Abstract
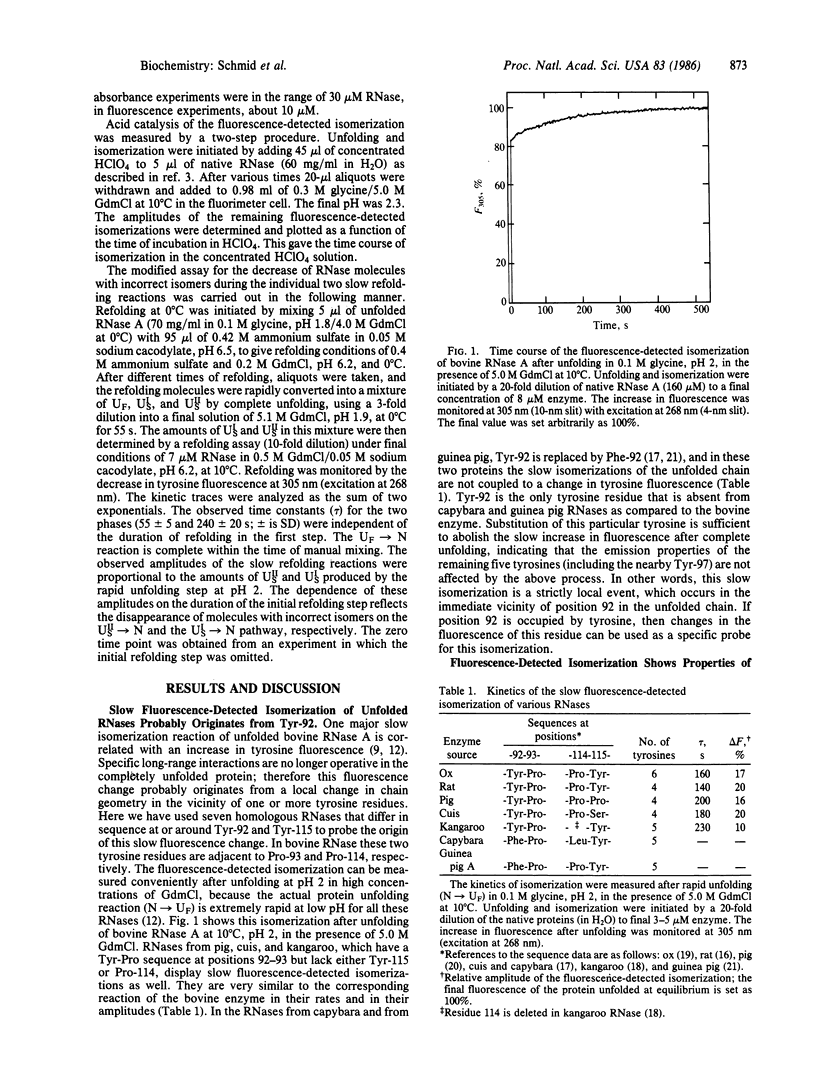
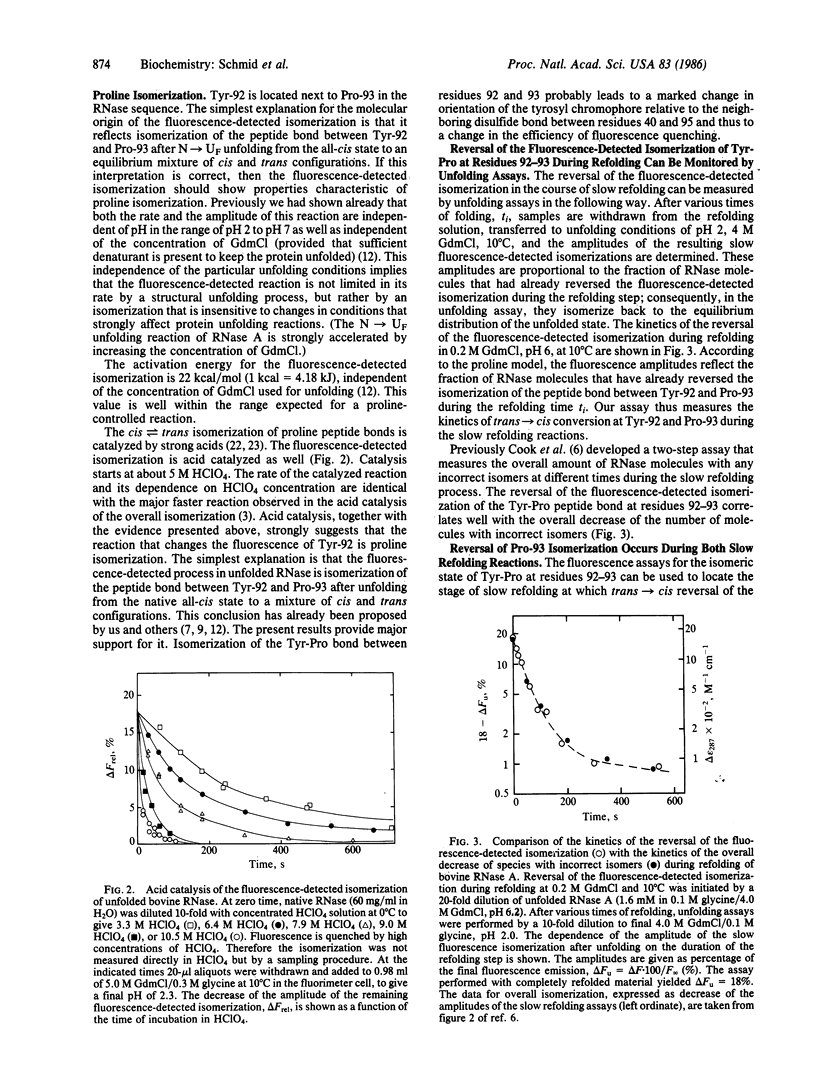
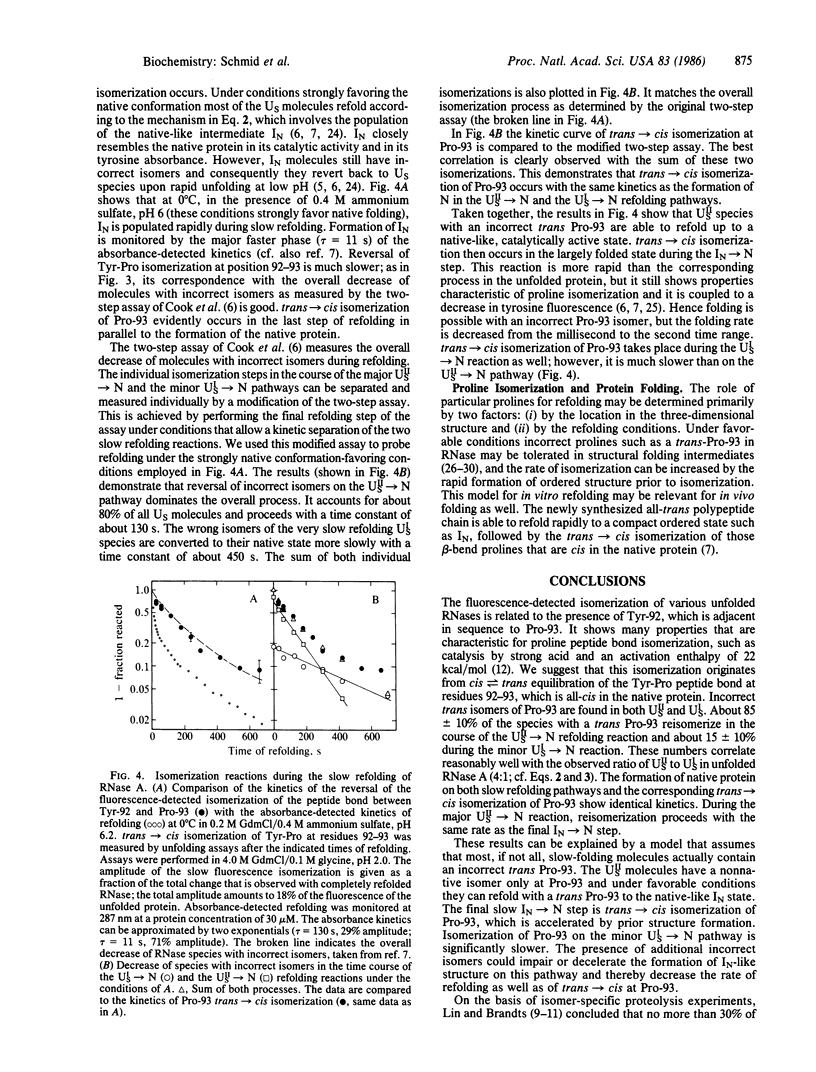
The isomerization of the proline peptide bond between tyrosine-92 and proline-93 in bovine pancreatic ribonuclease A has been investigated in the unfolded protein as well as during the slow refolding process. This bond is in the cis state in the native protein. By comparison of various homologous ribonucleases we show that isomerization of proline-93 is associated with a change in fluorescence of tyrosine-92. This provides a spectroscopic probe to monitor this process in the disordered chain after unfolding as well as its reversal in the course of slow refolding. In unfolded ribonuclease incorrect trans isomers of proline-93 are found in both slow-folding species. trans----cis reversal of isomerization of this proline peptide bond during refolding shows kinetics that are identical with the time course of formation of native protein. Isomerization of proline-93 is slower than the formation of a native-like folded intermediate that accumulates on the major slow refolding pathway. Models to explain these results are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beintema J. J., Gruber M. Rat pancreatic ribonuclease. II. Amino acid sequence. Biochim Biophys Acta. 1973 May 17;310(1):161–173. doi: 10.1016/0005-2795(73)90020-2. [DOI] [PubMed] [Google Scholar]
- Beintema J. J., Neuteboom B. Origin of the duplicated ribonuclease gene in guinea-pig: comparison of the amino acid sequences with those of two close relatives: capybara and cuis ribonuclease. J Mol Evol. 1983;19(2):145–152. doi: 10.1007/BF02300752. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Cook K. H., Schmid F. X., Baldwin R. L. Role of proline isomerization in folding of ribonuclease A at low temperatures. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6157–6161. doi: 10.1073/pnas.76.12.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., Welling G. W., Beintema J. J. The amino-acid sequence of kangaroo pancreatic ribonuclease. Eur J Biochem. 1978 May;86(1):209–217. doi: 10.1111/j.1432-1033.1978.tb12301.x. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. Both the fast and slow refolding reactions of ribonuclease A yield native enzyme. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3347–3351. doi: 10.1073/pnas.70.12.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Hirs C. H. The primary structure of porcine pancreatic ribonuclease. II. The amino acid sequence of the reduced S-aminoethylated protein. J Biol Chem. 1970 Feb 10;245(3):637–653. [PubMed] [Google Scholar]
- Jullien M., Baldwin R. L. The role of proline residues in the folding kinetics of the bovine pancreatic trypsin inhibitor derivative RCAM(14-38). J Mol Biol. 1981 Jan 5;145(1):265–280. doi: 10.1016/0022-2836(81)90343-0. [DOI] [PubMed] [Google Scholar]
- Krebs H., Schmid F. X., Jaenicke R. Native-like folding intermediates of homologous ribonucleases. Biochemistry. 1985 Jul 16;24(15):3846–3852. doi: 10.1021/bi00336a005. [DOI] [PubMed] [Google Scholar]
- Levitt M. Effect of proline residues on protein folding. J Mol Biol. 1981 Jan 5;145(1):251–263. doi: 10.1016/0022-2836(81)90342-9. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Involvement of prolines-114 and -117 in the slow refolding phase of ribonuclease A as determined by isomer-specific proteolysis. Biochemistry. 1984 Nov 20;23(24):5713–5723. doi: 10.1021/bi00319a009. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Isomerization of proline-93 during the unfolding and refolding of ribonuclease A. Biochemistry. 1983 Feb 1;22(3):559–563. doi: 10.1021/bi00272a006. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Mechanism for the unfolding and refolding of ribonuclease A. Kinetic studies utilizing spectroscopic methods. Biochemistry. 1983 Feb 1;22(3):564–573. doi: 10.1021/bi00272a007. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Mechanism for the unfolding and refolding of ribonuclease A. Simulations using a simple model with no structural intermediates. Biochemistry. 1983 Feb 1;22(3):573–580. doi: 10.1021/bi00272a008. [DOI] [PubMed] [Google Scholar]
- Matthews C. R., Crisanti M. M., Manz J. T., Gepner G. L. Effect of a single amino acid substitution on the folding of the alpha subunit of tryptophan synthase. Biochemistry. 1983 Mar 15;22(6):1445–1452. doi: 10.1021/bi00275a019. [DOI] [PubMed] [Google Scholar]
- McPhie P. Swine pepsinogen folding intermediates are highly structured, motile molecules. Biochemistry. 1982 Oct 26;21(22):5509–5515. doi: 10.1021/bi00265a020. [DOI] [PubMed] [Google Scholar]
- Nall B. T. Structural intermediates in folding of yeast iso-2 cytochrome c. Biochemistry. 1983 Mar 15;22(6):1423–1429. doi: 10.1021/bi00275a016. [DOI] [PubMed] [Google Scholar]
- Rehage A., Schmid F. X. Fast- and slow-refolding forms of unfolded ribonuclease A differ in tyrosine fluorescence. Biochemistry. 1982 Mar 30;21(7):1499–1505. doi: 10.1021/bi00536a006. [DOI] [PubMed] [Google Scholar]
- SAGE H. J., SINGER S. J. The properties of bovine pancreatic ribonuclease in ethylene glycol solution. Biochemistry. 1962 Mar;1:305–317. doi: 10.1021/bi00908a018. [DOI] [PubMed] [Google Scholar]
- SMYTH D. G., STEIN W. H., MOORE S. The sequence of amino acid residues in bovine pancreatic ribonuclease: revisions and confirmations. J Biol Chem. 1963 Jan;238:227–234. [PubMed] [Google Scholar]
