Abstract
The extent to which bone marrow (BM) contributes to physiological cell renewal is still controversial. Using the marker human placental alkaline phosphatase (ALPP) which can readily be detected in paraffin and plastic sections by histochemistry or immunohistochemistry, and in ultrathin sections by electron microscopy after pre-embedding staining, we examined the role of endogenous BM in physiological cell renewal by analysing tissues from lethally irradiated wild-type inbred Fischer 344 (F344) rats transplanted (BMT) with unfractionated BM from ALPP-transgenic F344 rats ubiquitously expressing the marker. Histochemical, immunohistochemical and immunoelectron microscopic analysis showed that the proportion of ALPP+ capillary endothelial cells (EC) profoundly increased from 1 until 6 months after BMT in all organs except brain and adrenal medulla. In contrast, pericytes and EC in large blood vessels were ALPP–. Epithelial cells in kidney, liver, pancreas, intestine and brain were recipient-derived at all time-points. Similarly, osteoblasts, chondrocytes, striated muscle and smooth muscle cells were exclusively of recipient origin. The lack of mesenchymal BM-derived cells in peripheral tissues prompted us to examine whether BMT resulted in engraftment of mesenchymal precursors. Four weeks after BMT, all haematopoietic BM cells were of donor origin by flow cytometric analysis, whereas isolation of BM mesenchymal stem cells (MSC) failed to show engraftment of donor MSC. In conclusion, our data show that BM is an important source of physiological renewal of EC in adult rats, but raise doubt whether reconstituted irradiated rats are an apt model for BM-derived regeneration of mesenchymal cells in peripheral tissues.
Keywords: bone marrow transplantation, human placental alkaline phosphatase, endothelial cells, stem cells, physiological cell renewal
Introduction
Insights into the mechanisms of physiological cell renewal in adult organs are of fundamental importance for medicine and biology. Earlier reports indicate that bone marrow (BM)-derived precursor cells play a role in cell renewal and repair in many adult organs. For example, female patients receiving sex-mismatched bone marrow transplantation (BMT) show integration of male donor cells into brain as neurons, into heart as cardiomyocytes, into kidney as tubular epithelial cells, into liver as hepatocytes and cholangiocytes, and into lung as epithelial and endothelial cells (EC; reviewed in [1]). Similarly, irradiated mice reconstituted with green fluorescent protein (GFP) or lacZ-labelled BM show participation of BM-derived cells in the renewal of endothelial [2], epithelial [3, 4] and mesenchymal [5] cells in various organs. In addition, it was reported that mobilization of endogenous bone marrow cells (BMC) after acute myocardial infarction contributes to regeneration of cardiac muscle [6]. Collectively, these findings have lead to the concept that circulating BM-derived precursor cells participate in physiological cell renewal and repair in many adult organs. Furthermore, it was suggested that haematopoietic stem cells may trans-differentiate into epithelial and also mesenchymal lineage cells [4, 7–9]. However, these interpretations have been questioned on the ground of data indicating methodological problems involved in some of these studies [10–12]. Therefore, the role of BM in physiological cell renewal is still a matter of controversy.
Recent work from our laboratory has shown that human placental alkaline phosphatase (ALPP) is a highly suitable marker enzyme for histological tracking of genetically labelled cells in all tissues, including hard tissues. In contrast to endogenous heat-labile alkaline phosphatases, ALPP is heat-stable and therefore its enzymatic activity is retained after heat pre-treatment of paraffin and methylmethacrylate sections [13]. As a consequence, ALPP-labelled cells are easily detectable histologically in the total absence of background staining. To examine the role of BM in physiological cell renewal in various organs, we lethally irradiated 3-month-old wild-type (wt) Fischer 344 (F344) rats, and reconstituted them with unfractionated BM from R26-ALPP transgenic (ALPP-tg) sex-matched F344 donors. R26-ALPP-tg rats express ALPP in a ubiquitous and stable fashion under the R26 promoter, which is a 0.8 kb fragment of the ROSA βgeo 26 promoter sequence [13–15]. ALPP is mainly expressed in the cell membrane, irrespective of the cell type analysed [13, 16], and is developmentally neutral in transgenic rats and mice [14]. Graft-versus-host reactions can be ruled out a priori in this co-isogeneic BMT model, because F344 rats are an inbred strain. In this sequential study, the reconstituted rats were followed over a 6-month period after BMT.
Materials and Methods
Animals
All experimental procedures were conducted in compliance with prevailing animal welfare regulations. Hemizygous male or female R26-F344 ALPP-tg rats were mated with wt F344 rats, and the resulting wt and hemizygous tg offspring were genotyped as described [14]. Rats were housed in pairs at 24°C and a 12 hrs/12 hrs light/dark cycle with free access to tap water and commercial rat diets (Altromin, Lage, and Ssniff, Soest, Germany).
Lethal irradiation and bone marrow transplantation
Three-month-old wt F344 rats were lethally irradiated with a single dose of 8.5 Gy using a cobalt-60 irradiator (Eldorado, Atomic Energy of Canada, Ottawa, Canada), or with a single dose of 8.0 or 9.0 Gy using a linear accelerator (Siemens Primus, Munich, Germany). Four hours after irradiation, rats were intravenously injected with 4 × 106 unfractionated BMC isolated from sex-matched ALPP-tg co-isogeneic F344 donors. To rule out unsuccessful engraftment, injection of freshly prepared tg BMC was repeated 24 hrs after irradiation. For the time course study, groups of four to six rats each were killed 1, 2, 4 and 6 months after BMT through exsanguination from the abdominal aorta under ketamine/xylazine anaesthesia. For mesenchymal stem cells (MSC) isolation experiments, animals were killed 4 weeks after BMT.
Flow cytometric detection of ALPP
To determine the degree of chimerism in haematopoietic BMC after BMT, unfractionated BMC were harvested and analysed by fluorescence-activated cell sorting (FACS) as described [16], using a monoclonal anti-ALPP antibody (Chemicon, Temecula, CA, USA) and rat-adsorbed, fluorescein isothiocyanate (FITC)-labelled goat antimouse IgG antibody (Sigma-Aldrich, Deisenhofen, Germany). The standard curve for determination of the degree of chimerism was obtained by mixing wt BMC with BMC from ALPP-tg rats at various known ratios.
