Abstract
Background
The clinical significance of magnetic resonance imaged (MRI) plaque characteristics in the superficial femoral artery (SFA) is not well established. We studied associations of the ankle brachial index (ABI) and leg symptoms with MRI-measured plaque area and percent lumen area in the SFA in participants with and without lower extremity peripheral arterial disease (PAD).
Methods and Results
Four hundred twenty-seven participants (393 with PAD) underwent plaque imaging of the first 30 millimeters of the SFA. Twelve 2.5 millimeter cross-sectional images of the SFA were obtained. Outcomes were normalized plaque area, adjusted for artery size (0–1 scale, 1=greatest plaque), and lumen area, expressed as a percent of the total artery area. Adjusting for age, sex, race, smoking, statins, cholesterol, and other covariates, lower ABI values were associated with higher normalized mean plaque area (ABI < 0.50:0.79; ABI 0.50 to 0.69:0.73; ABI 0.70 to 0.89:0.65; ABI 0.90 to 0.99:0.62; ABI 1.00 to 1.09:0.48; ABI 1.10–1.30:0.47 (P trend <0.001)) and smaller mean percent lumen area (P trend<0.001). Compared to PAD participants with intermittent claudication, asymptomatic PAD participants had lower normalized mean plaque area (0.72 vs. 0.65, p=0.005) and larger mean percent lumen area (0.30 vs. 0.36, p=0.01), adjusting for the ABI and other confounders.
Conclusions
Lower ABI values are associated with greater MRI-measured plaque burden and smaller lumen area in the first 30 millimeters of the SFA. Compared to PAD participants with claudication, asymptomatic PAD participants have smaller plaque area and larger lumen area in the SFA.
Keywords: atherosclerosis, magnetic resonance imaging, peripheral vascular disease, plaque
Direct atherosclerotic plaque visualization has improved our understanding of the development and progression of atherosclerosis (1,2). However, little is known about clinical correlates of lower extremity atherosclerotic plaque characteristics in patients with lower extremity peripheral arterial disease (PAD).
High resolution magnetic resonance imaging (MRI) has emerged as the most promising modality for direct atherosclerotic plaque imaging (3,4). In this study, we used MRI to directly image consecutive cross-sectional slices of the superficial femoral artery (SFA). We assessed associations of the ankle brachial index (ABI), an established clinical tool for assessing presence and severity of PAD, with MRI-measured plaque area and lumen area in the SFA among participants with and without PAD. We hypothesized that lower ABI values would be associated with greater plaque area and smaller percent lumen area in directly imaged cross-sections of the proximal SFA. We also studied associations of the ABI with plaque area and percent lumen area in the SFA in the subset of participants with PAD. We determined whether plaque area and percent lumen area differed between PAD participants with distinct types of leg symptoms. Finally, we studied associations of the ABI with plaque measures in PAD participants with and without diabetes mellitus, to determine whether associations of lower ABI values with plaque area and percent lumen area were similar in PAD participants with vs. without diabetes mellitus.
Methods
Subjects
Participants with PAD were identified from among consecutive PAD patients in the noninvasive vascular laboratories at Northwestern Memorial Hospital, Jesse Brown Veterans Administration (VA), Rush Medical Center, and Mt. Sinai Hospital in Chicago. Additionally, lists of consecutive patients with a diagnosis of PAD in the vascular surgery, cardiology, endocrinology, general medicine, and geriatric practices at Northwestern Medical Faculty Foundation and in the vascular surgery practice at the Jesse Brown VA were contacted and invited to participate. Participants without PAD were identified from among consecutive patients age 65 and older in Northwestern’s general internal medicine practice who had no history of smoking, diabetes mellitus, or established cardiovascular disease, including PAD. The protocol was Institutional Review Board-approved by Northwestern University Feinberg School of Medicine and all participating sites. Participants gave informed consent. Enrollment occurred between 10/26/07 and 12/22/09.
Inclusion Criteria
For participants with PAD, the inclusion criterion was an ABI < 1.00. This inclusion criterion was selected because truly normal ABI values are 1.10–1.30 or 1.10–1.40 (5–6) and because including participants with ABI < 1.00 ensured a broad range of severity of lower extremity atherosclerosis. For participants without PAD, the inclusion criterion was ABI 1.00–1.30 (7).
Exclusion Criteria
Potential participants with dementia and those with a mini-mental status examination (MMSE) score < 23 (8), were excluded because of concerns about their cognitive function. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because of their severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded. Patients with contraindications to MRI testing were excluded. We excluded potential participants requiring oxygen therapy, those who stopped during a six-minute walk test due to shortness of breath, and those with severe knee osteoarthritis, measured by reported pain in or around the knee joint combined with a radiograph-measured osteoarthritis K/L score of four (9). PAD patients with bilateral SFA stents were excluded, because the stents interfered with plaque imaging. Other lower extremity revascularizations were not exclusion criteria.
Ankle Brachial Index Measurement
After participants rested supine for five minutes, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to measure systolic pressures in this order: right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures (10). Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg in at least one measurement set, since in such cases subclavian stenosis was possible (11). For the dorsalis pedis and posterior tibial vessels, zero values and values of incompressible arteries were excluded. Lowest leg ABI was used in analyses.
