Abstract
The purpose of this study was to investigate whether brain activity related to the presence of stuttering can be identified with rapid functional MRI (fMRI) sequences that involved overt and covert speech processing tasks. The long-term goal is to develop sensitive fMRI approaches with developmentally appropriate tasks to identify deviant speech motor and auditory brain activity in children who stutter closer to the age at which recovery from stuttering is documented. Rapid sequences may be preferred for individuals or populations who do not tolerate long scanning sessions. In this report, we document the application of a picture naming and phoneme monitoring task in three minute fMRI sequences with adults who stutter (AWS). If relevant brain differences are found in AWS with these approaches that conform to previous reports, then these approaches can be extended to younger populations. Pairwise contrasts of brain BOLD activity between AWS and normally fluent adults indicated the AWS showed higher BOLD activity in the right inferior frontal gyrus (IFG), right temporal lobe and sensorimotor cortices during picture naming and and higher activity in the right IFG during phoneme monitoring. The right lateralized pattern of BOLD activity together with higher activity in sensorimotor cortices is consistent with previous reports, which indicates rapid fMRI sequences can be considered for investigating stuttering in younger participants.
Keywords: stuttering, fMRI, lateralization, inferior frontal gyrus
Developmental stuttering impairs speech production and negatively impacts quality of life in more than three million Americans (Craig, Blumgart & Tran, 2009; Yaruss, 2010). The underlying nature of the disorder appears to involve a genetic predisposition that is expressed neurologically in altered brain structure and function (Ambrose, Cox & Yairi, 1997; De Nil, Kroll, Kapur & Houle, 2000; Sommer, Koch, Paulus, Weiller & Buchel, 2002). In this study, we discuss the initial results of a neuroimaging project aimed at developing rapid optimized imaging protocols to study persistent developmental stuttering in children and adults. A rapid functional sequence that reliably identifies speech production activity in the speech system has multiple applications including imaging individuals who do not tolerate long scans and permits more time during an MRI session for a battery of imaging protocols.
Varied functional imaging investigations have documented an atypical speech production system in AWS that comprises both aberrant increases in brain activity and relative deactivations in speech relevant brain regions. Certain differences in functional activity appear to reflect a general state of the speech production system in AWS, because they have been observed across studies and during fluent speech production or silent verbal processing (De Nil, Kroll, Kapur & Houle, 2000). AWS have shown higher brain activity in left and right cortical motor areas, and the right inferior frontal gyrus during speech tasks relative to normally fluent adults or NFA (Brown, Ingham, Ingham, Laird, & Fox, 2005). In contrast, a relative reduction or suppression of cortical activity has been detected in the bilateral temporoparietal cortex of AWS (auditory association areas) and left inferior frontal gyrus during overt speech (Braun, Varga, Stager, Schulz, Selbie, Maisog, et al., 1997; Fox et al., 1996; Neumann, Preibisch, Euler, Gudenberg, Lanfermann, Gall, et al., 2005) in many of the same studies showing over-activity in other areas. The distribution of cortical activity in AWS also appears more bilaterally symmetrical across the two hemispheres or is right hemisphere lateralized in contrast with the left predominant activity of NFA (Fox et al., 1996; Neumann et al., 2005). These functional anomalies in brain activity during speech are increasingly being linked to atypical neuroanatomical development in the same cortical regions or adjacent white matter connections (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008; Sommer et al., 2002).
Despite progress in identifying an aberrant cerebral system for speech production in adults, it is uncertain whether these functional differences are causal or an adaptation to stuttering. This vexing issue undermines attempts to determine the cause and mechanisms of recovery. Moreover, functional imaging results of a single task on its own may not be suited for identifying causal mechanisms unless supplemented with other methods, including structural scans or a battery of functional tasks. Another need is to study brain activity in younger groups such as children closer to the point of documented recovery from stuttering or documented persistency. Numerous challenges, however, confront neuroimaging studies of younger participants as they will more frequently be reluctant to enter the scanner or will only tolerate short imaging protocols. Yet, in other pediatric populations, fMRI sequences have been successfully employed to identify cortical language areas (O’Shaugnessy, Berl, Moore & Gaillard, 2008).