ALPP histology and detection
Tissue samples of heart, lung, liver, kidney, lymph nodes, spleen, brain, skeletal muscle, skin and bones were fixed in 40% ethanol at 4°C for 48 hrs, dehydrated and embedded in paraffin or modified methylmethacrylate [15]. Five-micrometre-thick sections were mounted on slides pre-treated with 3-aminopropyltriethoxy-silane (Sigma-Aldrich). Deparaffinated or deplasticized sections were rehydrated and heated at 65°C for 30 min. in deionized water to block endogenous ALPP activity. Cells expressing ALPP were histochemically stained by incubation with an alkaline phosphatase (AP) substrate (0.1 M Tris-HCl, pH 9.5, 0.1 M NaCl, 5 mM MgCl2, containing 0.175 mg/ml of the substrate 5-bromo-4-chloro-3-indolyl phosphate [BCIP, Sigma] and 0.45 mg/ml nitrotetrazolium blue chloride [NBT, Sigma]) at room temperature (RT) overnight. Subsequently, sections were counterstained with nuclear fast red (Sigma-Aldrich), dehydrated, and cover-slipped using Vectamount (Vector, Burlingame, CA, USA).
The combination of histochemistry for ALPP detection and immunohistochemistry (IHC) for the detection of various antigens was performed as follows: In a first step, histochemical detection of ALPP+ cells was performed after heat inactivation of endogenous ALPP as described above by incubating the slides for 4 hrs with the AP substrate Vector Blue (Vector) at RT in the dark. For vimentin staining, slides were pre-treated in the microwave for 2 × 3 min. in citrate buffer pH 6. After quenching of endogenous peroxidase activity by using 3% H2O2 in phosphate-buffered saline (PBS) for 15 min., slides were incubated with 20% horse, goat or rabbit serum (Vector) for 20 min. Thereafter, slides were incubated with mouse anti-human smooth muscle actin (SMA; Dako, Glostrup, Denmark) diluted 1:200, mouse anti-vimentin (Dako) diluted 1:200, goat anti-rat CD34 (R&D, Wiesbaden-Nordenstadt, Germany) diluted 1:50, or mouse anti-rat CD68 (Serotec, Harwell, UK) diluted 1:100 in PBS containing 5% of the appropriate serum at 4°C overnight. For double staining of pancreatic samples, slides were incubated with guinea pig anti-porcine insulin (Dako) diluted 1:2000, rabbit anti-human pancreatic polypeptide (Dako) diluted 1:700 or rabbit anti-human somatostatin (Dako) diluted 1:700 for 2 hrs in PBS containing 5% rabbit respective goat serum at RT. Bound antibody was detected by incubation for 0.5–1 hrs at RT with biotinylated rat-adsorbed horse antimouse antibody 1:50 (Vector), horse anti-goat antibody 1:50 (Vector), rabbit anti-guinea pig IgG 1:100 (Dako) or biotinylated goat anti-rabbit antibody 1:200 (Vector), as appropriate. Staining was accomplished by applying avidin-biotin complex (ABC)-peroxidase (Vector) for 30 min., followed by incubation with the peroxidase substrate VIP (Vector) for 0.5 to 2 min., resulting in purple staining of positive cells. For vimentin and SMA co-staining, we first performed SMA-IHC, visualized by ABC-AP-complex (Vector) and Vector Blue using levamisole (Vector) for inhibition of endogenous APs and ALPP enzyme activity. Subsequently, after blocking with avidin/biotin (Vector), vimentin-IHC was performed as described, using ABC-peroxidase complex and VIP as substrate. For the staining of EC with the tomato lectin Lycopersicon esculentum, slides were incubated with the biotinylated lectin [Vector; 1:200 in hydroxethyl-piperazine-ethanesulfonic acid (HEPES) buffer for 2 hrs at RT] after histochemical ALPP staining and blocking of endogenous peroxidase. Visualization was performed with the ABC-peroxidase kit as described above. No counterstaining was carried out, and slides were cover-slipped using aqueous gelatine (Merck, Darmstadt, Germany).
ALPP immuno-electron microscopic analysis
For transmission electron microscopy, tissue samples from heart and kidney were fixed in 4% paraformaldehyde for 24 hrs at 4°C. Samples were frozen in liquid nitrogen with Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Zoeterwoude, Netherlands), and 45-μm-thick sections were cut using a cryotome (Leica 1800 CM, Bensheim, Germany). Sections were post-fixed in 4% paraformaldehyde for 1 hrs and rinsed in 0.1 M phosphate buffer 3 × 10 min. Peroxidase activity was inhibited by 3% H2O2 in PBS for 30 min. and non-specific binding was minimized by incubation with 3% normal goat serum containing 1% bovine serum albumin (BSA) for 60 min. at RT. Incubation with anti-ALPP (Genetex, Irvine, CA, USA), diluted 1:50 in blocking solution, was carried out at 4°C overnight. As a control, in order to visualize EC in general, sections were stained with anti-human von Willebrand factor (vWF, Dako), 1:600, or CD34 1:50. For negative controls, the primary antibody was omitted. Peroxidase-labelled rabbit PowerVision™ (ImmunoVision Technologies, Burlingame, CA, USA) secondary system was employed for antibody detection with subsequent DAB staining (Sigma-Aldrich). The reaction was stopped by rinsing with PBS 3 × 10 min. Sections were placed in 0.1 M phosphate buffer and kept at 4°C before embedding. Post-fixation was performed in 1% osmium tetroxide for 2 hrs at RT followed by dehydration and incubation in propylene oxide, propylene oxide-epon and subsequent embedding in pure epon 812. Thin sections were stained with lead citrate, and were investigated under a transmission electron microscope (Zeiss EM 900; Zeiss, Oberkochen, Germany).
Quantification of endothelial cells in myocardium
The percentage of ALPP+ capillary EC was quantified in ALPP-stained sections in seven optical fields per animal at ×400 magnification, evenly distributed over the left ventricular (five fields) and septal (two fields) myocardium. Using a one-phase exponential model, nonlinear regression analyses were performed with Prism 5.03 (GraphPad Software, Inc., San Diego, CA, USA).
MSC isolation, cultivation and staining
MSC were isolated from long bones of wt, ALPP-tg, and BMT rats, using an isolation protocol described elsewhere [17]. After 24 hrs, the non-adherent cell fraction was removed by washing twice with D-PBS (Invitrogen, Paisley, UK). After the primary culture had reached confluence, cells were washed twice with D-PBS, and subsequently treated with 0.05% trypsin / 1 mM ethylenediaminetetraacetic acid (EDTA) (Invitrogen) for 5 min. at 37°C. Cells were harvested, washed once in MEM and further expanded. For histochemical staining of cultivated MSC, MSC were seeded at 100,000/cm2 in culture flasks or at 1300/cm2 in 12-well plates and cultivated for 3 to 7 days until confluency. After fixation with ice-cold acetone-methanol (30/70), plates were washed several times with PBS and endogenous APs were inactivated at 65°C for 30 min. Histochemical staining for heat-stable ALPP was performed with the AP substrate BCIP/NBT as described above. Each cell preparation was stained at least in triplicates, MSC isolated from ALPP-tg and wt donors served as controls.
