Abstract
Spinal epidural abscess (SEA) is a rare, but serious, condition with multiple causes. We prospectively studied the aetiology, predisposing factors, and clinical outcomes of SEA in all patients with SEA treated in our hospital’s neurosurgical service from 2004 to 2008. For each patient, we recorded the medical history, comorbidities, focus of infection, pathogen(s), and outcome. The 36 patients (19 women and 17 men) ranged in age from 34 to 80 years old (mean 57; median 56). The SEA was primary (i.e., due to haematogenous spread) in 16 patients (44%); it was secondary to elective spinal procedures, either injections or surgery, in 20 patients (56%). The duration of follow-up was 12–60 months (mean 36; median 37.5). The most common pathogen, Staphylococcus aureus, was found in 18 patients (50%). Patients with primary SEA had different underlying diseases and a wider range of pathogens than those with secondary SEA. Only five patients (14%) had no major comorbidity; 16 of the 20 patients with secondary SEA (44% of the overall group) had undergone spinal surgery before developing the SEA; the treatment of the SEA involved multiple surgical operations in all 16 of these patients, and spinal instrumentation in 5 (14%); 22 patients (61% of the overall group) recovered fully.
Keywords: Spinal epidural abscess (SEA), Surgical site infection (SSI), Focal infection, Staphylococcus aureus, Instrumented surgery
Introduction
Spinal epidural abscess (SEA) is found in 0.2–2.8 cases per 10,000 hospital admissions per year [1, 2]. Its outcome is often poor and sometimes fatal. So-called primary SEA is due to the haematogenous spread of pathogens from a distant focus of infection into the spinal epidural space. Microorganisms are normally eliminated from the bloodstream in minutes by the reticuloendothelial system [3]; under certain conditions, however, they can settle and proliferate far away from their site of origin, giving rise to a local primary infection, such as SEA. The risk of SEA is higher in immunocompromised persons, e.g., persons who have the acquired immune deficiency syndrome (AIDS) or are under immunosuppressive treatment after organ transplantation [4]. The risk of SEA is also elevated by drug addiction, alcoholism, cancer, and systemic inflammation or infection [4–6]. The source of infection remains unclear in many cases of primary SEA [7].
So-called secondary SEA occurs after spinal trauma, injections, or surgery, or after the direct introduction of pathogens into the epidural space. Secondary SEA after surgery is a type of surgical site infection (SSI) [8]. The most common pathogen in both primary and secondary SEA is Staphylococcus aureus [2, 9].
Methods
Patients and hospital setting
We prospectively studied all 36 patients treated for SEA by the neurosurgery service of our hospital from January 2004 to December 2008, with the participation of the oral–maxillofacial surgery and infectious disease services. There were 19 women and 17 men (53 and 47% of the total), with ages ranging from 34 to 80 years (mean 57; median 56). They were followed up for periods ranging from 12 to 60 months (mean 36; median 37.5). Our hospital is the only academic tertiary care centre serving an area with a population of about 500,000 people; thus, we can roughly estimate the local incidence of SEA at 1.8/100,000 persons per year.
This purely observational study did not affect the diagnostic or therapeutic steps taken for any patient with SEA. It was designed to meet the ethical standards of the Declaration of Helsinki.
Diagnostic evaluation
SEA was first suspected on clinical grounds (from the history, abnormal physical findings, and/or laboratory abnormalities), then confirmed by an imaging study. Most patients presenting as an emergency initially underwent contrast-enhanced computed tomography (CT). As CT does not always provide conclusive evidence of SEA [2, 5], the diagnosis was definitively established in all patients by magnetic resonance imaging (MRI) with and without contrast medium [10]. All patients had standard laboratory tests on admission, and all underwent a clinical dental examination, including orthopantomography or dental CT. Potential oral foci of infection were sought within 24 h after the diagnosis of SEA through consultation with the oral–maxillofacial surgery and infectious disease services. Swabs from potential intraoral foci of infection were put in aerobic and anaerobic bacterial swab tubes (Copan M40 Transystem®, Copan Italia, Brescia, Italy) for bacterial and fungal cultures. The same system was used to collect samples of infectious material from the SEAs during surgery. Bacterial identification and susceptibility testing were performed according to the guidelines of the Clinical and Laboratory Standards Institute®.
