Abstract
Objective
To exercise testing in AD and possible disease-related change over time. Though physical activity and fitness are receiving increased attention as a possible adjunct treatment for Alzheimer’s disease (AD), relatively little work has been done characterizing their physiologic response to exercise
Design
Retrospective assessment of a 2-year, observational study
Setting
University medical center
Participants
50 nondemented individuals and 31 with AD
Interventions
None
Main Outcome Measures
Participants underwent a clinical dementia evaluation and performed an incremental exercise test using a treadmill and the modified Bruce protocol at baseline and at a two year follow-up. We examined oxygen consumption, minute ventilation, heart rate and ventilatory equivalents for oxygen and carbon dioxide at submaximal and peak exercise intensities to determine if the measures were different between groups or over time.
Results
AD and nondemented participants performed similarly at submaximal effort and both groups showed similar change in exercise response over 2 years. However, nondemented individuals had consistently higher values of oxygen consumption (p≤0.02) and minute ventilation at peak effort at baseline (p=0.003).
Conclusions
Individuals with AD demonstrate physiologic responses to submaximal exercise effort that are not significantly different than individuals without dementia. However, differences are apparent at the extreme of effort.
Keywords: dementia, peak oxygen consumption
There is increasing evidence to support a relationship between cardiorespiratory (CR) fitness and brain health. Higher CR fitness has been repeatedly linked to improved cognitive performance in both healthy older adults1–3 and those with Alzheimer’s disease (AD).4–7 Further, in individuals with AD, CR fitness has been associated with whole brain volume.8 However, other reports have demonstrated disease-related brain change may also have systemic consequences, such body composition change, metabolic change and a decline in physical activity that could result in lower CR fitness.8–13 These findings highlight the reverse relationship whereby brain disease may result in dysfunction beyond the nervous system and impact cardiorespiratory health.
As noted by the American College of Sports Medicine, recommendations for exercise testing individuals with AD are based solely on anecdote and procedures for older adults or those with emotional lability.14 Currently, there are only a few reports using standard maximal exercise testing procedures to assess CR fitness in those with dementia.4, 8, 15 The increasing interest on this topic, coupled with observed systemic differences necessitates further characterization and comparison of cardiopulmonary response during exercise testing in those with AD. In addition, therapeutic exercise interventions are routinely prescribed at intensities below maximal performance from an exercise test or as a percentage of age-predicted heart rate (HR) max.16 Despite the demonstrated benefits for exercise in those with AD, there is no comparison of cardiovascular and pulmonary performance at submaximal effort in individuals with AD to nondemented peers. Understanding the exercise response in those with AD may help guide exercise prescription recommendations and inform appropriate conclusions about factors affecting fitness over the course of the disease.
The purpose of the present report is two-fold: 1) to assess the cardiovascular and pulmonary response during submaximal work and at peak effort of an incremental treadmill exercise test between those with AD and nondemented individuals, and 2) to describe change in exercise testing performance with time and disease progression. We examined a 2-year change in the cardiopulmonary system in an early AD and nondemented population generally considered to be underactive.12
METHODS
Sample and recruitment
The sample for this secondary analysis was drawn from a larger pool of participants enrolled in the University of Kansas Brain Aging Project (BAP). The BAP is a longitudinal observational study of older adults, both nondemented individuals and those with early-stage AD, aged 65 years and older. Study exclusion criteria have been discussed previously8 and include neurologic disease other than AD that might account for dementia, major depression (Geriatric Depression Scale > 4), current or past history of diabetes mellitus, recent history of cardiovascular disease, or systemic illness or orthopedic impairment that could interfere with completion of the study. Institutionally approved informed consent was obtained from all participants or their legal representative as appropriate before enrollment into the study.
Participants (n=113) attended a baseline clinical and exercise evaluation and an identical evaluation approximately 24 months later. Based on our interest in submaximal performance and change over 2-years, we included only the 81 individuals who completed at least 3 full stages (6 minutes) of the exercise test at both timepoints (n=23 failed to meet these criteria). We also excluded 9 individuals who began experiencing cognitive impairment at follow-up.