DNA isolation and Southern blot analysis of cultivated MSC
For DNA isolation, cells were covered with 100 mM Tris-HCl, pH 8.5, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, 100 μg/ml proteinase K and incubated at 55°C for 2 hrs. Before phenol/chloroform/iso-amylalcohol (PCI) extraction, debris was removed by centrifugation at 10,000 ×g. PCI extraction was repeated at least twice in order to yield a clear aqueous phase and interphase. Before alcohol precipitation, the solution was extracted once with chloroform/iso-amylalcohol. Large amounts of precipitated DNA were removed using a pipette tip, otherwise the precipitate was centrifuged and the pellet washed in 70% ethanol. The moist pellet was dissolved in 10 mM Tris-HCl pH 7.5, 1 mM EDTA by incubation overnight at 55°C and the solution was stored at 4°C. For Southern analysis, 10 μg of genomic DNA were restricted with 20 units of endonuclease EcoRI (NEB Biolabs, Ipswitch, MA, USA) together with 1 μg/μl RNAseA (Fermentas, St. Leon-Rot, Germany) in an appropriate buffer at 37°C overnight. The DNA was then separated by 1% Tris-acetate-EDTA (TAE)/agarose gel electrophoresis, and the ethidium bromide-stained gel was photo documented under UV irradiation. Before transfer to Biodyne® Plus Nylon membrane (Pall Corporation, Dreieich, Germany) in 10× SSPE, the gel was immersed for 15 min. in 0.25 N HCl, followed by 30 min. 0.4 N NaOH/0.6 M NaCl, and a 30 min. incubation in 1.5 M NaCl/0.5 M Tris-HCl, pH 7.5. The transferred DNA was immobilized onto the membrane by UV irradiation (0.12 J/cm2; Stratalinker, Stratgene, La Jolla, CA, USA). The dried membrane was prehybridized for 2 hrs at 65°C with 5× SSC, 5× Denhardt, 1% SDS. After replacement of buffer, a 33P-labelled probe specific to the transgene insert was hybridized for 18 hrs at 65°C. The membrane was washed (0.2× SSPE, 0.1% SDS at 65°C) and exposed for 72 hrs to a Fuji imaging plate (MS30170095), which was subsequently scanned by a Fuji Bas 1800-II phosphoimager (Fuji, Tokyo, Japan).
Results
The current study employed two different radiation sources, namely a cobalt-60 source and a linear accelerator. We reported previously that irradiation of F344 rats at a single dose of 8.5 Gy using a cobalt-60 irradiator followed by transplantation with ALPP-tg BM results in full replacement of the haematopoietic compartment as documented by FACS analysis of BMC [16]. In dose–response experiments with the linear accelerator, we found in two independent experiments that a single irradiation dose of 9 Gy was necessary to obtain wt F344 rats in which host BMC were fully replaced by ALPP-tg BMC (Fig. 1H, I).
Fig 1.
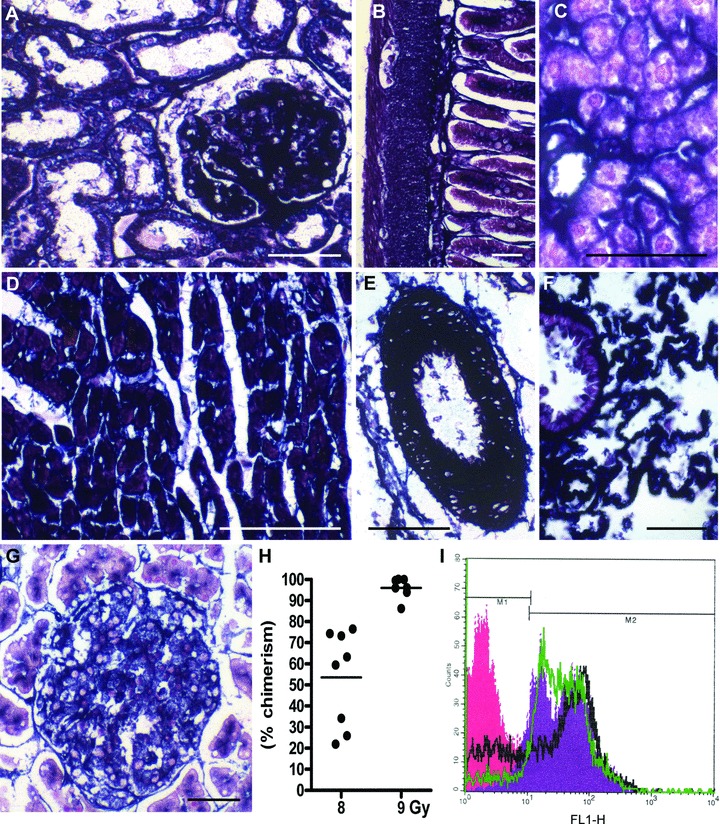
Expression of the marker enzyme ALPP in tissues of ALPP-transgenic (ALPP-tg) F344 rats and chimerism after reconstitution of irradiated wt rats with ALPP-tg BM. (A)–(G) Histochemical staining showed strong and ubiquitous expression of ALPP in cells of epithelial, endothelial and mesenchymal origin in kidney (A), gut (B), liver (C), heart muscle (D), arteries (E), lung (F), as well as exocrine and endocrine pancreas (G) in ALPP-tg rats. The 5-μm-thick paraffin sections shown in (A)–(G) were stained for ALPP enzyme activity (BCIP/NBT, purple) overnight at RT after heat pre-treatment, and were counterstained with nuclear fast red. Bars represent 50 μm. (H)–(I) FACS analysis of BMC isolated from wt rats lethally irradiated with a linear accelerator, using a single dose of 8 or 9 Gy, and reconstituted with unfractionated BM harvested from ALPP-tg donors. Irradiation with 9 Gy resulted in high ALPP chimerism (96.9 ± 6.4%), whereas chimerism was much lower using a single dose of 8 Gy (53.6 ± 22.7%). Chimerism was determined on the basis of a standard curve. Representative single parameter fluorescence histogram (I) shows hPLAP expression (marker M2) on nearly all BMC in reconstituted wt rats after irradiation with 9 Gy (green line), similar to BMC from hPLAP-tg rats (violet area). Irradiation with 8 Gy (black line) resulted in lower degree of chimerism. The red area depicts ALPP– (marker M1) BMC from wt control rats.
A prerequisite for our study was that ALPP is ubiquitously expressed in the target tissues to be analysed. Our earlier work had shown that ALPP is strongly expressed in haematopoietic cells, lung, spleen, lymph nodes and in BM stromal cells such as osteoblasts, osteocytes and chondrocytes in R26-ALPP-tg rats [13, 16]. In the current study, we examined the expression of ALPP in kidney, gut, liver, heart, arteries, lung and pancreas of ALPP-tg rats. We found strong expression of the marker enzyme in endothelial, epithelial and mesenchymal cells in all these tissues (Fig. 1A–G).