Leg Symptoms
We used the San Diego claudication questionnaire to classify participants into one of five leg symptom categories, based on prior study (12,13): 1) Intermittent claudication (exertional calf pain that does not begin at rest, causes the participant to stop walking, and resolves within ten minutes of rest); 2) Atypical exertional leg pain/carry on (exertional leg symptoms that do not begin at rest and do not stop the individual from walking); 3) Atypical exertional leg pain/stop (exertional leg symptoms that do not begin at rest, stop the individual from walking, and do not involve the calves or resolve within ten minutes of rest); 4) Leg pain on exertion and rest (exertional leg pain that sometimes begins at rest); 5) Asymptomatic (no exertional leg symptoms) (13).
Magnetic Resonance Imaging
We imaged the SFA because it is the most common site of lower extremity atherosclerosis (14) and because it supplies calf muscle, which is typically symptomatic in PAD. The leg with the lowest ABI was imaged. However, if the leg with the lowest ABI had an SFA stent, the opposite leg was imaged. The MRI was performed using a 1.5 Tesla (Siemens) platform with four-element phased-array surface coils. The proximal SFA was imaged because it is more amenable to high quality images than the more deeply located distal SFA. Data were collected using a standard, Turbo Spin Echo acquisition proton density weighted images (TR/TE= 2160 milliseconds/8 milliseconds, Bandwidth 230 Hz/pixel, Turbo factor 15). The field of view was 120 × 120 mm2 and images were acquired in matrix 192, yielding an in-plane spatial resolution of 0.625 × 0.625 mm2. Three signal averages were acquired. Regional signal saturation bands were played out superiorly and inferiorly to suppress signal from inflowing blood, ensuring dark blood contrast. Chemically selective lipid saturation pulses eliminated signal from peri-adventitial fat. Twelve sequential 2.5 millimeter cross-sectional images were obtained, beginning at the bifurcation of the common femoral artery into the SFA and moving distally without gap using 2-dimensional bright blood time-of-flight and proton-density weighted images. Bright-blood 2D time of flight images (TR/TE=31.0 ms/7.2 ms) were registered to the proton density images and acquired using an identical field of view, slice thickness, and imaging matrix. This method has excellent test re-test reliability (15).
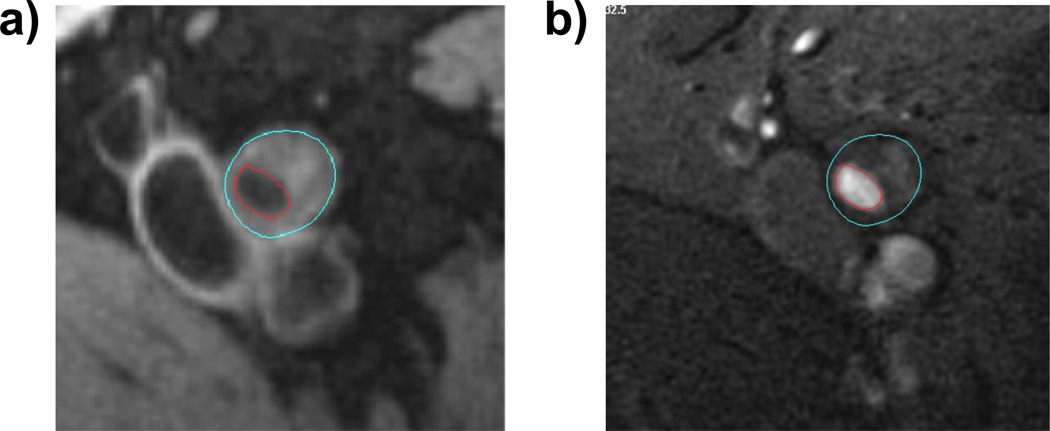
Two physicians used CASCADE software (Seattle, WA) to trace the outer boundary and lumen of each cross-sectional image, to quantify plaque and lumen area. Measurement of plaque composition was not available in the version of CASCADE used for this study. The lumen was identified in time-of-flight images and was copied onto the proton-density weighted image, where the artery outer wall boundary was traced. Images for each participant were assigned to one primary physician reviewer. Tracings were assessed by the second physician reviewer to ensure accuracy. An example image is shown in Figure 1.
Figure 1.
A representative image of a participant with peripheral arterial disease.
Panel a shows proton weighted MRI images TR/TE = 2160 ms/8 ms). Panel b shows time of flight images (TR/TE=38 ms/8.7ms). The blue contours define the outer wall area of the vessel. The red contours define the lumen boundary.
To adjust for the fact that a given plaque area will have a different effect on different sized arteries, plaque measurements were normalized for artery size (16). Mean and maximum plaque area were normalized by dividing the average and maximum plaque area, respectively, by the median of the outer wall area. Mean and minimum percent lumen area were normalized by dividing the mean and minimum lumen area, respectively, by the outer wall area for each arterial slice.