Results
Symptoms and findings on presentation
The patients’ presenting complaints ranged in severity from nonspecific back pain to marked weakness; the neurological examination revealed sensory, motor, and/or reflex deficits in all patients. Two were incontinent of urine, three were tetraparetic, one was hemiplegic, and three were paraplegic. All had local spinal tenderness and pain on percussion. Fever, if present, was only mild, but all patients had an elevated C-reactive protein (CRP) concentration and an increased white blood cell count. All SEAs were diagnosed by contrast-enhanced spinal MRI: the regional distribution of SEAs is shown in Tables 1 and 2. Thirty-one patients (86%) had one or more comorbidities, as listed in Table 3. Twenty-six patients (72%) had chronic inflammatory diseases, endocarditis, or another septic condition. The two youngest patients (both roughly 35 years old) were both under immunosuppressive treatment.
Table 1.
Abscess localisation correlated to primary or secondary origin
| Abscess localisation | |
|---|---|
| Primary SEA cervical | 4 |
| Primary SEA thoracic | 5 |
| Primary SEA lumbar | 11 |
| Primary SEA sacral | 2 |
| Primary SEA with spondylodiscitis | 2 |
| Secondary SEA cervical | 1 |
| Secondary SEA thoracic | 4 |
| Secondary SEA lumbar | 17 |
| Secondary SEA sacral | 3 |
| Secondary SEA with spondylodiscitis | 3 |
| Total number of abscess lesions | 52 |
The total number of lesions (n = 52) is higher than the number of patients (n = 36) due to SEAs might be located in two or more regions, as well as to the fact that the spondylodiscitis cases are counted twice. This extension is shown in Table 2
Table 2.
Numbers of the abscess-involved levels of the spine
| Extension of abscesses in spinal levels | |
|---|---|
| One level | 3 |
| Two levels | 20 |
| Three levels | 9 |
| Four levels | 1 |
| More levels | 3 |
| Total number of lesions | 36 |
Mostly two levels were involved (20/36 cases; 55.6%); in one patient 13 spinal levels were affected
Table 3.
Underlying diseases detected in the 36 SEA patients
| Frequency of comorbidities (n = 36 patients, n = 21 distinct diagnosis) | |
|---|---|
| Previous spine surgery | 16 |
| Septic disease in case history | 14 |
| Chronic inflammatory disease | 9 |
| Neoplasma | 8 |
| Immunosuppressive therapy | 8 |
| Infiltrations/Injections in actual history | 9 |
| Diabetes mellitus | 7 |
| Status after radiation | 6 |
| Metabolic disorders | 5 |
| Addiction diseases | 4 |
| Vascular disease | 4 |
| Obesity | 4 |
| Endocarditis | 3 |
| Psychiatric disorders | 3 |
| Immunosuppressive disease | 3 |
| Previous surgery (excluding spine) | 3 |
| Status after transplantation | 2 |
| Hepatopathy | 1 |
| Pulmonary embolism | 1 |
| Pulmonary insufficiency | 1 |
| Renal insufficiency | 1 |
| Dialysis | 1 |
| Total number of comorbidities | 113 |
Most of the patients suffered from more than one comorbidity, only five patients had none
Primary and secondary SEA
Sixteen patients (44%) had a primary SEA. In five of them (14% of the overall group), the SEA arose after injections at distant sites: repeated antibiotic injections in the limbs (1 patient), intramuscular injections (2 patients), and indwelling venous catheters for chemotherapy and renal failure (1 patient each).