When we started to examine the occurrence of ALPP+ cells in various organs of irradiated wt rats reconstituted with unfractionated BM from ALPP-tg donors, the most striking finding was a high number of ALPP+ endothelial-like cells in capillaries of kidney (Fig. 2A), lung (data not shown), pancreas (Fig. 2B), liver (Fig. 2C) and heart (Fig. 2D–F). In contrast, ALPP labelling of EC was absent in the medulla of adrenal glands (data not shown), in brain (data not shown) and in large blood vessels such as aorta (Fig. 2G), arteries, or veins (not shown). To explore further the nature of the ALPP-labelled endothelial-like cells, we performed co-staining experiments. Co-staining of ALPP enzyme activity with tomato lectin (Fig. 2H) or monoclonal mouse anti-CD34 (data not shown) suggested that the majority of the ALPP+, endothelial-like cells were indeed EC in the heart. However, in kidneys we observed many ALPP+ endothelial-like cells which stained negative for tomato lectin and CD34. In addition, in most organs we found ALPP+ cells located in the immediate vicinity to blood vessels (Fig. 2I), staining negative for tomato lectin or CD34, as shown here for liver. We hypothesized that these cells might represent pericytes. In order to answer the question whether EC in lymph capillaries were ALPP+, we tried to specifically stain lymph vessels by using an antibody specific to lymphatic vessel endothelial hyaluronan receptor-1. However, specific immunostaining of lymph vessels failed in our ethanol-fixed tissue samples (data not shown). Similarly, anti-human vWF staining did not work reliably in our ethanol-fixed paraffin sections (data not shown).
Fig 2.
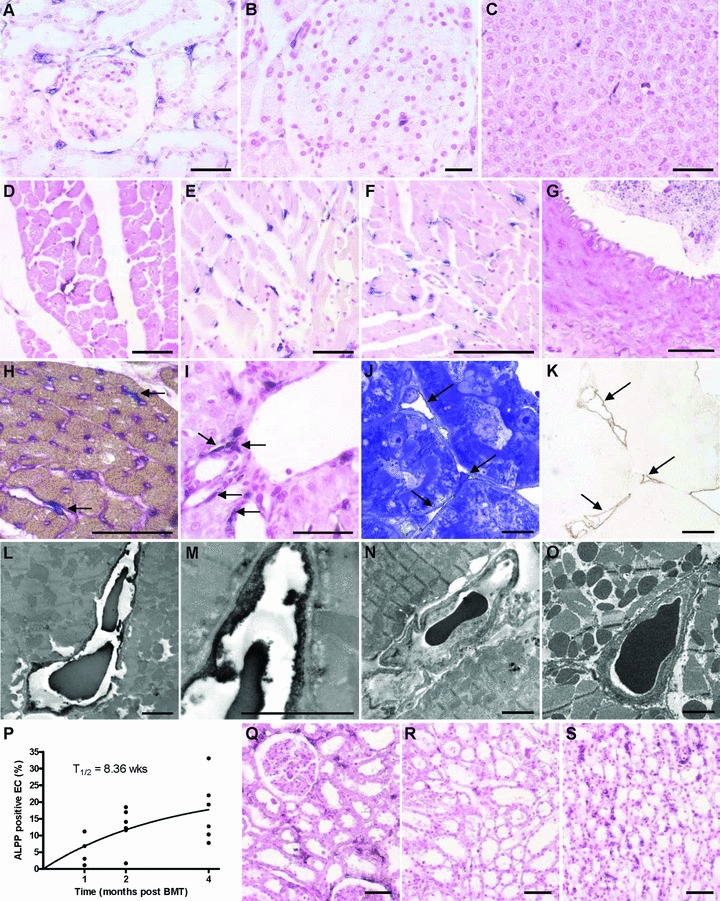
Light and electron microscopic analysis of tissues from wt F344 rats, lethally irradiated and reconstituted with BM from ALPP-tg F344 donors. ALPP+ endothelial-like cells in capillaries are evident in kidney (A), pancreas (B) and liver (C), 2 months after BM transplantation (BMT). The number of ALPP+ endothelial-like cells increased with time in all organs as shown for heart tissue 1 (D), 2 (E) and 4 (F) months after BMT. In contrast, ALPP+ EC were absent in large blood vessels such as the Aorta thoracica (G), arteries or veins (not shown). (H) Double staining (arrows) for ALPP by histochemistry (blue) and for tomato lectin Lycopersicon esculentum (purple) confirmed the endothelial nature of the putative BM-derived capillary EC in the myocardium, 2 months after BMT. (I) ALPP+ cells in the vicinity of blood vessels as shown here in liver. Semi-thin serial sections of epon-embedded, 45-μm-thick kidney cryosections stained immunohistochemically against ALPP by a pre-embedding protocol and counterstained with toluidine blue (J) or left without counterstaining (K, brown DAB immunoprecipitate) show that the anti-ALPP staining (arrows in K) co-localized with capillaries situated between renal tubuli (arrows in J), 6 months after BMT. (L)–(O) Transmission electron microscopy (TEM) of ultra-thin sections of the heart muscle after pre-embedding anti-ALPP staining clearly showed that the BM-derived ALPP+ endothelial-like cells are indeed capillary EC, 6 months after BMT. Higher magnification is shown in (M). Anti-ALPP staining was absent in control sections of hearts from BMT rats when the primary antibody was omitted (N) or in wt controls (O). (P) Nonlinear regression analysis of the time dependent increase in BM-derived EC in the heart muscle revealed a half time of 8.36 weeks for these cells. (Q)–(S) In the kidney, EC turnover showed marked inhomogeneity. BM-derived ALPP+ EC were more frequent in cortical regions (Q) and in the Vasa recta of the papilla (S) than in medullary areas (R) throughout the study period, as demonstrated here at 2 months after BMT. The 5-μm-thick paraffin sections shown in (A)–(G), (I) and (Q)–(S) were stained for ALPP enzyme activity with BCIP/NBT (purple) overnight at RT after heat pre-treatment, and were counterstained with nuclear fast red. Semi-thin and ultra-thin sections shown in (J)–(O) were immunostained using a monoclonal anti-ALPP antibody by the pre-embedding protocol described in ‘Methods’. Bars represent 50 μm for light microscopy and 2.5 μm for transmission electron microscopy.
In order to unequivocally document the nature of the ALPP-expressing endothelial- and pericyte-like cells, we developed a pre-embedding anti-ALPP immunostaining method for electron microscopic analysis. Similar to paraffin histology, semi-thin sections of epon-embedded thick kidney cryosections stained with anti-ALPP antibody by a pre-embedding protocol suggested that the BM-derived ALPP+ cells represent capillary EC situated between the renal tubuli (Fig. 2J, K). Figure 2L and M shows a representative ultra-thin section of the immunostained heart with the appropriate negative controls (Fig. 2N, O). The capillary EC shown in Figure 2L and at higher magnification in Figure 2M stained clearly positive for the marker enzyme, whereas staining was absent in the negative controls. In heart and kidney, we found no evidence for ALPP-labelled pericytes or ALPP-labelled EC in lymph capillaries by immuno-electron microscopy.