Poor image quality was identified by the physician reviewers, who were blinded to all other participant characteristics. When poor image quality was identified, every attempt was made to have the participant return to the medical center for repeat imaging. In some cases, poor image quality was related to artifact from artificial joints, large body size, or inability of the participant to remain still during imaging. In some of these cases, even repeat imaging did not result in a readable image. Each poor quality image was reviewed at least twice by the physician reviewers to confirm that the data were not usable.
Approximately six percent of participants returned on a second day for test re-test reliability assessment of the MRI plaque measurement. The coefficient of variation percent values for these test re-test reliability assessments were 5.8 for mean plaque area, 8.9 for maximum plaque area, 7.9 for mean percent lumen area, and 12.9 for minimum percent lumen area.
Comorbidities
Medical record review, participant questionnaires, and a primary care physician questionnaire were used to identify and confirm comorbidities using established methods (17). Hypertension was defined as participant report of physician-diagnosed high blood pressure or physician report of hypertension on the primary care physician questionnaire. Diabetes mellitus was defined as a) use of a diabetes medication or b) participant report of diabetes mellitus that was confirmed by the primary care questionnaire or medical record review.
Low-Density Lipoprotein Levels and High-Density Lipoprotein levels
Low Density Lipoprotein Cholesterol (LDL-C) was determined by a homogenous direct method from Roche Diagnostics (Indianapolis, IN) (18). High density lipoprotein cholesterol (HDL-C) was measured using a direct enzymatic colorimetric assay (19).
Other Measures
Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight kg/(height (meters))2. Seated systolic blood pressure was measured with an Omron automatic blood pressure machine after a five minute rest period using an established protocol (20). Cigarette smoking history was measured with self-report. Participants brought their medication bottles or a medication list to their study visit. Medication names were recorded. The study principal investigator (MMM) identified which participants were taking statin medications, blinded to all other participant characteristics.
Statistical Analyses
Based on previous study (7,21,22), the following ABI categories were defined in an a priori fashion: ABI < 0.50; ABI 0.50–0.69, ABI 0.70–0.89; ABI 0.90 to 0.99; ABI 1.00–1.09; ABI 1.10–1.30. Differences in continuous and dichotomous variables were compared across the ABI categories using analyses of variance and chi square tests, respectively. Mean plaque area, maximum plaque area, mean percent lumen area and minimum percent lumen area were compared across each ABI category and across each leg symptom category, adjusting for age, race, sex, smoking, BMI, statins, diabetes, hypertension, systolic blood pressure, LDL-C, and HDL-C. Analyses of leg symptoms additionally adjusted for the ABI. BMI, systolic blood pressure, LDL-C, and HDL-C were included as continuous variables because these characteristics were measured at the study visit. In each set of analyses, pair-wise comparisons were performed between the highest ABI category and the remaining ABI categories. Analyses were repeated in the subset of participants with PAD. Among PAD participants, pair-wise comparisons were performed between those with intermittent claudication and each remaining leg symptom category. Analyses of ABI and plaque measures were repeated among PAD participants with and without diabetes mellitus, respectively. A test for interaction was performed to determine whether associations of ABI with plaque measures differed between PAD participants with vs. without diabetes mellitus.
Analyses were performed using SAS Statistical Software version 9.2 (SAS Inc, Cary, NC).
Results
We mailed recruitment letters to 3,391 consecutive patients identified from recruitment sources with an established diagnosis of PAD. Of these, 1161 did not respond, 504 met one or more exclusion criteria, 954 were not interested, and 304 could not be scheduled or did not show for their study visit, leaving 468 participants with PAD. An additional five PAD participants were identified from among participants identified from the internal medicine practice who were screened with the ABI (total=473 PAD participants). We mailed recruitment letters to 558 consecutively identified patients age 65 and older in the internal medicine practice without history of diabetes, smoking, or established atherosclerosis. Of these, 283 did not respond to the recruitment letter, 62 met one or more exclusion criteria, 125 were not interested in participating, 41 could not be scheduled or did not show for their visit, and five had a low ABI consistent with PAD, leaving 42 participants without PAD. Of the 515 participants with and without PAD, 16 had poor quality MR images, and 72 were missing covariate data used in our analyses, leaving 427 participants for the current analyses.
Table 1 shows characteristics of the cohort. Compared to participants with ABI 1.00 to 1.30, those with ABI <1.00 included higher proportions of men and participants with intermittent claudication and lower proportions of participants who were asymptomatic (Table 1).
Table 1.