Twenty patients (56%) had a secondary SEA. Four (11%) had undergone local spinal injections (3 facet or periradicular injections, 1 epidural injection), and 16 (44%) had undergone elective microsurgical discectomy. No patient had undergone endoscopic or instrumented spinal surgery. These 16 cases corresponded to a 1.1% rate of postsurgical SEA among the 1,482 elective spinal operations performed on our neurosurgical service over the period of the study. The time from elective discectomy to the appearance of an abscess ranged from 1 to 8 weeks, with a peak at 2 weeks; in a single, exceptional patient, the first symptoms of a fungal abscess (pathogen: Candida albicans) arose 31 weeks after discectomy.
Primary SEA was more common in men than in women: 12 of the 17 men in the study (71%), but only 4 of the 19 women (21%), had a primary SEA. This difference was statistically significant [p = 0.006 by Fisher’s exact test (two-tailed)].
Initial surgical treatment
Two patients with primary SEA were not operated on because of their poor general condition and were treated with antibiotics alone. The remaining 34 patients in the study (94%) underwent surgical removal of the abscess, including decompression of the dural sac and the spinal nerves. The surgical approach was via laminotomy, interlaminar fenestration, hemilaminectomy, or laminectomy, depending on whether liquid pus or granulation tissue was present. As soon as infectious material had been taken for culture, empirical treatment with ceftriaxone (4 g/day IV) and rifampicin (1.2 g/day IV) was begun intraoperatively. A system of two epidurally placed tubes for continuous postoperative irrigation and simultaneous suction drainage was used whenever necessary.
Pathogens causing SEA
The responsible pathogens are listed in Table 4. The most common pathogen causing SEA was Staphylococcus aureus [8 of 16 primary SEA, or 50%; 11 of 20 secondary SEA, or 55%; p = 1.00 by Fisher’s exact test (two-tailed)]. Mixed flora was found in 11 patients (31%). The three rare pathogens at the bottom of Table 4 were all found in a 35-year-old man with severe ulcerative colitis who was taking high-dose prednisone and was also eating yoghurt to build up his enteric flora. The sites of origin of infection are listed in Table 5.
Table 4.
SEA-causing germs listed by frequency
| Proven groups of SEA-causing microorganisms (MO; n = 36 patients) | |
|---|---|
| Staphylococcus aureus a | 19 |
| Coagulasenegative staphylococci a | 5 |
| Corynebacterium a | 4 |
| Streptococcus mitis (Viridans group)b | 3 |
| Enterococcus c | 3 |
| Enterobacter cloace c | 2 |
| Streptococcus agalactiae b | 2 |
| Candida albicans a | 2 |
| Staphylococcus epidermidis a | 1 |
| Staphylococcus capitis c | 1 |
| Propionibacterium acnes c | 1 |
| Finegoldia magna (Peptostreptococcus magnus)b | 1 |
| Streptococcus milleri b | 1 |
| Actinomyces species (DNA-sequenced)b | 1 |
| Actinomyces neuii subsp. antitratus b | 1 |
| Pseudomonas aeruginosa d | 1 |
| Proteus mirabilis c | 1 |
| Salmonella enteridis c | 1 |
| Lactobacillus Morphotype 1 | 1 |
| Lactobacillus Morphotype 2 | 1 |
| Candida Kefyr | 1 |
| None | 2 |
| Total | 53 |
The last three MOs represent an inhuman group of MOs from milk products. In two cases treated exclusively with antibiotics, there was no find, due to missing proof of specimen. In most cases there was more than only one sample for MO present; there were up to six different relevant MOs detected per patient
aTypical dermal MOs
bOral MOs
cCentral MOs
dA typical inhuman hospital pathogen
Table 5.