Two other observations made in this experiment are noteworthy. First, the number of labelled capillary EC increased with time after transplantation in all analysed tissues, as demonstrated for heart muscle in Figure 2D–F. Under the assumption of a steady state the increase in labelling index should follow a one-phase exponential model [18]. Nonlinear regression analysis of the increase in labelling index over time revealed a half time of 8.36 weeks (Fig. 2P), and a plateau of 24 ± 14%. These findings suggest that about a fourth of the total capillary EC in the heart are BM derived, and that this myocardial EC compartment undergoes rapid turnover. Second, in the kidney, BM-derived regeneration of EC was not uniform, but followed a distinct pattern: Replacement was most intense in the cortical regions (Fig. 2Q), in the glomerula (Fig. 2Q), and in the vasa recta of the renal papilla (Fig. 2S), but low in the medullary regions (Fig. 2R).
Epithelial cells in BMT rats are host-derived
While epithelial cells show strong ALPP expression in ALPP-tg rats (Fig. 1A–C, F–G), we never observed a single ALPP+ epithelial cell at any time-point in gut (Fig. 3A), kidney (Fig. 3B), liver (Fig. 3C), skin (Fig. 3D), brain (not shown), lung (Fig. 3E) or pancreas (Fig. 3F–H) in our BMT rat model. The ALPP+ cells in these tissues were EC, leucocytes, and some glial cells in the brain (data not shown). The number of ALPP-labelled Kupffer cells in the liver (data not shown) and of alveolar macrophages (Fig. 3E) in the lung distinctly increased with time after BMT, showing that there is a high turnover of these tissue-specific macrophages in liver and lung. However, ALPP– CD68+ alveolar macrophages could be found even 4 months after BMT, suggesting that some of these cells have a lifespan exceeding 4 months in the adult rat, even after irradiation (Fig. 3E). Figure 3F to H shows that ALPP+ cells found in islets of Langerhans did not stain positive for insulin (F), somatostatin (G) or pancreatic polypeptide (H), 6 months after BMT.
Fig 3.
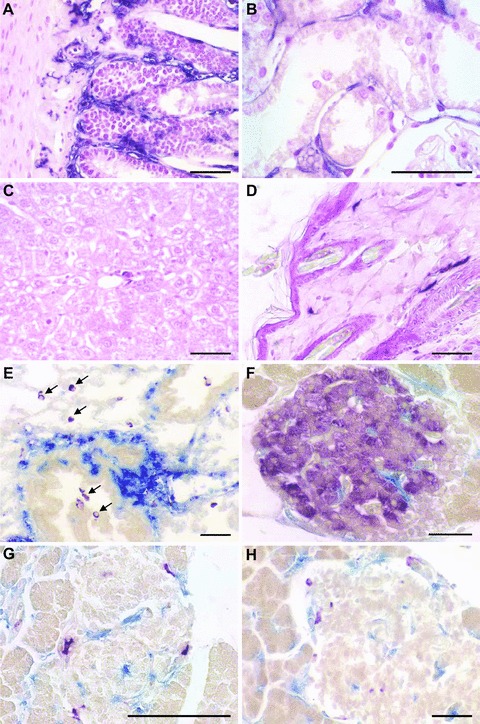
Lack of BM-derived ALPP+ epithelial cells in irradiated wt F344 rats reconstituted with unfractionated BM from ALPP-tg F344 donors. Histochemical detection of ALPP enzyme activity (BCIP/NBT, purple) reveals absence of BM-derived epithelial cells in gut (A), kidney tubules (B), liver (C), or skin (D), 6 months after BMT. (E) Co-staining of ALPP enzyme activity (blue) and anti-CD68 antibody (purple) in lung sections shows many double positive alveolar macrophages (centre), but also some CD68+ ALPP– macrophages as indicated by arrows, 4 months after BMT. Double staining of ALPP enzyme activity (blue) with anti-insulin (F), anti-somatostatin (G) or anti-pancreatic polypeptide (H) (purple) demonstrates lack of BM-derived endocrine cells in islets of Langerhans. The paraffin sections shown in (A–D) were stained for ALPP enzyme activity with BCIP/NBT (purple) overnight at RT after heat pre-treatment, and were counterstained with nuclear fast red. The paraffin sections in (E–H) were stained for ALPP enzyme activity using Vector Blue, and immunostained against CD68, insulin, somatostatin, or pancreatic polypeptide using Vector VIP (purple) as substrate. Bars represent 50 μm.
Lack of BM-derived mesenchymal cells in BMT rats
Osteoblasts, osteocytes and chondrocytes were exclusively ALPP– in bones of BMT rats until the end of study, i.e. 6 months after BMT (Fig 4A–C). Histochemical staining of blood vessels (Fig. 4D) and skeletal muscle (Fig. 4E) did not provide evidence for BM-derived, ALPP+ smooth muscle cells or muscle fibres. Similarly, when we examined smooth muscle cells in the intestine, only extremely few ALPP+ cells stained also positive for SMA (Fig. 4F). Most ALPP+ cells were located between SMA-stained smooth muscle cells, and likely represent EC (Fig. 4G).
Fig 4.