Characteristics of Study Participants
| Characteristic | Entire Cohort | Participants with ABI <1.00 | Participants with ABI 1.00–1.30 | P Value* |
|---|---|---|---|---|
| N=427 | N=393 | N=34 | ||
| Age (years) | 69.7 (9.9) | 69.4 (10.1) | 72.1 (6.8) | 0.138 |
| ABI | 0.70 (0.21) | 0.67 (0.17) | 1.12 (0.07) | <.001 |
| Male sex (%) | 66.3 | 69.2 | 32.4 | <.001 |
| African-American (%) | 28.8 | 29.8 | 17.7 | 0.134 |
| Current Smoker (%) | 21.6 | 23.4 | 0.00 | NA** |
| Diabetes Mellitus (%) | 36.5 | 39.7 | 0.00 | NA** |
| Prior lower extremity revascularization (%) | 39.3 | 42.8 | 0.00 | NA** |
| Intermittent claudication (%) | 22.5 | 24.4 | 0.00 | 0.001 |
| Asymptomatic (%) | 23.4 | 19.6 | 67.7 | <.001 |
| Atypical exertional leg symptoms/carry on (%) | 9.8 | 10.4 | 2.9 | 0.231 |
| Atypical exertional leg symptoms/stop (%) | 18.7 | 19.3 | 11.8 | 0.278 |
| Leg pain on exertion and rest (%) | 25.5 | 26.2 | 17.7 | 0.272 |
Comparison of participants with Ankle Brachial Index (ABI) < 1.00 vs. those with ABI 1.00–1.30. Data shown are means (standard deviations).
Inclusion criteria for potential participants with ABI 1.00–1.30 included absence of diabetes mellitus and cigarette smoking. Therefore statistical comparisons are not meaningful for these variables.
Table 2 shows associations of baseline ABI values with participant characteristics. Lower ABI values were associated with higher prevalences of males, hypertension, and statin use (Table 2). Lower ABI values were associated with lower HDL and LDL cholesterol levels, respectively.
Table 2.
Associations of Participants Characteristics with Ankle Brachial Index Values*
| ABI < 0.50 (N=77) |
ABI 0.50–0.69 (N=143) |
ABI 0.70–0.89 (N=131) |
ABI 0.90–0.99 (N=42) |
ABI 1.00–1.09 (N=14) |
ABI- 1.10–1.30 (N=20) |
P Trend | |
|---|---|---|---|---|---|---|---|
| Age (years) | 70.7 (9.0) | 70.8 (9.8) | 67.7 (10.9) | 67.9 (9.5) | 74.14(6.4) | 70.6 (6.9) | 0.390 |
| Ankle Brachial Index | 0.41 (0.08) | 0.61 (0.06) | 0.79 (0.06) | 0.94 (0.03) | 1.05 (0.03) | 1.16 (0.04) | <.001 |
| Male Sex (%) | 67.5 | 77.6 | 66.4 | 52.4 | 28.6 | 35.0 | <.001 |
| African-American (%) | 33.8 | 30.8 | 26.0 | 31.0 | 21.4 | 15.0 | 0.090 |
| Current Smoker (%) | 23.4 | 21.7 | 23.7 | 28.6 | 0.00 | 0.00 | NA |
| Diabetes Mellitus (%) | 49.4 | 42.0 | 30.5 | 42.9 | 0.00 | 0.00 | NA |
| Hypertension (%) | 97.4 | 90.9 | 86.3 | 88.1 | 78.6 | 65.0 | <.001 |
| HDL Cholesterol (mgs/dl) | 51.2 (16.7) | 48.5 (17.2) | 50.4 (16.5) | 49.5 (14.3) | 67.1 (18.9) | 67.1 (21.0) | 0.001 |
| LDL Cholesterol (mgs/dl) | 93.9 (30.1) | 83.7 (32.7) | 94.9 (29.6) | 103.1 (37.9) | 110.1 (34.3) | 123.2 (37.0) | <.001 |
| Statin Use (%) | 80.5 | 76.2 | 73.3 | 73.8 | 64.3 | 10.0 | <.001 |
| Prior lower extremity revascularization (%) | 49.4 | 39.2 | 41.2 | 47.6 | 0.00 | 0.00 | NA |
| Intermittent Claudication (%) | 37.7 | 24.5 | 18.3 | 19.1 | 0.00 | 0.00 | <.001 |
| Asymptomatic (%) | 9.1 | 19.6 | 22.9 | 28.6 | 71.4 | 65.0 | <.001 |
| Leg Pain/Carry on (%) | 15.6 | 8.4 | 9.2 | 11.9 | 0.00 | 5.0 | 0.133 |
| Atypical exertional leg pain (%) | 14.3 | 19.6 | 23.7 | 14.3 | 14.3 | 10.0 | 0.787 |
| Pain on exertion and rest (%) | 23.4 | 28.0 | 26.0 | 26.2 | 14.3 | 20.0 | 0.571 |
| Normalized mean plaque area (0–1 scale, 1= most plaque). | 0.79 (0.19) | 0.72 (0.17) | 0.65 (0.14) | 0.61 (0.14) | 0.51 (0.04) | 0.48 (0.05) | <.001 |
| Normalized maximum plaque area (0–1 scale, 1= most plaque). | 1.1 (0.55) | 0.94 (0.30) | 0.84 (0.26) | 0.81 (0.30) | 0.64 (0.09) | 0.59 (0.07) | <.001 |
| Mean percent lumen area | 0.24 (0.14) | 0.30 (0.14) | 0.36 (0.13) | 0.42 (0.07) | 0.50 (0.05) | 0.52 (0.05) | <.001 |
| Minimum percent lumen area | 0.16 (0.13) | 0.22 (0.13) | 0.28 (0.13) | 0.33 (0.10) | 0.42 (0.06) | 0.46 (0.06) | <.001 |
Data shown are means and standard deviations.