To address some of these challenges, we developed a three minute fMRI sequence that employs either an oral picture naming task or a phoneme monitoring task to test whether potential neural correlates of stuttering can be identified in AWS with a time-optimized sequence. The naming task is predicted to elicit higher activity in the bilateral oral motor cortex, and right inferior frontal gyrus and relatively decreased responses in auditory processing areas in AWS relative to NFA. The auditory monitoring task is used as a non-motor contrast for the auditory processing prediction. The tasks were selected because young children have performed similar tasks in fMRI experiments and the tasks are sensitive to developmental changes in brain activity (Wilke, Lidzba, Staudt, Buchenau, Grodd & Krageloh-Mann, 2006).
Methods
Participants
Eleven AWS (10 males, 1 female; mean age = 25.9 years, sd = 4.4) and ten normally fluent adults (9 males, 1 female ; mean age = 25.2 years, sd = 3.8) participated in the study. Each subject was right-handed and a monolingual English speaker. None of the participants had a history of psychological, psychiatric, neurological or communicative disorders other than a diagnosis of persistent developmental stuttering in the experimental group. All methods were approved by the Institutional Review Board of the University of Illinois at Urbana/Champaign.
Tasks and Analyses
The two tasks were oral picture-naming and silent auditory-phoneme monitoring. A block design was used to detect blood-oxygen-level-dependent (BOLD) activity during both tasks. A 10 second ON/OFF period was utilized which minimizes movement artifacts during speech tasks (Birn, Cox, & Bandettini, 2004). During each 10 second task block, either 5 pictures were named by the participant or 5 words were presented acoustically and the participant listened for the /s/ sound (Run duration = 3 minutes). The active tasks were contrasted with silent rest blocks. All images were acquired with a 3T Siemens Allegra Headscanner: 32 axial slices, TR=2000 ms, TE=30 ms, flip angle = 90, FOV=240 mm. Separate pairwise contrasts were used to compare BOLD activity between the stuttering and control groups during oral naming and phoneme monitoring. The whole brain contrasts were corrected for multiple comparisons and reported at a corrected alpha level of p<0.05.
Results
Participants in both groups showed robust and distinct activity in speech-relevant brain areas during the 3 minute scanning period for each task. The hemodynamic responses in Figure 1A from a healthy control indicate clear task related responses were present in the primary oral motor cortex for the oral naming task and in the superior temporal gyrus for the auditory monitoring task. In Figure 1B-C, whole brain activity for each group is shown separately for both tasks. During oral naming, both groups showed bilateral cortical activity in the precentral gyrus, superior temporal gyrus (STG), inferior frontal gyrus, insula and supplementary motor area (SMA) along with bilateral subcortical activity in the thalamus and basal ganglia (Figure 1B). During auditory monitoring, the predominant BOLD activity for both groups was detected bilaterally in the STG encompassing the primary auditory cortex and planum temporale (Figure 1C). Less intense BOLD activity was noted in inferior frontal areas and insula bilaterally (Figure 1C). Overall, the BOLD activity for both groups was characterized by a high degree of similarity in both tasks.
Figure 1.