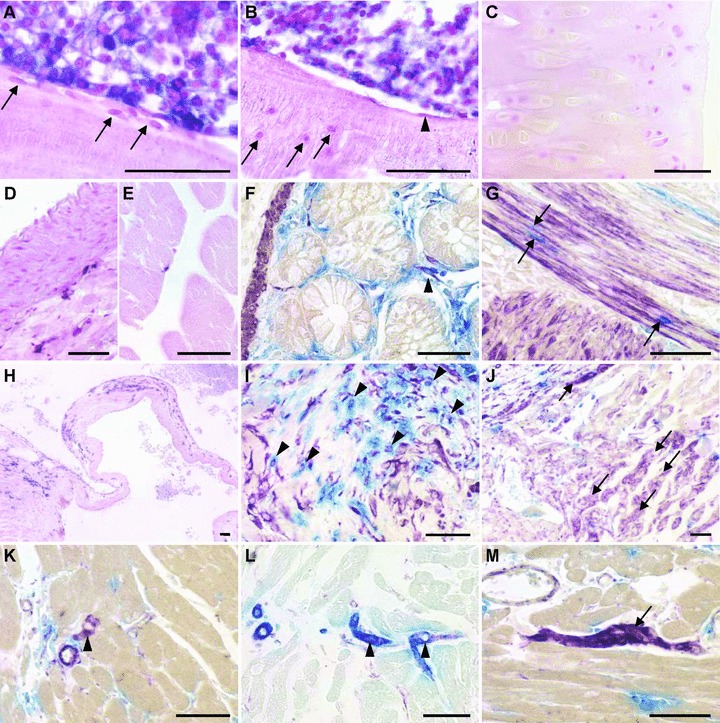
Renewal of mesenchymal cells in irradiated wt F344 rats reconstituted with unfractionated BM from ALPP-tg F344 donors, 4–6 months after BMT. Sections of methylmethacrylate-embedded bones from reconstituted wt F344 rats do not show evidence of ALPP+ BM-derived osteoblasts (A, arrows), osteocytes (B, arrows), bone lining cells (B, arrowhead) or chondrocytes (C). In contrast, BMC were strongly positive for the marker enzyme (A, B). Histochemical detection of the marker enzyme in paraffin sections shows absence of BM-derived smooth muscle cells in aorta (D), and absence of ALPP-expressing muscle fibres in skeletal muscle (E). Cells staining double positive (arrowhead) for ALPP activity (blue) and anti- SMA (purple) were rarely present in the gut (F). In contrast, BM-derived single ALPP+ endothelial-like cells (blue) located between the smooth muscle cells could be frequently found (G, arrows). In the heart, we found numerous ALPP+ cells nearby valvular insertion sites and in the valvular leaflets, here at 4 months after BMT (H). Combination of ALPP-staining (blue) with immunohistochemical staining against vimentin (purple) or SMA (not shown) demonstrated that the BM-derived cells in the valvular insertion areas partially also stained positive for vimentin, suggesting a fibroblast-like nature (I, arrowheads). Numerous single vimentin+ cells with cardiomyocyte-like morphology could also be found at the valvular insertion sites, indicated by arrows (J), as well as rarely in the working myocardium (arrowhead in K), throughout the study period. In rare cases, these vimentin+ (purple) cardiomyocyte-like cells stained also positive for SMA (blue), possibly reflecting early differentiated cardiomyocytes (L). Moreover, in epicardial areas of the heart base, we found several clusters of vimentin+ ALPP– cells (M), potentially representing a cardiac resident stem cell pool. The 5-μm-thick paraffin and methylmethacrylate sections shown in (A–E) and (H) were stained for ALPP enzyme activity with BCIP/NBT (purple) overnight at RT after heat pre-treatment, and were counterstained with nuclear fast red. The paraffin sections in (F–G) and (I–M) were stained for ALPP enzyme activity using Vector Blue, and immunostained against vimentin or SMA using Vector VIP (purple) or Vector Blue (L) as substrate. Bars represent 50 μm.
All cardiomyocytes in BMT animals were ALPP– throughout the study. However, we found a substantial number of ALPP+ cells near the insertion of the heart valves and in the valvular leaflets (Fig. 4H). Some of these ALPP+ cells stained positive for vimentin (Fig. 4I), and some also for SMA (data not shown), suggesting differentiation of BM-derived cells into cardiac fibroblasts or myofibroblasts. In addition, we observed numerous vimentin+ but ALPP– cardiomyocyte-like cells (Fig. 4J), also in regions of valvular insertions. In the remaining heart muscle vimentin+ and ALPP– cardiomyocytes-like cells were rarely seen (Fig. 4K). These vimentin-expressing cardiomyocyte-like cells might represent early differentiated cardiomyocytes, situated especially at sites of peak mechanical stress such as at the valvular insertion sites. In rare cases, we found SMA and vimentin double positive cardiomyocytes (Fig. 4L). Interestingly, we found several clusters of ALPP–, vimentin+ cells in epicardial areas of the heart basis (Fig. 4M) which may represent a pool of undifferentiated endogenous cardiac-resident stem cells.
Mesenchymal precursor cells do not engraft in BMT rats
The failure to detect any ALPP+, BM-derived mesenchymal lineage cells in bone or striated and smooth muscle of BMT rats irrespective of the time after BMT prompted us to ask the question whether stromal precursor cells engraft after BMT with unfractionated BM. To answer this question, we isolated and cultivated MSC from BM of wt, ALPP-tg and BMT rats, 4 weeks after BMT. In line with our earlier report [13], MSC from ALPP-tg animals showed strong expression of ALPP as revealed by histochemistry (Fig. 5C). MSC cultivated from the BM of wt (Fig. 5A) and also of BMT (Fig. 5B) rats did not show positive ALPP staining. To rule out a down-regulation of marker enzyme expression in ALPP-tg cells in a wt environment, we performed Southern blot analysis of DNA extracted from the MSC cultures. Southern blot analysis clearly documented the absence of the ALPP transgene in MSC cultures of BMT rats. These findings indicate that mesenchymal precursor cells do not engraft after lethal irradiation of wt rats and reconstitution with unfractionated BM from ALPP-tg donor rats.
Fig 5.
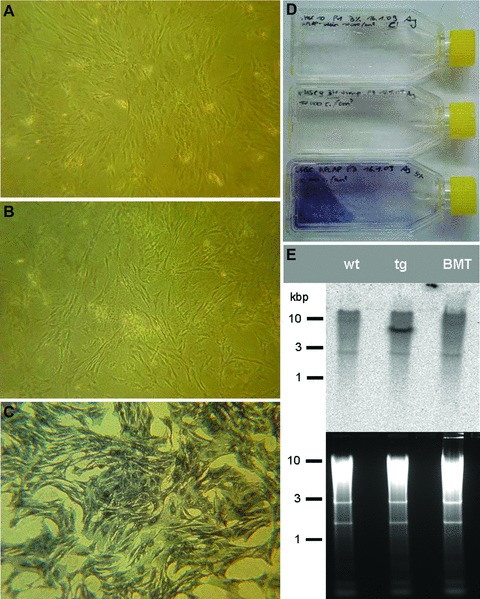
MSC culture reveals lack of MSC engraftment in reconstituted rats. Histochemical staining after heat pre-treatment of cultured and fixed MSC isolated from wt F344 rats (A), from lethally irradiated wt F344 rats reconstituted with unfractionated BM of ALPP-tg co-isogeneic donors, 4 weeks after BMT (BMT, B), or from ALPP-tg F344 rats (C). ALPP staining was absent in wt (A) and BMT MSC (B), whereas MSC of ALPP-tg donors showed strong ALPP expression (C). (D) Fixed and stained MSC in 25 cm2 culture flasks from top to bottom: MSC of wt, BMT and ALPP-tg F344 rats. (E) Southern blot analysis of DNA isolated from cultured MSC confirms the absence of the marker transgene in DNA samples isolated from wt (left line) and BMT rats (right line), in contrast to the ALPP-tg donor (middle line).
