons is improved by antibodies against the myelin-associated neurite growth inhibitors NI-35/250. Eur J Neurosci. 1991;3:825–832. doi: 10.1111/j.1460-9568.1991.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Weibel D, Cadelli D, Schwab ME. Regeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitors. Brain Res. 1994;642:259–266. doi: 10.1016/0006-8993(94)90930-X. [DOI] [PubMed] [Google Scholar]
- Huber AB, van der Haar ME, Bröesamle C, Schnell L, Weinmann O, Schwab ME. Subcellular localisation of Nogo-A, a major myelin-associated neurite outgrowth inhibitor. Soc Neurosci Abstr. 2000;26:217–212. [Google Scholar]
- Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Schnell L. Channeling of developing rat corticospinal tract axons by myelin-associated neurite growth inhibitors. J Neurosci. 1991;11:709–721. doi: 10.1523/JNEUROSCI.11-03-00709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Z'Graggen WJ, Thallmair M, Schwab ME. Sprouting and regeneration after pyramidotomy and blockade of the myelin-associated neurite growth inhibitors NI 35/250 in adult rats. Eur J Neurosci. 1999;11:1486–1490. doi: 10.1046/j.1460-9568.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Friedman B, Hockfield S, Black JA, Woodruff KA, Waxman SG. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2:380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- Humason GL. Animal Tissue Techniques San Francisco, CA: W H Freeman and Company; 1972.