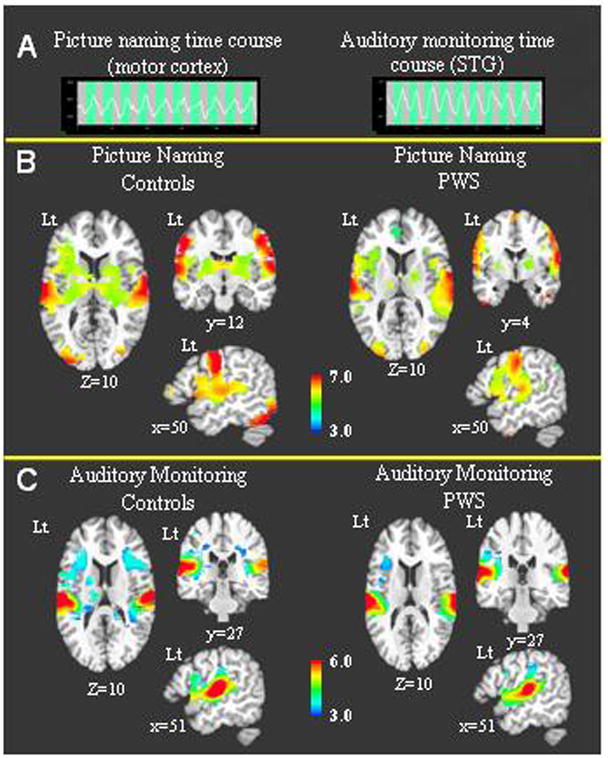
1A – BOLD signal time courses from single voxels are shown for a control participant. The first time course is from the oral motor cortex during the picture naming task and the second is from the superior temporal gyrus during the auditory monitoring task. 1B –The average BOLD activity during the picture naming task is shown for each group. 1C - The average BOLD activity during the auditory monitoring task is shown for each group.
The results of the group contrast for oral naming indicated that AWS show significantly higher BOLD activity across both hemispheres (p<.05 corrected). On the left side, AWS had higher BOLD activity than controls in the precentral gyrus (BA 4, 6) and the superior temporal sulcus (STS) (Figure 2). The group differences were more prominent in the right hemisphere as AWS showed higher activity in the inferior frontal gyrus (IFG), insula, superior temporal sulcus (STS) and middle temporal gyrus (MTG). AWS further showed higher BOLD activity in the subthalamic nucleus bilaterally. The group contrast for auditory monitoring indicated AWS had higher BOLD activity in the right inferior frontal gyrus (p<.05 corrected). AWNS did not show higher activity than AWS in either task contrast.
Figure 2.
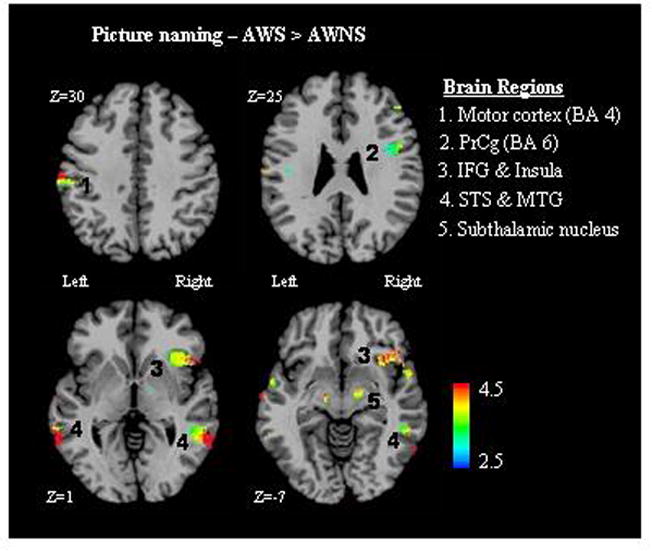
The results of the group contrast between AWS and NFA for the picture naming task are presented. Clusters of significant voxels that indicate higher BOLD activity for the AWS are labeled numerically
Discussion
The purpose of this study was to evaluate whether a rapid fMRI scan would be sensitive to relevant differences in brain activity between AWS and NFA that conform to previous functional imaging reports. The AWS showed prominently higher BOLD activity in the right IFG, including Brodmann areas (BA) 44, 45 & 46, and the insula. An increase in right IFG activity in AWS is a recurring finding in neuroimaging studies (Neumann et al. 2005). The shift shown by AWS away from Broca’s area to the right hemisphere homologue could hinder rapid temporal sequencing required for fluent speech. The right shift in IFG activity may also be related to the increased white matter volume found in this cortical region in AWS (Jancke, Hanggi, & Steinmetz, 2004). Although, the contribution of right IFG activity to stuttering is debated, the consistency with which it is observed (Brown et al., 2005) point to the IFG as a candidate region for explaining part of the deviant speech production representation in stuttering.