Discussion
The goal of the current study was to monitor the renewal of peripheral tissues by BM-derived cells in adult rats, using a marker protein which can readily be detected in semi-thin paraffin and plastic sections by histochemistry or IHC, and in ultrathin sections by transmission electron microscopy after pre-embedding immunostaining. In analogy to a plethora of similarly designed earlier studies using fluorescent proteins or LacZ as genetic markers of BM-derived cells, our study was undertaken under the premise that lethal irradiation and reconstitution with unfractionated BM would result in engraftment of mesenchymal precursor cells in the recipient. However, we found that this premise is wrong, at least in rats. Therefore, we could only examine cellular turnover originating from the haematopoietic BM compartment. Our data show that the haematopoietic BM compartment is an important source of EC renewal in capillaries of many, but not all organs. However, haematopoietic BMC do not contribute to physiological regeneration of muscle, bone, cartilage or epithelial tissues.
The role of BM in EC turnover is a controversial issue. While it was reported that BM-derived cells contribute to blood and lymphatic capillary endothelium [19], and to neovascularization in wound healing [20] or in scar remodelling after myocardial infarction [21], other authors failed to find BM-derived EC in mouse models of tumour vascularization or revascularization of ischemic tissue [22, 23]. Some mouse studies suggested that BM-derived cells in blood vessels represent pericytes [22, 23]. Using immuno-electron microscopy, we found no evidence of BM-derived pericytes or lymphatic EC in heart and kidney. Rather, with the exception of circulating blood cells all ALPP-labelled cells were unequivocally capillary EC in blood vessels of heart and kidney. It is currently unclear whether the discrepancies between some mouse and our rat study could be explained by species differences in BM-derived EC turnover.
Interestingly, we observed organ-specific turnover patterns of capillary EC in our study. Replacement of capillary EC by BM-derived cells was highest in heart and kidney. Low turnover was seen in brain and adrenal medulla. In accordance with our findings, studies in mice transplanted with lacZ-expressing BM reported low values for physiological replacement of EC by BM-derived precursors in skin and brain [24]. In most organs, the number of ALPP-labelled EC increased with time, and, with the exception of kidney, showed a homogenous distribution pattern. The reason for the regional differences in renal BM-derived EC turnover is currently unclear. It is known that lethal irradiation damages EC (reviewed in [25]). Therefore, replacement of EC seen at 4 weeks after transplantation could be due to cell damage induced by irradiation. However, it is unlikely that radiation-induced cell damage can explain the increase in labelled EC at the later time-points.
In the current study, we calculated a half time of 8.36 weeks for BM-derived EC replacement in cardiac muscle. Using a completely different methodology, namely assessment of cellular half life by analysing the decline in labelling index after long-term 5-bromo-2′-deoxyuridine (BrdU) labelling in non-irradiated rats, we reported a cardiac capillary EC half life of 2.2 weeks [18]. Although both methods are not directly comparable because different cellular compartments might be assessed (BrdU labels proliferating cells irrespective of their origin), they nevertheless show that there is a rapid turnover of capillary EC in the heart. However, we demonstrated that labelled EC were absent in large blood vessels and in the adrenal medulla throughout the 6-month experimental period. It remains unclear whether EC turnover does not take place at these sites or whether the replacement is driven exclusively by mitosis of local cells. In this context, it is interesting to note that we never found BrdU+ EC in arterial or venous blood vessels (unpublished data) in the long-term BrdU-labelling study mentioned above [18]. Collectively, these observations suggest that physiological EC turnover is absent or very low in larger vessels.
Several reports from sex-mismatched BMT in human beings and from mice reconstituted with GFP or lacZ-labelled BM have suggested that BM-derived cells participate in cell renewal of epithelial [4, 8, 9, 26–29] and also mesenchymal cells [30] in various organs. In our experiment BM-derived cells did not contribute to epithelial or mesenchymal repair processes. In accordance with our results, more recent reports in reconstituted mouse models have suggested that turnover and regeneration of epithelial cells in the adult endocrine pancreas [31], kidney [10], upper respiratory tract [32] and lung [12], even after tissue damage, does not involve BM-derived cells. Technical problems with the histological detection of the Y-chromosome, or with fluorescent marker proteins such as GFP and non-specific tissue auto-fluorescence might be the reason for the discrepant findings.
It is still controversial whether stromal cells are engrafted during BMT with unfractionated BM. Although some contrasting findings have been reported [33], the majority of studies in patients after allogeneic BMT reported that BM-derived MSC in these patients are of recipient origin [34–37]. The methods used to determine the origin of stromal cells in these studies ranged from demonstration of a Y-chromosome after sex-mismatched BMT [34, 35, 37] to DNA fingerprinting methods [37]. In accordance with the clinical reports, our data did neither provide evidence for ALPP-expressing mesenchymal cells of donor origin in bone or peripheral tissues of BMT rats irrespective of the time-point after BMT, nor of ALPP+ MSC after culture of BM harvested from BMT rats. Along similar lines, Wang et al. [38] showed that in irradiated mice reconstituted with unfractionated BM of Col1a1-GFP reporter transgenic mice, GFP-expressing cells did not differentiate into osteocytes. However, other studies in mice reported donor-derived chimerism of MSC after irradiation and transplantation of unfractionated BMC or MSC [39–41]. The reason for the contrasting findings between some mouse experiments and studies in human beings and rats remains unclear. However, there is evidence that strain-related differences in the sensitivity of murine MSC to irradiation may be involved [40].
Why do stromal precursors, although certainly present in unfractionated BM, not engraft in lethally irradiated human beings and rats? MSC show relative radioresistance in vitro and may not be affected by the irradiation regimen [42]. Therefore, the most likely explanation is that, in contrast to haematopoietic stem cells, a niche for stromal precursor cells is lacking in BM of irradiated animals. Given the fact that the MSC pool remained recipient-derived in our study, how can the occurrence of BM-derived ALPP+ fibroblasts in certain organs such as heart or intestine be explained? Other investigators have also reported the presence of BM-derived fibroblasts in kidney, valvular leaflets of the heart or skin during wound healing after transplantation of unfractionated BM or even haematopoietic stem cells in mice [43–45]. Moreover, studies in a mouse model of cardiac fibrosis have provided firm evidence of a blood-borne, BM-derived fibroblast precursor population of haematopoietic origin, giving rise to α-SMA, type I collagen, CD34 and CD45+ cardiac fibroblasts [46]. Thus, our data corroborate the existence of a distinct blood-borne population of fibroblast precursors of haematopoietic origin also in the rat.
In the current study, we found some ALPP–, vimentin and/or SMA-staining cardiomyocyte-like cells in the heart, especially in areas of high mechanical stress. This finding may implicate that, given the assumption that these vimentin-expressing cells can be defined as early differentiated cardiomyocytes [47], the heart may be capable of limited regeneration through differentiation of cardiac stem cells [48]. However, although these repair mechanisms may be suitable to compensate physiologic cell death especially at sites of high mechanical demand, they without doubt fail to compensate extensive loss of cardiomyocytes after ischemia-reperfusion injury [21]. We demonstrated that in our model engraftment of donor-derived MSC did not take place. Therefore, we cannot rule out a possible recruitment of BM-derived mesenchymal precursors in mesenchymal tissue turnover, because the recipient-derived BM-MSC could not be tracked in our study. However, a study of heterotopic transplantation of wt rat hearts into transgenic rats ubiquitously expressing GFP showed that BM-derived cardiomyocyte turnover represents a negligible event under more or less physiologic conditions [49].