Clinical assessment
The clinical assessment included a semi-structured interview with the participant and a knowledgeable informant. Medications, past medical history, education, demographic information, and family history were collected from the informant. Dementia status of the participant was based on clinical evaluation.17 NINCDS-ADRDA diagnostic criteria for AD were used,18 and the severity of dementia was graded using the Clinical Dementia Rating (CDR).19 The CDR assesses impairment in multiple domains. An algorithm is used to generate a Global dementia severity score (very mild=0.5, mild=1, moderate=2, severe=3), or the domains can be summed to create a more sensitive measure of (CDR Sum of Boxes, range 0–18). All participants with dementia in the present study enrolled in the earliest stages of AD, CDR of 0.5 or 1.
Exercise testing
Participants performed a graded treadmill exercise test within 1 month of their clinical evaluation. Individuals were instructed to abstain from consuming food and caffeine 3 hours prior the scheduled test. Calibration procedures were performed on the metabolic cart before each test according to the manufacturer specifications. An exercise physiologist familiarized each participant with the exercise equipment, testing protocol and explained the Borg Rating of Perceived Exertion (RPE) Scale. An incremental treadmill test using a modified Bruce protocol designed for older adults was employed.20 Participants began walking at a pace of 1.7 miles per hour at 0% incline. At each 2-minute interval, the grade, speed or both was increased. Subjects were attached to a 12-lead electrocardiograph to continuously monitor heart rate and rhythm. A 2-way, non-rebreathing valve, headgear, mouthpiece and nose clip were worn. Blood pressure and RPE were acquired during the last 30 seconds of each stage. Expired gases were collected continuously and oxygen uptake and carbon dioxide production was averaged at 15-second intervals (Parvomedics, Sandy, UT). The exercise test was terminated if the participant reached volitional exhaustion or met absolute test termination criteria according to ACSM guidelines.16
The outcome measures selected provide a comprehensive overview of the cardiopulmonary physiologic response to exercise. Key exercise testing variables were: oxygen consumption (VO2; ml*kg−1*min−1), heart rate (HR), minute ventilation (VE in L*min−1), which is the amount of air moved in and out of the lungs per minute, and ventilatory equivalent for oxygen (VE/VO2) and ventilatory equivalent for carbon dioxide (VE/VCO2) as an indices of ventilatory efficiency.21 Because exercise effort is dependent in part on participant motivation, we compared submaximal performance over the first 6 minutes of the exercise test, averaged at 1 minute intervals, indicative of oxygen consumption at lower intensity activities, such as community ambulation.
The same performance measures, as well as respiratory exchange ratio (RER) and rating of perceived exertion (RPE) were also assessed at peak effort; the highest value of oxygen consumption measured during the test (VO2peak; ml*kg−1*min−1). Criteria for peak effort consisted of achieving RER >=1.0 and 85% of age-predicted maximal HR at VO2peak. Peak values reflect a subgroup of participants at each timepoint as not all individuals achieved these criteria. At baseline, 5 nondemented and 2 individuals with AD failed to meet these criteria. At the 2-year follow-up visit, 7 nondemented and 6 individuals with AD failed to meet criteria.
Statistical Analysis
Group differences in demographic measures (age, gender, cognitive status) were tested with Student’s t or Mann-Whitney U for non-normally distributed data and Chi square tests for categorical measures. Submaximal performance was assessed using an ANOVA with Dementia Group (AD, nondemented) as a between-subjects factor and Test Minute (minutes 1–6) and Study Visit (Baseline and Follow-up) as within-subject factors, corrected for repeated measures. We employed 3-way mixed model ANOVAs to reduce the number of tests necessary and therefore the likelihood of Type I error. Further, assessment of submaximal performance over each minute of the initial testing period is a useful method of examining early response to exercise in clinical populations.21 Greenhouse-Geisser correction was used to address violations of sphericity, α=0.05. Post-hoc testing was performed when necessary for those measures showing significant interaction effects. Because, those who met peak exercise criteria were only a sub sample of each group at each study visit, we used a one-way ANOVA to test measures at peak oxygen consumption with Dementia Group as the between-subjects factor, rather than a repeated measures model. To aid interpretation, general eta squared (η2 G)22 is provided for interpretation of effect size in repeated measures ANOVA, and Cohen’s d is provided for one-way ANOVA. We additionally described who was and was not able to meet criteria for peak effort based on medication use and dementia status.