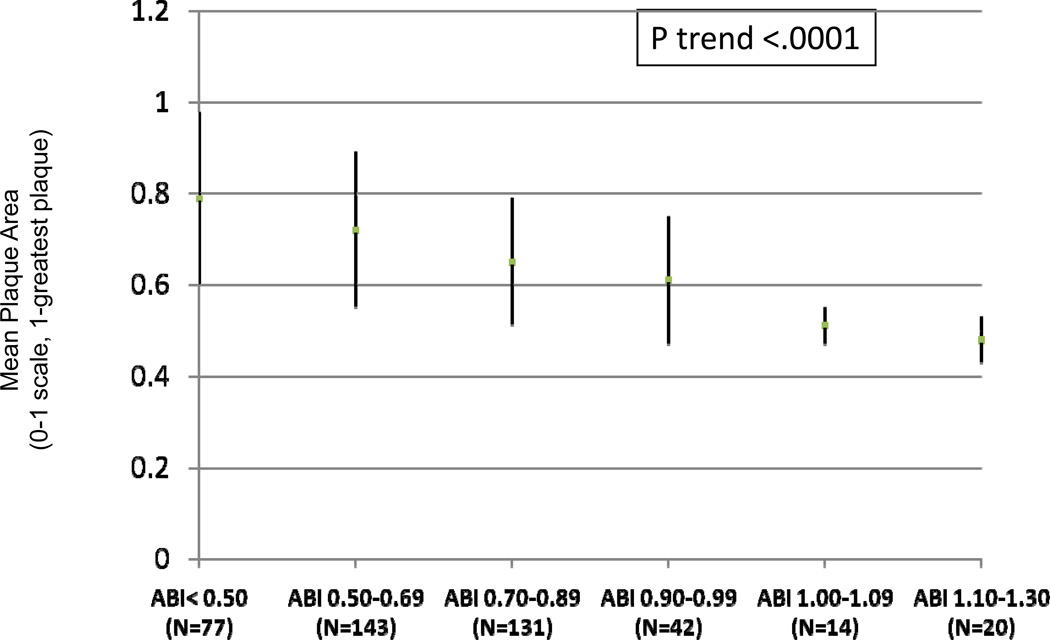
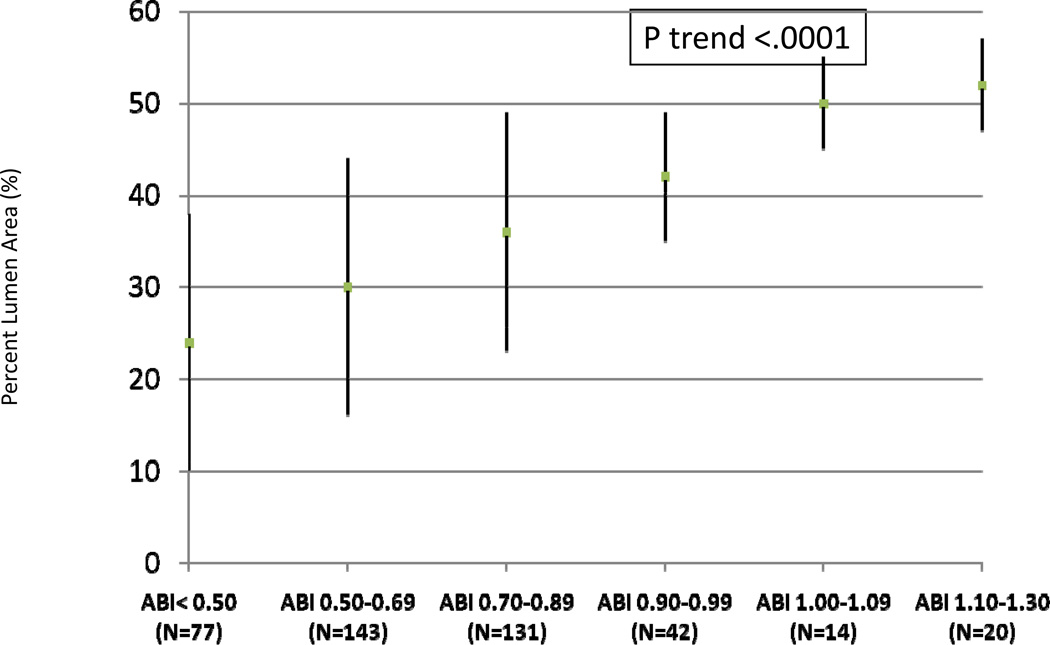
Figures 2 and 3 show unadjusted associations of normalized mean plaque area and mean percent lumen area across ABI categories. Lower ABI values were associated with greater mean plaque area and smaller mean percent lumen area.
Figure 2. Associations of the Ankle Brachial Index with Mean Plaque Area (N=427)*.
Unadjusted mean plaque area across ankle brachial index categories (N=427)
Mean plaque area has been normalized for artery size by dividing average wall area by the median outer wall area normalized for artery size.
*Data shown are means and standard deviations.
Figure 3. Associations of the Ankle Brachial Index with Mean Lumen Area (N=427)*.
