Abstract
The "nonspecific" esterases are a family of enzymes that were originally identified because of their reaction with synthetic O-acetyl ester substrates. While the electrophoretic polymorphisms of these enzymes have been extremely useful for genetic studies, their biological functions have remained completely unknown. Esterase D is characterized by its reactivity with 4-methylumbelliferyl acetate. This enzyme has recently been of particular interest because of its tight linkage to the putative recessive gene causing retinoblastomas, and to the recessive gene causing Wilson disease. We describe here the partial purification of a human erythrocyte esterase that appears to be highly specific for O-acetylated sialic acids. We next present evidence that suggests that esterase D is identical to this sialic acid-specific O-acetylesterase. First, both activities copurify from human erythrocyte lysates through several different purification steps, each of which use different principles of separation. Second, both activities show a remarkably similar profile of inhibition with a variety of different agents. Third, they both show a nearly identical heat-inactivation profile. This cytosolic sialic acid-specific O-acetylesterase appears to be involved in the "recycling" of O-acetylated sialic acid molecules. Thus, esterase D may be the first nonspecific esterase for which a specific biological role can be predicted.
Full text
PDF
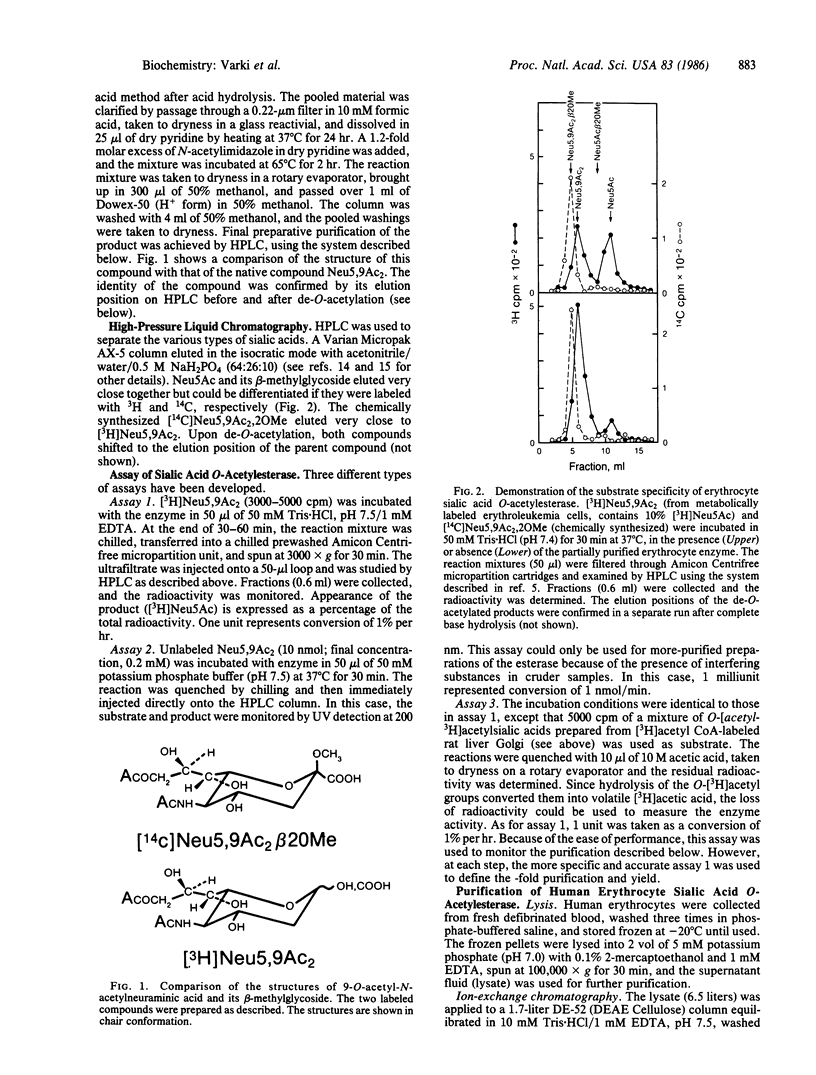

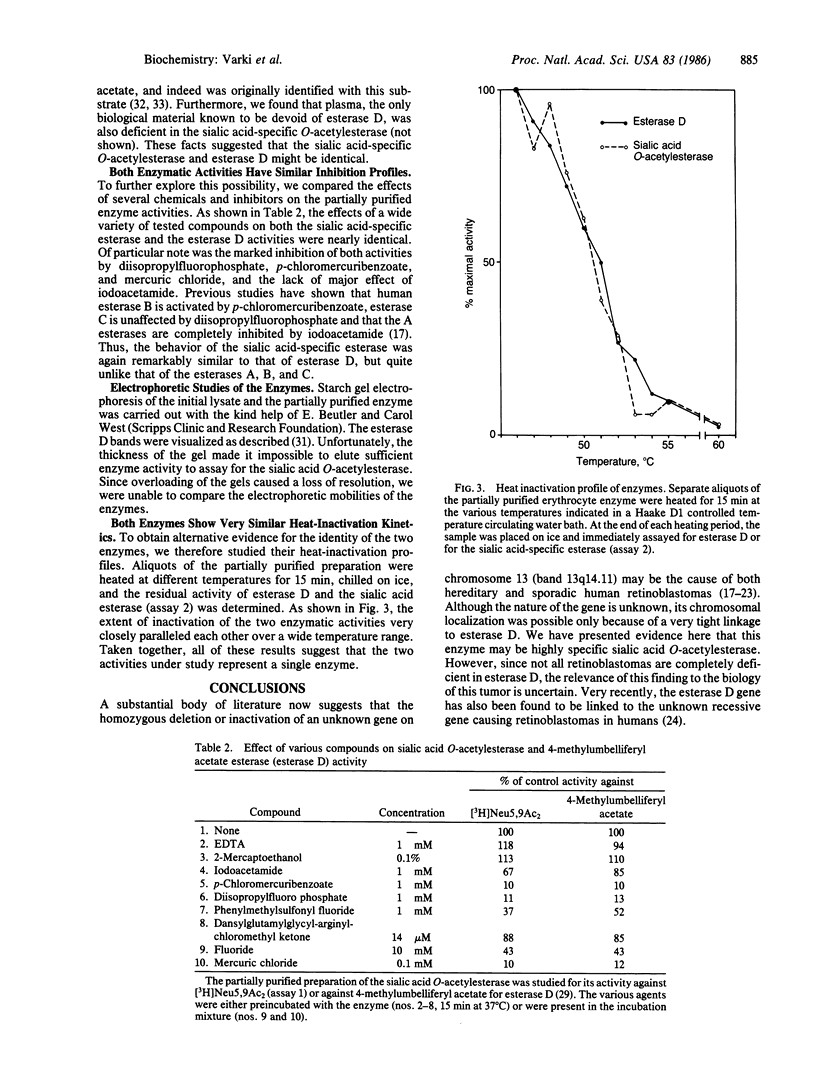

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960 Sep;235:2529–2537. [PubMed] [Google Scholar]
- Cheresh D. A., Reisfeld R. A., Varki A. P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984 Aug 24;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Corfield A. P., Veh R. W., Wember M., Michalski J. C., Schauer R. The release of N-acetyl- and N-glycolloyl-neuraminic acid from soluble complex carbohydrates and erythrocytes by bacterial, viral and mammalian sialidases. Biochem J. 1981 Aug 1;197(2):293–299. doi: 10.1042/bj1970293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S., Varki A. Metabolic labeling of sialic acids in tissue culture cell lines: methods to identify substituted and modified radioactive neuraminic acids. Anal Biochem. 