Origins of primary and secondary SEAs
| Origin of spine abscess | Primary SEAs (n = 16) | Secondary SEAs (n = 20) |
|---|---|---|
| Number of foci | Number of foci | |
| Iatrogen (injections/catheters) | 5 | 4 |
| Oral focus | 4 | 1 |
| Potential oral lesion | 3 | 2 |
| Skin lesion | 4 | 2 |
| Enteral lesion | 3 | – |
| Surgical approach | – | 16 |
| Unknown | 2 | – |
| Total | 21 | 25 |
In two cases of primary SEA (n = 16) no origin was demonstrable. 5 of the remaining 14 patients showed clinically and by the MO proof two different foci; this was reflected in a mixed flora on the SEA side from distinct origins. Beside the surgical approach, the secondary SEAs (n = 20) showed lesions in five cases which were more probable to be the causative origin of the SEA. This was depending on the confirmed MO on the abscess side and the clinically obvious distinct focal lesion, again reflected in a mixed flora on the SEA side. Mixed flora led to more origins in both kinds of abscesses than expected by the numbers of patients
Oral pathogens
Dental examination revealed oral pathogens in eight patients (22%), including five with primary and three with secondary SEA. Among the five patients with primary SEA, only one was found to have the same pathogen (Streptococcus agalactiae) in the mouth and in the SEA; one patient was found to have three different oral pathogens; and the remaining three patients suffered from bacterial endocarditis and were found to have Streptococcus viridans in their SEA, apparently without any relation to their oral foci. On the other hand, in all three patients with oral pathogens and secondary SEA, the oral pathogens were shown to be the cause of the SEA. One of them had a Streptococcus agalactiae infection that was apparently due to an odontogenic cyst. Another, an 80-year-old woman with multiple comorbidities (hepatitis C, hepatocellular carcinoma, myeloproliferative syndrome and diabetes mellitus), developed a secondary SEA containing three different oral pathogens after an epidural infiltration. The third patient in this group had Corynebacterium in both the oral focus and the SEA. A total of six different oral pathogens were found in SEAs (primary or secondary), sometimes in a mixed infection (cf. Table 5).
Further treatment and outcome
After surgery, the neurosurgeons and infectious disease specialists followed the patients clinically and radiologically and regularly checked the culture results and infection-related laboratory values. All oral foci of infection were eradicated (this approach was based on previous research on brain abscesses within our group [11]). The antibiotic regimen was adapted as needed, and reoperation was performed in case of clinical or radiological progression of the SEA. These steps were reiterated until the SEA and other infectious foci (if present) were eradicated.
Among the 16 patients with primary SEA, 14 were cured by a single operation with open decompression and evacuation of the abscess, followed by long-term, specific antibiotic treatment. The two who were not operated on because of their poor general condition made a good recovery with antibiotics alone.
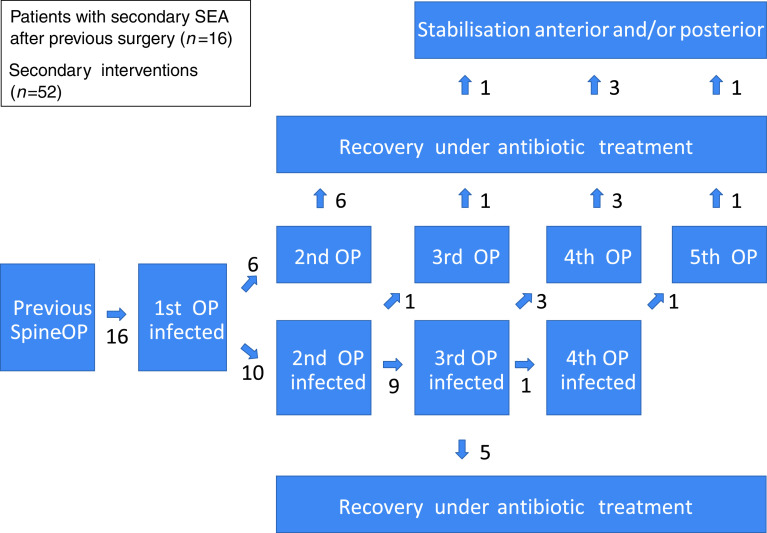
Among the 20 patients with secondary SEA, all 4 whose SEAs were caused by injections were successfully treated with a single operation and antibiotics. On the other hand, all 16 whose secondary SEA had followed an elective discectomy needed two or more operations to treat it (cf. the flow diagram in the Fig. 1). Ten of them needed three or more operations, and five went on to undergo an instrumented spinal stabilisation operation for the elimination of movement-related pain due to spinal instability. Whenever more than three operations were performed, the surgical instruments were specially sterilised according to the recommended MRSA precautions, and the patients were isolated on the ward as if they had MRSA.