Unadjusted mean percent lumen area across ankle brachial index categories (N=427)
*Data shown are means and standard deviations.
In the entire cohort, lower ABI values were associated with greater mean and maximal plaque area and smaller mean and minimum percent lumen area, adjusting for age, sex, race, hypertension, systolic blood pressure, diabetes mellitus, cholesterol levels, BMI, smoking, and statin use (Table 3). Furthermore, participants with ABI values of < 0.50, 0.50 to 0.69, 0.70 to 0.89, and 0.90 to 0.99, respectively, had significantly greater mean and maximum plaque area and smaller mean and minimum percent lumen area, compared to the reference group of participants with ABI values of 1.10–1.30 (Table 3).
Table 3.
Adjusted Associations of the Ankle Brachial Index with Plaque Characteristics in the Proximal Superficial Femoral Artery.*
| Plaque Characteristics | ABI < 0.50 (N=77) |
ABI 0.50–0.69 (N=143) |
ABI 0.70–0.99 (N=131) |
ABI 0.90–0.99 (N=42) |
ABI 1.00–1.09 (N=14) |
ABI- 1.10–1.30 (N=20) |
P Trend |
|---|---|---|---|---|---|---|---|
| Participants with and without Peripheral Arterial Disease (ABI < 1.30) (N=427) | |||||||
| Mean Vessel Plaque Area | 0.79 (0.02)1 | 0.73 (0.01)1 | 0.65 (0.01)1 | 0.62 (0.02)1 | 0.48 (0.04) | 0.47 (0.04) | <0.001 |
| Maximal Vessel Plaque Area | 1.09 (0.04)1 | 0.95 (0.03)1 | 0.84 (0.03)2 | 0.81 (0.05)3 | 0.59 (0.09) | 0.56 (0.08) | <0.001 |
| Mean Percent Lumen Area | 0.25 (0.01)1 | 0.30 (0.01)1 | 0.36 (0.01)1 | 0.42 (0.02)2 | 0.51 (0.03) | 0.53 (0.03) | <0.001 |
| Minimum Percent Lumen Area | 0.16 (0.01)1 | 0.21 (0.01)1 | 0.28 (0.01)1 | 0.32 (0.02)1 | 0.43 (0.03) | 0.46 (0.03) | <0.001 |
| Participants with Peripheral Arterial Disease (ABI < 1.00) (N=393) | |||||||
| Plaque Characteristics |
ABI < 0.50 (N=77) |
ABI 0.50–0.69 (N=143) |
ABI 0.70–0.99 (N=131) |
ABI 0.90–0.99 (N=42) |
ABI 1.00–1.09 (N=0) |
ABI- 1.10–1.30 (N=0) |
P Trend |
| Mean Vessel Plaque Area | 0.79 (0.02)1 | 0.73 (0.01)1 | 0.65 (0.01) | 0.61 (0.03) | N/A | N/A | <0.001 |
| Maximal Vessel Plaque Area | 1.08 (0.04)1 | 0.95 (0.03)4 | 0.83 (0.03) | 0.81 (0.05) | N/A | N/A | <0.001 |
| Mean Percent Lumen Area | 0.25 (0.02)1 | 0.30 (0.01)1 | 0.36 (0.01)5 | 0.42 (0.02) | N/A | N/A | <0.001 |
| Minimum Relative Percent Lumen Area | 0.16 (0.01)1 | 0.22 (0.01)1 | 0.28 (0.01) | 0.32 (0.02) | N/A | N/A | <0.001 |
Data are adjusted for age, sex, race, hypertension, systolic blood pressure, diabetes mellitus, HDL cholesterol, LDL cholesterol, body mass index, smoking, and statin use.
Pairwise comparisons were performed between the highest ABI category and each lower ABI category: 1 P≤0.001;
P=0.002,
P=0.01,
P=0.02,
P=0.03. Plaque area is normalized for total artery size (range 0–1, 1=greatest plaque). Percent lumen area represents lumen area divided by total arterial wall area. N/A denotes not applicable.
In the subset of participants with ABI < 1.00, lower ABI values remained associated with greater mean and maximum plaque area and smaller mean and minimum percent lumen area, adjusting for age, sex, race, hypertension, systolic blood pressure, diabetes mellitus, cholesterol levels, BMI, smoking, and statin use (Table 3). In pairwise comparisons, PAD participants with ABI values < 0.50 and those with ABI 0.50 to 0.69 had significantly greater mean and maximum plaque area and significantly smaller mean and minimum percent lumen area, compared to PAD participants with ABI values of 0.90–0.99 (Table 3). PAD participants with ABI values of 0.70 to 0.89 had significantly smaller mean percent lumen area compared to those with ABI of 0.90–0.99 (Table 3).
Among PAD participants with and without diabetes mellitus, respectively, lower ABI values were associated with greater mean and maximum plaque area and smaller mean and minimum percent lumen area, adjusting for age, sex, race, hypertension, systolic blood pressure, cholesterol, BMI, smoking, and statin use (data not shown). There were no significant interactions between presence vs. absence of diabetes mellitus and the association of ABI with any of the plaque characteristics (data not shown).