1985 Oct;150(1):32–46. doi: 10.1016/0003-2697(85)90438-5. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Substrate specificity of neuraminidases. Histochem J. 1973 May;5(3):271–290. doi: 10.1007/BF01004994. [DOI] [PubMed] [Google Scholar]
- Frydman M., Bonné-Tamir B., Farrer L. A., Conneally P. M., Magazanik A., Ashbel S., Goldwitch Z. Assignment of the gene for Wilson disease to chromosome 13: linkage to the esterase D locus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1819–1821. doi: 10.1073/pnas.82.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Haverkamp J., Schauer R., Wember M., Kamerling J. P., Vliegenthart J. F. Synthesis of 9-O-acetyl- and 4,9-di-O-acetyl derivatives of the methyl ester of N-acetyl-beta-D-neuraminic acid methylglycoside. Their use as models in periodate oxidation studies. Hoppe Seylers Z Physiol Chem. 1975 Oct;356(10):1575–1583. doi: 10.1515/bchm2.1975.356.2.1575. [DOI] [PubMed] [Google Scholar]
- Higa H. H., Paulson J. C. Sialylation of glycoprotein oligosaccharides with N-acetyl-, N-glycolyl-, and N-O-diacetylneuraminic acids. J Biol Chem. 1985 Jul 25;260(15):8838–8849. [PubMed] [Google Scholar]
- Hopkinson D. A., Mestriner M. A., Cortner J., Harris H. Esterase D: a new human polymorphism. Ann Hum Genet. 1973 Oct;37(2):119–137. doi: 10.1111/j.1469-1809.1973.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Differential enzyme activities in human esterase D phenotypes. Hum Genet. 1984;66(2-3):168–170. doi: 10.1007/BF00286594. [DOI] [PubMed] [Google Scholar]
- KARKAS J. D., CHARGAFF E. STUDIES ON THE STABILITY OF SIMPLE DERIVATIVES OF SIALIC ACID. J Biol Chem. 1964 Apr;239:949–957. [PubMed] [Google Scholar]
- Kozioł P., Stepien J. Atypical segregation of esterase D: evidence of a rare "silent" allele EsD0. Hum Genet. 1980 Feb;53(2):223–225. doi: 10.1007/BF00273500. [DOI] [PubMed] [Google Scholar]