RESULTS
Demographics and Clinical Dementia Presentation
Fifty individuals were nondemented (CDR 0) and 31 had AD. Dementia severity baseline for those with AD was very mild (CDR 0.5, n=28) to mild (CDR 1, n=3), progressing in several individuals (CDR 0.5, n=19; CDR 1, n=9; CDR 2, n=3) over the course of the study. Nondemented participants and those with AD were similar in age (t=0.778 [79], p=0.44) and gender distribution (χ2=0.24, p=0.65). The AD group had significantly lower cognitive function (MMSE) scores at baseline (U=179.0, p < 0.001) and follow-up (U=159.0, p < 0.001) evaluations. A summary of demographic information can be found in Table 1.
Table 1.
Demographics of study participants
| Nondemented (n=50) |
Alzheimer's disease (n=31) |
|||
|---|---|---|---|---|
| Age | 73.1 (6.7) | 74.2 (5.7) | ||
| Gender (% female) | 54% | 48% | ||
| Baseline Visit | Follow-up Visit | Baseline Visit | Follow-up Visit | |
| CDR Sum of Boxes | 0 (0.1) | 0 (0.1) | 2.8 (1.1) | 4.5 (3.7) |
| Mini-Mental Status Exam* | 29.5 (0.8) | 29.2 (1.3) | 26.6 (2.5) | 23.1 (7.8) |
| Β-blocker use (n) | 1 | 1 | 3 | 4 |
| Acetycholinesterase Inhibitor use (n) | 0 | 0 | 15 | 26 |
p<0.001 between groups at baseline and follow-up. Values for age, CDR Sum of Boxes and Mini-mental Status Exam represent means (standard deviation). Pharmaceutical use expressed in number of individuals taking drug at time of exercise test.
Exercise Response
We have grouped both submaximal and peak results by cardiopulmonary measure. Submaximal data include all participants whereas peak data include only those meeting peak exercise test criteria (RER >= 1.0 and HR at highest VO2 > 85% of age predicted maximal HR). Peak exercise values are listed in Table 2.
Table 2.
Cardiorespiratory Response at Peak Effort
| Nondemented | Alzheimer's disease | |||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Individuals Meeting Peak Criteria (n) | 45 | 43 | 29 | 25 |
| Peak Oxygen Consumption * | 24.0 (6.0) | 22.5 (5.8) | 21.0 (3.6) | 18.4 (4.1) |
| Heart Rate at Peak † | 150.5 (14.6) | 139.7 (26.2) | 137.6 (21.6) | 129.2 (34.2) |
| Minute Ventilation at Peak ‡ | 53.5 (18.9) | 49.2 (17.1) | 41.6 (10.7) | 41.9 (10.6) |
| Ventilatory Equivalent for Oxygen at Peak | 30.1 (4.3) | 30.2 (4.3) | 28.1 (2.9) | 30.4 (4.5) |
| Ventilatory Equivalent for Carbon Dioxide at Peak | 27.3 (3.2) | 27.7 (3.5) | 26.3 (2.4) | 27.7 (3.2) |
| Respiratory Exchange Ratio at Peak ‖ | 1.10 (0.07) | 1.09 (0.05) | 1.07 (0.05) | 1.10 (0.07) |
| Exercise Test End Time (minutes) ¶ | 12.4 (3.3) | 11.3 (3.5) | 10.6 (2.4) | 9.0 (2.2) |
| Rating of Perceived Exertion at Peak | 17.3 (1.6) | 16.1 (2.0) | 17.4 (1.9) | 16.8 (2.6) |
Values are group means (standard deviation) except for counts of individuals meeting peak criteria.