Adjusting for age, sex, race, hypertension, systolic blood pressure, cholesterol, BMI, smoking, ABI, and statin use, PAD participants who were asymptomatic had smaller mean plaque area, larger mean percent lumen area, and larger minimum percent lumen area compared to PAD participants with intermittent claudication (Table 4). PAD participants with atypical exertional leg pain/carry on had less maximum plaque area, compared to PAD participants with claudication (Table 4).
Table 4.
Adjusted Associations of Leg Symptoms with Plaque Area and Lumen Area in Superficial Femoral Artery in Participants with Peripheral Arterial Disease.*
| Plaque Characteristics | Asymptomatic (N=77) |
Atypical Exertional Leg Pain/Carry on (N=41) |
Atypical Exertional Leg Pain (N=76) |
Leg Pain on Exertion and Rest (N=103) |
Intermittent Claudication (N=96) |
P Value* |
|---|---|---|---|---|---|---|
| Normalized Mean Plaque Area | 0.65 (0.02) 1 | 0.68 (0.02) | 0.71 (0.02) | 0.71 (0.02) | 0.72 (0.02) | 0.058 |
| Normalized Maximal Plaque Area | 0.85 (0.04) 2 | 0.84 (0.06) 3 | 0.94 (0.04) | 0.95 (0.04) | 0.97 (0.04) | 0.121 |
| Mean Percent Lumen Area | 0.36 (0.02) 4 | 0.33 (0.02) | 0.33 (0.01) | 0.32 (0.01) | 0.30 (0.01) | 0.134 |
| Minimum Percent Lumen Area | 0.27 (0.02) 4 | 0.26 (0.02) | 0.23 (0.01) | 0.23 (0.01) | 0.22 (0.01) | 0.091 |
Data are adjusted for age, sex, race, hypertension, systolic blood pressure, HDL cholesterol, LDL cholesterol, body mass index, smoking, the ankle brachial index, and statin use.
The P value shown in the final column represents a test for homogeneity across all leg symptom categories. Pairwise comparisons were performed between participants with intermittent claudication (reference group) and each remaining leg symptom category: 1 P=0.005;
P=0.03;
P=0.045;
P=0.01.
Discussion
Among 427 men and women with an ABI < 1.30, lower ABI values were associated independently and significantly with greater mean and maximum normalized plaque area and smaller mean and minimum percent lumen area in the proximal superficial femoral artery, compared to higher ABI values. Findings were independent of potential confounders including age, sex, race, BMI, atherosclerotic disease risk factors, and statin use. These associations were maintained even when analyses were restricted to PAD participants only. PAD participants who were asymptomatic had smaller mean and maximum plaque area and greater mean and minimum percent lumen area as compared to PAD participants with intermittent claudication. PAD participants with atypical leg pain/carry on had smaller normalized maximum plaque area than PAD participants with intermittent claudication. Our results suggest that plaque measures visualized in a short segment of the proximal superficial femoral artery may be surrogate measures for total lower extremity plaque burden as measured by the ABI.
A previous study of 87 PAD participants who underwent MRI imaging of the superficial femoral artery demonstrated a correlation between the ABI and plaque area in the superficial femoral artery of −0.26 (16). However, this correlation was not adjusted for confounders (16). In addition, this prior study did not assess associations of leg symptoms with MRI-measured atherosclerotic plaque.
The ABI, a ratio of Doppler recorded systolic blood pressures in the lower and upper extremities, is an important prognostic indicator in people with and without PAD (23,24). Lower ABI values are associated with greater functional impairment, increased cardiovascular event rates, increased all-cause mortality, and greater functional decline among men and women with and without PAD (21–24). Further study is needed to determine whether greater plaque area and smaller lumen area in the proximal superficial femoral artery, measured by MRI, are associated with increased cardiovascular event rates and greater functional decline in patients with PAD.
In certain settings, measuring atherosclerotic plaque directly with MRI has potential advantages over the ABI. For example, the ABI can be influenced by medial arterial calcinosis. Stiff lower extremity arteries can increase the ABI measurement independently of presence or progression of lower extremity atherosclerosis, resulting in insensitivity of the ABI to progression of lower extremity atherosclerosis over time. Consistent with this phenomenon, previous study shows that the ABI does not readily change over time, even as functional performance deteriorates among individuals with PAD (25). In addition, the ABI may be influenced by collateral vessel flow. Thus, direct measurement of atherosclerotic plaque in the superficial femoral artery may better assess progression or regression of plaque than changes in the ABI over time. Further study is needed to determine whether MRI is more sensitive to progression of atherosclerotic plaque than changes in the ABI.
Our MRI imaging techniques did not allow us to separate the arterial wall from atherosclerotic plaque when quantifying total plaque area or percent lumen area. Because of individual variation in artery size, our measures of plaque area and percent lumen area were normalized to total artery size. Our results show that in men and women with ABI values of 1.00–1.30, mean lumen area comprised 50% of total artery size. However, our images do not allow us to quantify atherosclerotic plaque separately from wall area.