AWS exhibited higher BOLD activity in the bilateral primary sensory and motor cortices during oral naming. Most reports have documented increased sensorimotor activity during speech in AWS (Chang, Kenney, Loucks, & Ludlow, 2009; Fox et al., 1996; DeNil et al., 2004). This cortical motor activity difference in the stuttering group was accompanied by higher activity in the subthalamic nucleus bilaterally. Possible explanations for increased sensorimotor activity are that stuttering results from instabilities in the generation of feedforward motor commands or that AWS are more dependent on sensorimotor feedback to maintain fluency. Higher motor activity is even present during non-speech oral tasks in AWS (Chang et al., 2009) suggesting it might be related to the underlying disorder; although it may represent a strategy adopted in childhood.
The right hemisphere activity detected in the STS and MTG of the AWS is important given this was a picture naming task. Research in normal subjects indicate these regions are associated with word storage and retrieval, but much more so for the left hemisphere (Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996). Activation of the right hemisphere homologues in AWS further confirms their language system has a right hemisphere bias.
Decreased auditory activity in the Planum temporale was not evident in the group contrast. However, robust activity in this region was found bilaterally for both groups during oral naming. This certainly does not eliminate the possibility that reduced auditory activity is a functional marker of stuttering, but may reflect the variability of stuttering and emphasizes the highly task dependent nature of functional imaging results.
During auditory monitoring, the predominant BOLD activity for both groups was predictably within the STG encompassing both the primary auditory cortex and planum temporale. Less intense BOLD activity was noted in inferior frontal areas and insula that tended to be left lateralized. The left frontal activity may be related to an automatic engagement of articulatory planning areas even though the task is nonverbal (Chang, Kenney, Loucks, Poletto, & Ludlow, 2009). The group contrast indicated that AWS exhibited increased BOLD activity in the right homologue of Broca’s area (BA 44) during phoneme monitoring relative to the controls. Activity in this area is of interest because it suggests some phonological processing in AWS may be mediated in the right hemisphere. Recently, Weber-Fox et al. (2008) reported that children who stutter show some preference for processing rhyming effects in the right hemisphere. Again this points to a right hemisphere bias in AWS that is even present during covert processing.
The right hemisphere presentation is consistent with numerous reports of brain function in AWS (Fox et al., 1996, Moore, 1993). It is consistent with the long-standing suggestion that right hemisphere functional lateralization may be a compensatory mechanism (Braun et al., 1997) that prompts a neuroplastic reorganization of language activity. Other researchers have proposed that right hemisphere involvement interferes with speech fluency pathways and may be a cause of stuttering (Andrews, Quinn, & Sorby, 1972; Webster, 1990). “In the adult system it may be difficult to distinguish between mechanisms responsible for stuttering and those developed to compensate” (Ludlow, 2000). Whether the right hemisphere activity is predominately an attribute of AWS is a central research question that studies of children who stutter can address.
The picture naming and phoneme-monitoring tasks are designed to test a range of ages from 5 years and up. This study was successful in showing that an efficient three minute scan with distinct but straightforward tasks can identify neural correlates of stuttering. The results did not find a decrease in auditory activity or increased cerebellar activity, which have been previously linked to stuttering (Brown et al., 2005). Other tasks or structural scans may be necessary to supplement the protocol used here, but can be implemented with this time efficient approach.
Acknowledgments
1) 2007 Research Grant for New Investigators, American Speech-Language-Hearing Foundation, P.I. Torrey Loucks, PhD; 2) 2007 Research Board Award, University of Illinois at Urbana-Champaign, P.I. Torrey Loucks, PhD; 3) Subtypes and Associated Risk Factors in Stuttering, RO1 DC005210-06, P.I. Nicoline Ambrose, PhD.
Biographies
Torrey Loucks, PhD
Dr. Loucks is an assistant professor in the Department of Speech and Hearing Science at the University of Illinois. He is the director of the Neurospeech Lab and a member of the Illinois Stuttering Research Program. He combines neuroimaging and motor control paradigms to study sensory feedback and motor mechanisms in normal speech production and speech disorders.