Group difference (p<0.02) at both timepoints
Group difference (p=0.003) at baseline
Group difference (p<0.06) at both timepoints
Group difference (p=0.032) at baseline
Group difference (p=0.052) at baseline
Group difference (p<0.01) at both timepoints
Oxygen consumption (VO2)
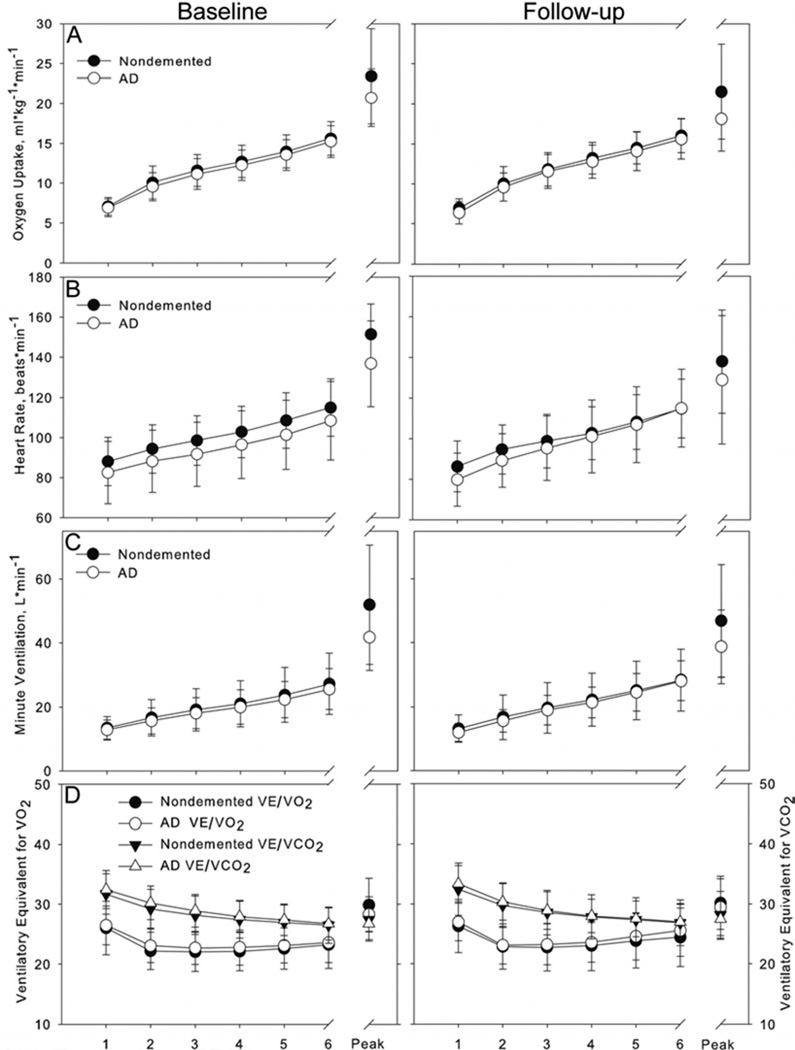
Examination of the submaximal response to exercise suggests during the second visit oxygen consumption increased faster in both groups than at the baseline visit, evident in an interaction of Study Visit×Test Minute (F=11.12 [2.28, 395], p < 0.001, η2G=0.006). VO2 over the initial 6 minutes of the test (i.e. submaximal effort) was not different between groups, evident in the absence of a main effect (F=1.2, p=0.28); Figure 1A). However, peak VO2 was significantly higher in the nondemented group at both baseline (F=6.00 [1, 73], p=0.02, d=0.56) and follow-up visits (F=9.46 [1, 67], p=0.003, d=0.73).
Figure 1.
Response to exercise in our 4 primary measures of interest are displayed for all participants for the initial six minutes of exercise and peak values for those who met 85% of age predicted maximal heart rate and RER > =1.0. Filled shapes represent the nondemented group and open shapes represent the group with AD. Row A) displays oxygen consumption, which rose significantly faster during the initial 6 minutes of the test during 2-year follow-up testing (right graph). VO2 peak was greater in the nondemented group at both timepoints. Row B) At follow-up testing, the AD group began testing with a lower heart rate but increased over the initial 6 minutes of testing to match the nondemented group (right graph), resulting in an interaction of Test Minute and Group. Peak heart rates were different between groups at baseline testing but not at follow-up. Row C) In both groups, VE rose at faster rate during the initial 6 minutes of exercise testing at follow-up compared to baseline, resulting in a significant interaction between Study Visit and Test Minute. Peak VE was greater in the nondemented group at baseline. Row D) Ventilatory equivalent for O2 (left Y axis) and CO2 (right Y axis) are presented together for ease of comparison. Interaction of Test Minute and Study Visit were evident for both measures, with steeper decline in VE/VCO2 and earlier rebound in VE/VO2 at follow-up testing. Peak values were not different between groups.