Fig. 1.
Treatment course of patients with secondary SEAs (n = 16); all had at least two operations to treat the SEA. The maximum number of surgical interventions in one patient was six, including instrumented surgery for anterior and posterior stabilisation
The overall outcome after SEA, both primary and secondary, is shown in Table 6. All patients ultimately had better neurological function than on admission. Recovery occurred without any neurologic deficit (not counting sensory irritative phenomena) in 11 of 16 patients with primary SEA (69%) and in 14 of 20 with secondary SEA (70%); clearly, primary and secondary SEA did not differ significantly in this respect [p = 1.00 by Fisher’s exact test (two-tailed)]. The maximum number of operations performed to treat SEA was six, in a single patient, including instrumented anterior and posterior spinal stabilisation. This patient made a full neurological recovery.
Table 6.
Overall outcome after primary and secondary SEAs (n = 36)
| Outcome | Primary SEAs (n = 16) | Secondary SEAs (n = 20) |
|---|---|---|
| Full recovery | 10 | 11 |
| Recovery with radicular irritation | 1 | 3 |
| Recovery with radicular deficits | 1 | 4 |
| Recovery with residual paraparesis | 2 | 1 |
| Recovery with residual tetraparesis | 2 | 1 |
| In total | 16 | 20 |
| Severe additional symptoms | ||
| Spinal ataxia | 2 | 1 |
| Urinary bladder dysfunction | 3 | 2 |
All patients in both groups improved compared to the status on admission; none was equal or worse
Discussion
We found many features of SEA that were similar to those reported in other studies. Our rough estimate of the incidence of SEA at 1.8/100,000 persons per year accords with other published estimates [1, 2], although its appearance after only 1.1% of the spinal operations performed by our neurosurgical service seems less frequent than others have reported [8]. We found that the commonest pathogen causing SEA was Staphylococcus aureus, just as in other reports [4, 5, 12, 13, 15, 21, 22]. SEA is usually associated with one or more comorbidities [7]; the list of our patients’ comorbidities resembles the corresponding lists in other reported series [4, 5, 8, 12]. The close association of chronic inflammatory diseases, endocarditis, and other septic conditions with SEA has already been pointed out [15], as has the risk of SEA in persons under immunosuppressive treatment [4, 8, 14]. SEA is known to become more common with advancing age because of the rising frequency of spinal disorders and of comorbidities [13], and the age distribution of our patients was similar to those previously reported [12, 13]. We saw no children with SEA; SEA is known to be rare in children [10].
SEA was previously reported to be more common in men [5, 12]. Our patients were nearly evenly split between men and women, yet we found men much more likely than women to have a primary SEA. This finding was highly statistically significant; we do not know the reason for it, nor have we found any comparable finding in previous reports.
Primary SEA has been reported to arise by haematogenous spread from skin lesions and from oral [3, 6, 11, 16, 17] and enteric foci, including bowel perforation in inflammatory bowel disease [18, 19], as well as from injections and infusion therapy at distant sites [15]. We found primary SEA of all these types among our patients. Secondary SEA is a well-known complication of spinal surgery [8]. It has been said to be a very rare complication of facet infiltration and epidural injections [20, 21], yet these procedures accounted for 4 out of 20 cases of secondary SEA in our series.