Shelly Jo Kraft, PhD
Shelly Jo Kraft, PhD, is a clinically certified speech pathologist specialized in stuttering. Her current research focuses on the identification and phenotypic role of genes associated with developmental stuttering. Additional research interests include anatomical and functional neurological features of people who stutter, auditory feedback, and stuttering severity.
Ai Leen Choo, MSc
Ai Leen Choo is a doctoral student at the University of Illinois at Urbana-Champaign. She holds a MSc in Psychology from the University of Canterbury, New Zealand. Her main interest lies in the area of speech fluency with a specific focus on developmental stuttering.
Harish Sharma, MSc
Harish Sharma is a biomedical engineer who was the physicist and research scientist at the Biomedical Imaging Center at the University of Illinois. He is currently a PhD student in the Institute of Medical Science at the University of Toronto.
Nicoline G. Ambrose, PhD
Nicoline G. Ambrose received her PhD from the University of Illinois. Her research centers on the etiology, onset and early development of stuttering, with particular reference to genetic factors underlying possible subtypes of stuttering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrose N, Cox N, Yairi E. The genetic basis of persistent and recovered stuttering. Journal of Speech, Language, and Hearing Research. 1997;40:567–80. doi: 10.1044/jslhr.4003.567. [DOI] [PubMed] [Google Scholar]
- Andrews G, Quin PT, Sorby W. Stuttering: An investigation into cerebral dominance for speech. Journal of Neurology, Neurosurgery and Psychiatry. 1972;35:414–18. doi: 10.1136/jnnp.35.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies of overt verbal responses. Neuroimage. 2002;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H215O positron emission tomography study. Brain. 1997;120:761–84. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping. 2005;25:105–17. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39:1333–44. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Kenney MK, Loucks TM, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. NeuroImage. 2009;46(1):201–12. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Kenney M, Loucks TM, Poletto C, Ludlow C. Common neural substrates support speech and nonspeech vocal tract gestures. Neuroimage. 2009;47(1):314–25. doi: 10.1016/j.neuroimage.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Blumgart E, Tran Y. The impact of stuttering on the quality of life in adults who stutter. Journal of Fluency Disorders. 2009;34(2):61–71. doi: 10.1016/j.jfludis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A Positron Emission Tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language and Hearing Research. 2000;43:1038–53. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Hunter Downs J, Martin C, Jerabek P, Glass T, Lancaster JL. A PET study of the neural systems of stuttering. Nature. 1996;382:158–62. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Jancke L, Hanggi J, Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BioMedCentral Neurology. 2004;4(23):1–8. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL. Stuttering: Dysfunction in a complex and dynamic system. Brain. 2000;123(10):1983–84. doi: 10.1093/brain/123.10.1983. [DOI] [PubMed] [Google Scholar]
- Moore WH. Hemisphere processing research. In: Boberg E, editor. Neuropsychology of stuttering. Edmonton (AB): University of Alberta Press; 1993. pp. 39–72. [Google Scholar]
- Neumann K, Preibisch C, Euler HA, Gudenberg A, Lanfermann H, Gall V, Giraud A. Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders. 2005;30(1):23–39. doi: 10.1016/j.jfludis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy ES, Berl MM, Moore EN, Gaillard WD. Pediatric functional magnetic resonance imaging (fMRI): issues and applications. Journal of Child Neurology. 2008;23(7):791–801. doi: 10.1177/0883073807313047. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch M, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. The Lancet. 2002;360:380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Vandenberge R, Price C, Wise R, Josephs O, Frackowiak R. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383(6597):254–56. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, III, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science. 2008;11(2):321–27. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster WG. Motor performance of stutterers: A search for mechanisms. Journal of Motor Behavior. 1990;22:553–71. doi: 10.1080/00222895.1990.10735528. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K, Staudt M, Buchenau K, Grodd W, Krageloh-Mann I. An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage. 2006;32(1):400–10. doi: 10.1016/j.neuroimage.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yaruss JS. Assessing quality of life in stuttering treatment outcomes research. Journal of Fluency Disorders. 2010;35(3):190–202. doi: 10.1016/j.jfludis.2010.05.010. [DOI] [PubMed] [Google Scholar]