Heart Rate (HR)
A three-way interaction of Study Visit, Test Minute and Dementia group (F=3.35 [2.7, 178.6], p=0.02, η2G=0.002; Figure 1B) for HR was evident. When we followed with post-hoc ANOVAs split by Study Visit, we found an interaction of Test Minute×Dementia Group in the follow-up visit that drove the interaction. Specifically, at the baseline visit, individuals with AD had started with a lower HR that remained lower throughout the initial 6 minutes of testing (Main effect of Group, F=4.05 [1, 77], p=0.048, η2G=0.05). However, in the follow-up visit individuals with dementia began with a lower HR, but had matched their nondemented peers by minute 6 of the exercise test (Test Minute×Dementia Group interaction, F=3.12 [2.5, 172.2], p=0.04, η2G=0.006). Peak HR was significantly higher in the nondemented group at the baseline visit (F=9.44 [1, 73], p=0.003, d=0.69) but not different at follow-up (F=2.0 [1, 67], p=0.16).
Minute Ventilation (VE)
VE rose faster in both groups at the follow-up test than the baseline test, evident in an interaction of Study Visit×Test Minute (F=15.58 [1.8, 142.4], p < 0.001, η2G=0.004; Figure 1C). There was no main effect of Group (F=0.5, p=0.48) at submaximal effort. VE at peak effort was greater in the nondemented group at baseline (F=9.50 [1, 73], p=0.003, d=0.69) but not at follow-up (F=3.77 [1, 67], p=0.056, d=0.48).
Ventilatory Equivalent for Oxygen (VE/VO2) and Carbon Dioxide (VE/VCO2)
Submaximal VE/VO2 was not different between groups (F=0.71 [1, 79], p=0.40). VE/VO2 had a steeper rate of increase at the follow-up test than the baseline test in minutes 4–6 of the test, evident in an interaction of Study Visit and Test Minute (F=6.65 [2.0, 157.1], p=0.002, η2G=0.004; Figure 1D). Peak VE/VO2 was greater in the nondemented group at baseline (F=4.77 [1, 73], p=0.032, d=0.51) but not at follow-up (F=0.02 [1, 67], p=0.88).
An interaction of Study Visit and Test Minute was present (F=3.60 [1.8, 141.8], p=0.03, η2G=0.001), driven by a steeper decline in VE/VCO2 in the follow-up test than the baseline test at submaximal effort. Submaximal performance was not different between groups (F=0.5 [1, 79], p=0.47). No differences in peak VE/VCO2 were detected between groups at either visit (F < 1.9, p > 0.15)
Respiratory Exchange Ratio (RER) & Rating of Perceived Exertion (RPE)
At the baseline visit, nondemented individuals achieved a higher RER at peak effort than those with AD (F=5.08 [1, 73], p=0.03, d=0.52). This difference did not carry to the follow-up visit (F=0.11 [1, 67], p=0.74). RPE at peak effort did not differ between groups at either time point (F < 2.0, p > 0.16).
Factors in Achieving Peak Effort Criteria
At baseline, 5 of the 50 nondemented individuals who completed at least 6 minutes of exercise testing did not meet our criteria for achieving peak effort (RER >= 1.0 and HR at highest VO2 > 85% of age predicted maximal HR). Failure to meet peak criteria was likely not a result of medications as only one nondemented individual was on a β-adrenergic receptor antagonist (β-blocker) and that person met peak criteria at both baseline and follow-up testing. At no time during the study was anyone in the nondemented group on an acetycholinesterase inhibitor (AChEI: Donepezil, Rivastigmine or Galantamine).
At the baseline visit, 2 of the 31 individuals with AD did not meet our peak effort criteria. As with the nondemented group, pharmaceutical use was likely not related to failure to meet peak criteria. All 3 individuals who were on a β-blocker met peak criteria. Fifteen individuals were taking an AChEI at testing, including 1 of the 2 individuals who did not meet criteria. Dementia severity, as indexed by CDR Sum of Boxes at baseline was 2.7 for those who did meet peak criteria and 4.3 for those who did not meet criteria.