In conclusion, our study clearly showed that haematopoietic BMC are an important source of physiological renewal of endothelial, but not of epithelial or mesenchymal, cells in many organs of adult rats. Thus, our data corroborate the notion that the major source of endothelial precursor cells in BM is of haematopoietic origin [50]. Because mesenchymal precursor cells did not engraft after BMT with unfractionated BM, our study questions the usefulness of reconstituted irradiated rats as a model for evaluating BM-driven regeneration of mesenchymal cells in peripheral tissues.
Acknowledgments
The authors thank Werner Panzer and Siegfried Kosik for help with the irradiation, and Magdalena Helmreich and Waltraud Tschulenk for establishment of protocols for IHC and electron microscopy, respectively. This research was supported by Deutsche Forschungsgemeinschaft (Er 223/8-1), by the University of Veterinary Medicine Vienna and by the Austrian Science Fund FWF (P 21904-B11). G.L. and A.J. were supported by the Austrian Science Fund FWF (FSP09309).
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100:11917–23. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 4.Lagasse E, Connors H, Al Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–34. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari G, Cusella-De Angelis G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa M, Larue AC, Watson PM, et al. Hematopoietic stem cell origin of connective tissues. Exp Hematol. 2010;38:540–7. doi: 10.1016/j.exphem.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 9.Lin F, Cordes K, Li L, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 10.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–64. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV, et al. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology. 2006;43:108–16. doi: 10.1002/hep.21005. [DOI] [PubMed] [Google Scholar]
- 12.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger NJ, Odörfer KI, Weber K, et al. Utility of human placental alkaline phosphatase as a genetic marker for cell tracking in bone and cartilage. Histochem Cell Biol. 2007;127:669–74. doi: 10.1007/s00418-007-0286-6. [DOI] [PubMed] [Google Scholar]
- 14.Kisseberth WC, Brettingen NT, Lohse JK, et al. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–38. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 15.Erben RG. Embedding of bone samples in methylmethacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem. 1997;45:307–13. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 16.Odörfer KI, Unger NJ, Weber K, et al. Marker tolerant, immunocompetent animals as a new tool for regenerative medicine and long-term cell tracking. BMC Biotechnol. 2007;7:30. doi: 10.1186/1472-6750-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell E, Byrne EM, Fischer J, et al. A comparison of the osteogenic potential of adult rat mesenchymal stem cells cultured in 2-D and on 3-D collagen glycosaminoglycan scaffolds. Technol Health Care. 2007;15:19–31. [PubMed] [Google Scholar]
- 18.Erben RG, Odörfer KI, Siebenhutter M, et al. Histological assessment of cellular half-life in tissues in vivo. Histochem Cell Biol. 2008;130:1041–6. doi: 10.1007/s00418-008-0470-3. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Bailey AS, Goldman DC, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One. 2008;3:e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bluff JE, Ferguson MW, O’Kane S, et al. Bone marrow-derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp Hematol. 2007;35:500–6. doi: 10.1016/j.exphem.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Odörfer KI, Walter I, Kleiter M, et al. Role of endogenous bone marrow cells in long-term repair mechanisms after myocardial infarction. J Cell Mol Med. 2008;12:2867–74. doi: 10.1111/j.1582-4934.2008.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajantie I, Ilmonen M, Alminaite A, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 24.Crosby JR, Kaminski WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–30. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 25.Woywodt A, Haubitz M, Buchholz S, et al. Counting the cost: markers of endothelial damage in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:1015–23. doi: 10.1038/sj.bmt.1704733. [DOI] [PubMed] [Google Scholar]
- 26.Korbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–7. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 28.Fang TC, Otto WR, Rao J, et al. Haematopoietic lineage-committed bone marrow cells, but not cloned cultured mesenchymal stem cells, contribute to regeneration of renal tubular epithelium after HgCl 2 -induced acute tubular injury. Cell Prolif. 2008;41:575–91. doi: 10.1111/j.1365-2184.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 30.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 31.Lechner A, Yang YG, Blacken RA, et al. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616–23. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 32.Davies JC, Potter M, Bush A, et al. Bone marrow stem cells do not repopulate the healthy upper respiratory tract. Pediatr Pulmonol. 2002;34:251–6. doi: 10.1002/ppul.10163. [DOI] [PubMed] [Google Scholar]
- 33.Keating A, Singer JW, Killen PD, et al. Donor origin of the in vitro haematopoietic microenvironment after marrow transplantation in man. Nature. 1982;298:280–3. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- 34.Laver J, Jhanwar SC, O’Reilly RJ, et al. Host origin of the human hematopoietic microenvironment following allogeneic bone marrow transplantation. Blood. 1987;70:1966–8. [PubMed] [Google Scholar]
- 35.Simmons PJ, Przepiorka D, Thomas ED, et al. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–32. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 36.Awaya N, Rupert K, Bryant E, et al. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30:937–42. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 37.Rieger K, Marinets O, Fietz T, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33:605–11. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Liu Y, Kalajzic Z, et al. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106:3650–7. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koide Y, Morikawa S, Mabuchi Y, et al. Two distinct stem cell lineages in murine bone marrow. Stem Cells. 2007;25:1213–21. doi: 10.1634/stemcells.2006-0325. [DOI] [PubMed] [Google Scholar]
- 40.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–70. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 41.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–96. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Kwong DL, Chan GC. The effects of various irradiation doses on the growth and differentiation of marrow-derived human mesenchymal stromal cells. Pediatr Transplant. 2007;11:379–87. doi: 10.1111/j.1399-3046.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 43.Direkze NC, Forbes SJ, Brittan M, et al. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–20. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 44.Visconti RP, Ebihara Y, Larue AC, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–6. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 45.Ishii G, Sangai T, Sugiyama K, et al. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells. 2005;23:699–706. doi: 10.1634/stemcells.2004-0183. [DOI] [PubMed] [Google Scholar]
- 46.Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babai F, Musevi-Aghdam J, Schurch W, et al. Coexpression of alpha-sarcomeric actin, alpha-smooth muscle actin and desmin during myogenesis in rat and mouse embryos I. Skeletal muscle. Differentiation. 1990;44:132–42. doi: 10.1111/j.1432-0436.1990.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 48.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ausoni S, Zaglia T, Dedja A, et al. Host-derived circulating cells do not significantly contribute to cardiac regeneration in heterotopic rat heart transplants. Cardiovasc Res. 2005;68:394–404. doi: 10.1016/j.cardiores.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]