- Schmid F. X. A native-like intermediate on the ribonuclease A folding pathway. 1. Detection by tyrosine fluorescence changes. Eur J Biochem. 1981;114(1):105–109. doi: 10.1111/j.1432-1033.1981.tb06179.x. [DOI] [PubMed] [Google Scholar]
- Schmid F. X., Baldwin R. L. Acid catalysis of the formation of the slow-folding species of RNase A: evidence that the reaction is proline isomerization. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4764–4768. doi: 10.1073/pnas.75.10.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid F. X., Baldwin R. L. The rate of interconversion between the two unfolded forms of ribonuclease A does not depend on guanidinium chloride concentration. J Mol Biol. 1979 Sep 15;133(2):285–287. doi: 10.1016/0022-2836(79)90536-9. [DOI] [PubMed] [Google Scholar]
- Schmid F. X., Blaschek H. A native-like intermediate on the ribonuclease A folding pathway. 2. Comparison of its properties to native ribonuclease A. Eur J Biochem. 1981;114(1):111–117. doi: 10.1111/j.1432-1033.1981.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Schmid F. X. Mechanism of folding of ribonuclease A. Slow refolding is a sequential reaction via structural intermediates. Biochemistry. 1983 Sep 27;22(20):4690–4696. doi: 10.1021/bi00289a013. [DOI] [PubMed] [Google Scholar]
- Van den Berg A., Van den Hende-Timmer L., Hofsteenge J., Gaastra W., Beintema J. J. Guinea-pig pancreatic ribonucleases. Isolation, properties, primary structure and glycosidation. Eur J Biochem. 1977 May 2;75(1):91–100. doi: 10.1111/j.1432-1033.1977.tb11507.x. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Huizinga J. D., Gaastra W., Welling G. W., Beintema J. J. Affinity chromatography of porcine pancreatic ribonuclease reinvestigation of the N-terminal amino acid sequence. FEBS Lett. 1973 Apr 15;31(2):181–185. doi: 10.1016/0014-5793(73)80098-5. [DOI] [PubMed] [Google Scholar]