At the follow-up visit, 6 of the 31 individuals with AD failed to meet our peak criteria. All of these individuals were taking an AChEI and none were on a β-blocker. Twenty of 25 individuals who did meet peak criteria were taking an AChEI and 4 of 25 were taking a β-blocker.
DISCUSSION
To our knowledge, there have been no comparisons of cardiopulmonary response along the exercise test continuum in those with AD. This is important because AD is associated with body composition change, metabolic change and a decline in physical activity.9, 11, 12, 23 Further, exercise and physical activity has received increasing attention as possible intervention for AD.24 The results inform both the use of standard exercise testing protocols for individuals with AD and potentially exercise response at submaximal intensity.
Submaximal Response
Both nondemented and individuals with AD responded similarly in the initial stages of exercise testing at both baseline and follow-up. This was true for VO2, VE, VE/VO2 and VE/VCO2. For example, during submaximal effort we observed that VE/VO2 over the initial 6 minutes of the exercise test followed a familiar pattern of early drop followed by slow increase in both groups. At lower exercise intensities, the AD group was working similarly to their non-demented peers.
HR differed slightly between the groups. HR was consistently lower at the baseline testing visit during exercise testing. At follow-up testing we found that though HR in the AD group started lower, HR rose more rapidly in response to exercise and did not differ between groups at peak. We initially thought that the lower observed HR could be attributed to AChEI use. Individuals with dementia taking AChEI are at greater risk for bradycardia.25 However, we found that at follow-up, when 26 of 31 AD participants were on an AChEI, HR response to exercise was more like that of the nondemented individuals. It is possible that this finding is a result of a selection bias, where those most affected by AChEI were unable to complete even 6 minutes of testing and were therefore excluded.
Peak Effort
In contrast to the submaximal exercise response data, notable differences were seen when we considered peak effort. In the group of those with early AD who met the criteria for peak effort, VO2 peak at baseline and 2-year follow-up was lower than their non-demented peers. Using normative values of aerobic fitness from septuagenarians provided by ACSM, mean peak VO2 at baseline was in approximately the 25th – 30th percentile for nondemented women and 10th – 15th percentile for women with AD. Mean peak VO2 at baseline was in approximately the 30th – 35th percentile for nondemented males and 15th – 20th percentile for males with AD.16
It is likely that differences in our outcome measures at peak are related in part to the lower work effort in the AD group. Individuals with AD terminated their exercise tests earlier than their peers without dementia. However, we believe it is premature to attribute these differences solely to reduced effort. For example, at baseline we reported differences in pulmonary performance (lower peak VE) in the AD group. An earlier study examined normal values and ranges for VE at maximal exercise.26 They report mean values for VE at 66 +/− 12 for males and 48 +/− 12 for females in the age range of 70–79. The data for our heterogenous AD group suggest that VE at VO2 peak is lower than the normative data for females. We also found that at baseline peak VE/VO2 was lower in the AD group. Systemic changes associated with AD are one possible contributing factor to this observed difference. We have previously reported lean mass loss in early-stage AD.13 Loss of lean tissue would reduce oxidative capacity and contribute to early muscle fatigue. Further study is warranted to explore these observed cardiorespiratory differences.
Study Limitations
The results reported should be considered carefully. First, we recognize that age and sex influence cardiorespiratory measures. Though the groups were similar in age and sex distribution, they nonetheless could interact with disease and cardiorespiratory fitness. Future studies may consider gender and age specific analyses. Second, it is possible that individuals with early AD prematurely end the exercise test based on perception of fatigue, or volitional exhaustion. However, we included exercise test results only for those who met criteria of an RER > 1.0 and reached 85% of age-predicted HR max. Finally, we did not systematically measure affect during or after the exercise test. If apprehension or anxiety resulted in early termination of the exercise test in the AD group we were unable to capture this.