Previous work from our group demonstrated that some asymptomatic patients with PAD have greater functional impairment and faster functional decline than PAD participants with intermittent claudication (26,27). However, results presented here indicate that asymptomatic PAD participants have less atherosclerotic plaque in the proximal SFA than PAD participants with intermittent claudication. Asymptomatic PAD participants may consist of a heterogeneous group of individuals, including individuals with mild PAD and those with more severe disease who have limited their walking speed or slowed their activity to avoid leg symptoms (26,27). Differences in study inclusion and exclusion criteria for the current cohort compared to our prior studies may have contributed to our finding that asymptomatic PAD participants had less atherosclerosis than those with intermittent claudication. Further study is needed to better characterize and define causes of asymptomatic PAD.
This study has limitations. First, data are cross-sectional. Further study is necessary to establish associations of changes in the ABI with changes in lower extremity atherosclerotic disease burden. Second, MRI data were obtained on a relatively small segment of the proximal superficial femoral artery. Associations of the ABI with directly-measured plaque in other lower extremity arteries are unknown. Third, data on plaque composition, such as lipid-rich necrotic core, were not available. Fourth, the relatively small number of participants without PAD may limit the representativeness of our findings regarding non-PAD participants.
In conclusion, MRI-measured plaque area and percent lumen area in the proximal superficial femoral artery are highly correlated with the ABI among men and women with PAD. PAD participants who were asymptomatic had smaller plaque area and greater percent lumen area compared to PAD participants with intermittent claudication. Prospective data are necessary to better identify the prognostic value and clinical significance of directly-visualized plaque in the superficial femoral artery.
Acknowledgments
Sources of Funding
Supported by the National Heart Lung and Blood Institute (R01-HL083064), the Intramural Research Program of the National Institute on Aging, and the Jesse Brown VA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Chun Yuan receives research support from VP Diagnostics and from Philips Healthcare. Christopher M. Kramer receives research support from Siemens Healthcare. Dongxiang Xu is a technical consultant for VP Diagnostics and owner of Imaging Biomarker Solutions. There are no disclosures from other authors.
References
- 1.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, Davis JW, Ellington E, Ferguson MS, Hatsukami TS. Identification of fibrous cap rupture with magnetic resonance imaging is associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–185. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque. Part II: Approaches by Noninvasive Computed Tomographic/Magnetic Resonance Imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 4.Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001;89:305–316. doi: 10.1161/hh1601.095596. [DOI] [PubMed] [Google Scholar]
- 5.Fung YC. Biodynamics – Circulation. New York: Springer-Verlag; 1984. Blood flow in arteries: pressure and velocity waves in large arteries and the effects of geometric nonuniformity; pp. 133–136. [Google Scholar]
- 6.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Brit J Surg. 1969;56:676–679. doi: 10.1002/bjs.1800560910. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huen R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Dieppe P, Altman RSD, Buckwalter JA. Standardization of methods used to assess the progression of osteoarthritis of the hip or knee joints. In: Kuettner KE, Goldberg VM, editors. Osteoarthritic Disorders. Rosemont, ILL: American Academy of Orthopaedic Surgeons; 1995. pp. 481–496. [Google Scholar]
- 10.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 11.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Garnst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 14.Hyvarinen S. Arteriographic findings of claudication patients. Ann Clin Res. 1984;16:1–45. [PubMed] [Google Scholar]
- 15.Isbell DC, Meyer CH, Rogers WJ, Epstein FH, DiMaria JM, Harthun NL, Wang H, Kramer CM. Reproducibility and reliability of atherosclerotic plaque area measurements in peripheral arterial disease with magnetic resonance imaging. J Cardiovasc Magn Reson. 2007;9:71–76. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, DiMaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease. J Am Coll Cardiol. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 18.Rifai N, Iannotti E, DeAnagelis K, Law T. Analytical and clinical performance of a homogenous enzymatic LDL-cholesterol assay compared with the ultracentrifugation-dextran sulfate-Mg++ method. Clin Chem. 1998;44:1242–1250. [PubMed] [Google Scholar]
- 19.Sugiuchi H, Ugi Y, Okabe H, Irie T, Uekama K, Kayahara N, Miyauchi K. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated a-cyclodextrin. Clin Chem. 1995;41:717–723. [PubMed] [Google Scholar]
- 20.Topouchian JA, El Assaad MA, Orobinskalia LV, El Feghali RN, Asmar RG. Validation of two automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension: The Omron M6 (HEM-7001-E) and the Omron R7 (HEM 637-IT) Blood Press Monit. 2006;11:165–171. doi: 10.1097/01.mbp.0000209078.17246.34. [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor L, Clark ET. The ankle brachial index as a measure of leg functioning and physical activity in peripheral arterial disease: The Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 23.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of Borderline and Low Normal Ankle-Brachial Index Values With Functional Decline at 5-Year Follow-Up: The WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner AW, Montgomery PS, Killewich LA. Natural history of physical function in older men with intermittent claudication. J Vasc Surg. 2004;40:73–78. doi: 10.1016/j.jvs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, Green D, Sufit R, Hoff F, Nishida T, Sharma L, Pearce WH, Schneider JR, Criqui MH. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Liao Y, Criqui MH. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58:1256–1262. doi: 10.1111/j.1532-5415.2010.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]