Previously published reports imply that oral foci of infection are only a rare cause of SEA [16, 17], yet we found oral foci in nearly one-quarter of our patients. We know of no previous reports of primary SEA with more than one focus of infection, which we found in 4 of our 16 patients with primary SEA (4 of 16 cases). Among our 20 patients with secondary SEA, 6 had distant foci of infection that were presumably causative, including 3 with oral foci. We know of no previous report of secondary SEA due to haematogenous spread from a distant focus.
Treatment and outcome
SEA has occasionally been treated successfully with minimally invasive techniques [23, 24] or with antibiotics alone [13, 22, 25], although the latter is considered controversial. Some of the patients reportedly cured in these ways had no neurological deficits. In contrast, nearly all patients with SEA on our neurosurgery service present with neurological deficits and are operated on as soon as SEA is diagnosed; previous reports imply that this yields a better outcome than giving antibiotics alone [10, 12, 15]. Nonetheless, our two patients whose poor condition precluded surgery did well on antibiotics alone.
Most of our patients had no residual deficit or other symptoms after the completion of treatment, including some of the patients with complicated secondary SEA. We treat SEA by an interdisciplinary approach involving immediate surgical intervention, as previously described and recommended [10, 12, 14, 15]. We know of no previously published comparisons of primary and secondary SEA regarding their origins, treatment, or outcome.
Conclusion
We found that an SEA could be due to more than one focus of infection, even if it is a secondary SEA. Secondary SEA is well known to be caused by skin pathogens that have been introduced by injections or previous surgery, yet one must also consider enteric and, especially, oral pathogens, and the foci from which they arise. To prevent secondary SEA, it would seem reasonable for all patients about to undergo elective spinal surgery to be evaluated for potential foci of infection, as is done before cardiac surgery. Patients with secondary SEA should also be informed early on that multiple operations, and even instrumented spinal stabilisation, might be necessary in the further course of their treatment. The optimal care of patients with SEA involves not only immediate surgery, but also the determination of the appropriate accompanying medical treatment through an early collaboration among neurosurgeons, infectious disease specialists, and the physicians treating the patient’s comorbid illnesses.
Acknowledgments
The authors would like to thank the staff members of the Departments for Neurosurgery, Maxillofacial-Surgery and the Division of Infectious Diseases and Hospital Epidemiology of the University Hospital Basel for their support and cooperation, as well as Petra Schwenzer and Virginia Dittrich for their support in editing.
Conflict of interest
None.
References
- 1.Fang WK, Chen SH, Huang DW, Huang KC. Post-traumatic osteomyelitis with spinal epidural abscess of cervical spine in a young man with no predisposing factor. J Chin Med Assoc. 2009;72:210–213. doi: 10.1016/S1726-4901(09)70057-7. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212. doi: 10.1136/jnnp.65.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartzell JD, Torres D, Kim P, Wortmann G. Incidence of bacteremia after routine tooth brushing. Am J Med Sci. 2005;329:178–180. doi: 10.1097/00000441-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chao D, Nanda A. Spinal epidural abscess: a diagnostic challenge. Am Fam Phys. 2002;65:1341–1346. [PubMed] [Google Scholar]
- 5.Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore) 1992;71:369–385. doi: 10.1097/00005792-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shimomura T, Hori S, Kasai N, Tsuruta K, Okada H. Meningioma associated with intratumoral abscess formation—case report. Neurol Med Chir (Tokyo) 1994;34:440–443. doi: 10.2176/nmc.34.440. [DOI] [PubMed] [Google Scholar]