CONCLUSION
The results suggest that individuals in the early stages of AD respond similarly to nondemented individuals during the initial stages of an exercise test. Because of the typical cardiopulmonary response to submaximal exercise demonstrated here and the well known benefits to overall health, individuals with early AD should be encouraged to participate in the same low to moderate intensity activities as their nondemented peers. However, these individuals demonstrated decreased peak exercise capacity during testing, suggesting that AD patients may have a reduced capacity for high-intensity exercise. These peak differences may represent a mix of real differences in the aerobic capacity of individuals with AD and environmental factors. Nevertheless, physical activity prescribed in an age and cognition-appropriate manner and at intensities described here may promote improvements in peak aerobic capacity and associated health benefits.
Acknowledgments
Support: This study was supported by grant R03AG026374 and R21AG029615 from the National Institutes of Aging, grant K23NS058252 from the National Institute on Neurological Disorders and Stroke, and support from the University of Kansas Endowment Association and William and Carolie Hougland. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, and nursing support. EDV is supported in part by a fellowship from the Foundation for Physical Therapy.
Abbreviations
- AD
Alzheimer’s disease
- CDR
Clinical Dementia Rating
- VO2
oxygen consumption in ml*kg−1*min−1
- HR
heart rate
- VE
minute ventilation
- VE/VO2
ventilatory equivalent for oxygen
- VE/VCO2
ventilatory equivalent for carbon dioxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Device Status: The manuscript submitted does not contain information about medical devices.
REFERENCES
- 1.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 2.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 7.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, et al. Physical activity and memory functions: An interventional study. Neurobiol Aging. 2009;32(7):1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF. Multiple defects in energy metabolism in Alzheimer's disease. Curr Drug Targets. 2010;11(10):1193–1206. doi: 10.2174/1389450111007011193. [DOI] [PubMed] [Google Scholar]
- 10.Cronk BB, Johnson DK, Burns JM. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2009;24(2):126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM. Bone density and brain atrophy in early Alzheimer's disease. J Alzheimers Dis. 2009;18(4):777–785. doi: 10.3233/JAD-2009-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang PN, Yang CL, Lin KN, Chen WT, Chwang LC, Liu HC. Weight loss, nutritional status and physical activity in patients with Alzheimer's disease. A controlled study. J Neurol. 2004;251(3):314–320. doi: 10.1007/s00415-004-0316-4. [DOI] [PubMed] [Google Scholar]
- 13.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimmer J, Smith D. Alzheimer's Disease. In: Durstine JL, Moore GE, Painter PL, Roberts SO, editors. ACSM's Exercise Management for Persons With Chronic Diseases and Disabilities. Champaign, IL: Human Kinetics; 2009. pp. 368–374. [Google Scholar]
- 15.Mancuso M, Filosto M, Bosetti F, Ceravolo R, Rocchi A, Tognoni G, et al. Decreased platelet cytochrome c oxidase activity is accompanied by increased blood lactate concentration during exercise in patients with Alzheimer disease. Exp Neurol. 2003;182(2):421–426. doi: 10.1016/s0014-4886(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 16.ACSM ACoSM. ACSM's guidelines for exercise testing and prescription. 8th ed. Philladelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 17.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11) doi: 10.1212/wnl.43.11.2412-a. 2412b-4. [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill Exercise Testing in an Epidepiologic Study of Elderly Subjects. J Gerontol Biol Sci. 1998;53A(4):259–267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- 21.Mossberg KA, Ayala D, Baker T, Heard J, Masel B. Aerobic capacity after traumatic brain injury: comparison with a nondisabled cohort. Arch Phys Med Rehabil. 2007;88(3):315–320. doi: 10.1016/j.apmr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37(3):379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- 23.Cronk BB, Johnson DK, Burns JM. Body Mass Index and Cognitive Decline in Mild Cognitive Impairment. Alzheimer Dis Assoc Disord. 2009;24(2):126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9(6):390–405. doi: 10.1016/j.jamda.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs new England healthcare system. J Am Geriatr Soc. 2009;57(11):1997–2003. doi: 10.1111/j.1532-5415.2009.02488.x. [DOI] [PubMed] [Google Scholar]
- 26.Blackie SP, Fairbarn MS, McElvaney NG, Wilcox PG, Morrison NJ, Pardy RL. Normal values and ranges for ventilation and breathing pattern at maximal exercise. Chest. 1991;100(1):136–142. doi: 10.1378/chest.100.1.136. [DOI] [PubMed] [Google Scholar]