- Levine J. M., Beasley L., Stallcup W. B. The D1.1 antigen: a cell surface marker for germinal cells of the central nervous system. J Neurosci. 1984 Mar;4(3):820–831. doi: 10.1523/JNEUROSCI.04-03-00820.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Nishigaki I., Itoh T., Ogasawara N. Quantitative variations in polymorphic types of human red cell esterase D. Ann Hum Genet. 1983 Jul;47(Pt 3):187–192. doi: 10.1111/j.1469-1809.1983.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. Nonspecific esterases of Mus musculus. Biochem Genet. 1982 Jun;20(5-6):585–606. doi: 10.1007/BF00484706. [DOI] [PubMed] [Google Scholar]
- Scott E. M., Wright R. C. Purification and substrate specificity of polymorphic forms of esterase D from human erythrocytes. Am J Hum Genet. 1978 Jan;30(1):14–18. [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Targum S., Gershon E., Sensabaugh G. F., Sparkes M. C., Crist M. Evidence for a null allele at the esterase D (EC 3.1.1.1) locus. Hum Genet. 1979 Feb 15;46(3):319–323. doi: 10.1007/BF00273315. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Varki A., Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983 Oct 25;258(20):12465–12471. [PubMed] [Google Scholar]
- Varki A., Diaz S. The transport and utilization of acetyl coenzyme A by rat liver Golgi vesicles. O-acetylated sialic acids are a major product. J Biol Chem. 1985 Jun 10;260(11):6600–6608. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. An autosomal dominant gene regulates the extent of 9-O-acetylation of murine erythrocyte sialic acids. A probable explanation for the variation in capacity to activate the human alternate complement pathway. J Exp Med. 1980 Sep 1;152(3):532–544. doi: 10.1084/jem.152.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Packman S., Loughman W., Sparkes M., Sparkes R., McMahon A., Gregory T., Ablin A. Location of the retinoblastoma susceptibility gene(s) and the human esterase D locus. J Med Genet. 1984 Apr;21(2):92–95. doi: 10.1136/jmg.21.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Deimling O., de Looze S. Human red cell butyrylesterase, and its homologies in thirteen other mammalian species. Hum Genet. 1983;63(3):241–246. doi: 10.1007/BF00284657. [DOI] [PubMed] [Google Scholar]