- 7.Vilke GM, Honingford EA. Cervical spine epidural abscess in a patient with no predisposing risk factors. Ann Emerg Med. 1996;27:777–780. doi: 10.1016/S0196-0644(96)70201-9. [DOI] [PubMed] [Google Scholar]
- 8.Pull Ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine. 2009;34:1422–1428. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 9.Martin RJ, Yuan HA. Neurosurgical care of spinal epidural, subdural, and intramedullary abscesses and arachnoiditis. Orthop Clin N Am. 1996;27:125–136. [PubMed] [Google Scholar]
- 10.Jacobsen FS, Sullivan B. Spinal epidural abscesses in children. Orthopedics. 1994;17:1131–1138. doi: 10.3928/0147-7447-19941201-09. [DOI] [PubMed] [Google Scholar]
- 11.Mueller AA, Saldamli B, Stubinger S, Walter C, Fluckiger U, Merlo A, Schwenzer-Zimmerer K, Zeilhofer HF, Zimmerer S. Oral bacterial cultures in nontraumatic brain abscesses: results of a first-line study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:469–476. doi: 10.1016/j.tripleo.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Curry WT, Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63:364–371. doi: 10.1016/j.surneu.2004.08.081. [DOI] [PubMed] [Google Scholar]
- 13.Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect. 2008;41:215–221. [PubMed] [Google Scholar]
- 14.Pradilla G, Ardila GP, Hsu W, Rigamonti D. Epidural abscesses of the CNS. Lancet Neurol. 2009;8:292–300. doi: 10.1016/S1474-4422(09)70044-4. [DOI] [PubMed] [Google Scholar]
- 15.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Lampen R, Bearman G. Epidural abscess caused by Streptococcus milleri in a pregnant woman. BMC Infect Dis. 2005;5:100. doi: 10.1186/1471-2334-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poullis A, Gould SR, Lim AG. It could only happen to a doctor—Haemophilus aphrophilus septicaemia complicated by a prevertebral infection after dental work. Postgrad Med J. 2001;77:261–262. doi: 10.1136/pmj.77.906.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CJ, Jaffer H, Jaffer N, Burul C, McLeod RS. Spinal epidural abscess—a rare complication of inflammatory bowel disease. Can J Gastroenterol. 2008;22:177–180. doi: 10.1155/2008/893757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conen A, Zimmerer S, Frei R, Battegay M, Elzi L, Trampuz A. A pain in the neck: probiotics for ulcerative colitis. Ann Intern Med. 2009;151:895–897. doi: 10.7326/0003-4819-151-12-200912150-00020. [DOI] [PubMed] [Google Scholar]
- 20.Goodman BS, Posecion LW, Mallempati S, Bayazitoglu M. Complications and pitfalls of lumbar interlaminar and transforaminal epidural injections. Curr Rev Musculoskelet Med. 2008;1:212–222. doi: 10.1007/s12178-008-9035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamiyama Y. Two cases of spinal epidural abscess with granulation tissue associated with epidural catheterization. J Anesth. 2006;20:102–105. doi: 10.1007/s00540-005-0370-9. [DOI] [PubMed] [Google Scholar]
- 22.Siddiq F, Chowfin A, Tight R, Sahmoun AE, Smego RA., Jr Medical vs surgical management of spinal epidural abscess. Arch Intern Med. 2004;164:2409–2412. doi: 10.1001/archinte.164.22.2409. [DOI] [PubMed] [Google Scholar]
- 23.Lyu RK, Chen CJ, Tang LM, Chen ST. Spinal epidural abscess successfully treated with percutaneous, computed tomography-guided, needle aspiration and parenteral antibiotic therapy: case report and review of the literature. Neurosurgery. 2002;51:509–512. [PubMed] [Google Scholar]
- 24.Panagiotopoulos V, Konstantinou D, Solomou E, Panagiotopoulos E, Marangos M, Maraziotis T. Extended cervicolumbar spinal epidural abscess associated with paraparesis successfully decompressed using a minimally invasive technique. Spine. 2004;29:E300–E303. doi: 10.1097/01.BRS.0000131215.46119.DD. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler D, Keiser P, Rigamonti D, Keay S. Medical management of spinal epidural abscesses: case report and review. Clin Infect Dis. 1992;15:22–27. doi: 10.1093/clinids/15.1.22. [DOI] [PubMed] [Google Scholar]