Abstract
Introduction
Anterior access to the lumbar spine is established for disc replacement surgery and anterior interbody fusion in the lumbar spine. The spine is accessed normally from the left side either by a transperitoneal or retroperitoneal approach through a midline or oblique skin incision. After reaching the retroperitoneum and depending on the level of exposure, the surgeon has to mobilise and retract the aorta or left common iliac artery, as well as the left common iliac vein or internal vena cava to the right lateral border to address the whole disc space. The left common iliac artery is especially stretched during intervertebral disc exposure putting it at a greater risk of adverse events. Not surprisingly, vascular adverse events like direct injuries, thrombosis and embolism are feared complications in anterior surgery. Permanent intra-operative left leg oxygen saturation surveillance via pulse oximetry can help detecting embolic situations thereby allowing immediate treatment minimising the leg ischemia or preventing limb loss.
Case report
In the presented case, a 61-year-old male patient undergoing a two-level anterior interbody fusion lost oxygen saturation in the left leg after vessel retraction for exposure. After cage insertion and release of the retractor blades, the pulse oximetry signal did not return and no pulses were found during instant Doppler investigation below the femoral artery, indicating severe embolism in the left leg. The left common iliac artery was clamped and opened showing a ruptured calcified plaque with adherent fresh thrombotic material. An endovascular embolectomy in the superficial and deep femoral artery revealed several small thrombi. An artherectomy of the common iliac artery followed by patch closure was performed. Immediately after clamp release, pulse oximetry returned and Doppler signals were detectable at the tibialis posterior and dorsalis pedis artery. Post-operative recovery was uneventful and pulses were palpable at all times.
Conclusion
Arterial adverse events in anterior access surgery are rare complications but none the less, it is of paramount importance to detect and treat these situations immediately. This case highlights the need of routine pulse monitoring during the whole anterior surgery to prevent embolic complications. Even manual pulse control might not be sufficient to rule out any distal embolic events creating severe leg ischemia.
Keywords: ALIF, Access complications, Embolectomy, Monitoring, Pulse oximetry, Lumbar disc replacement
Case presentation
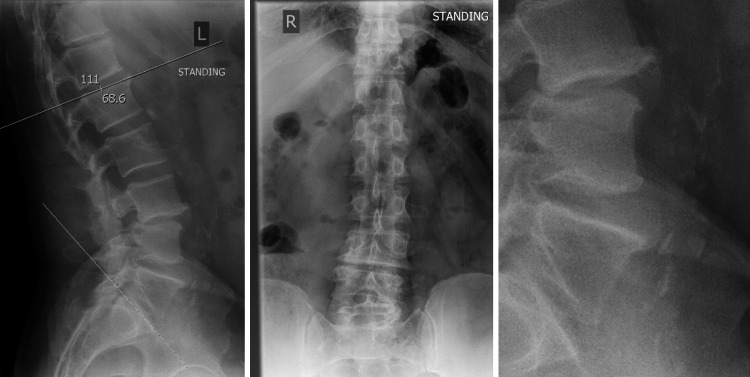
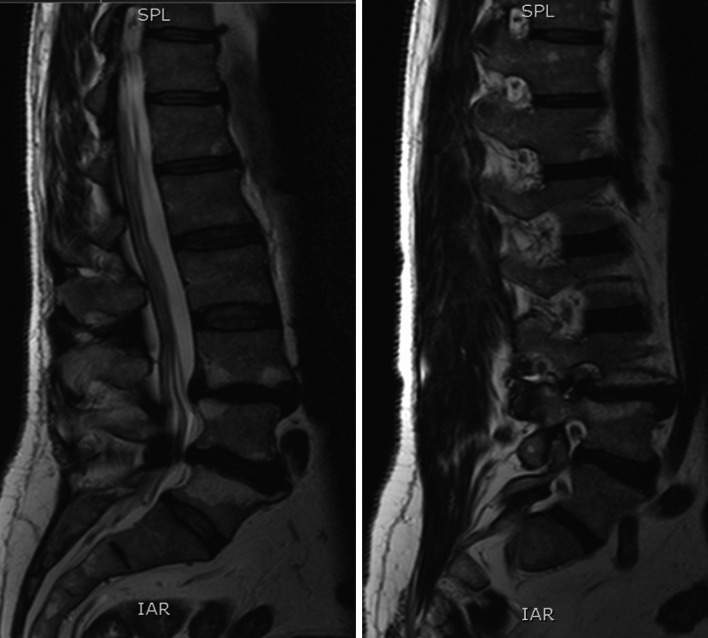
A 61-year-old man was worked up for low back pain and bilateral L5 sciatic symptoms. He had no bowel or bladder disturbance and the physical examination showed no sensory loss and full motor power. The past medical history contained an orchiectomy as a teenager but no other major abdominal surgeries. This non-diabetic had 12 pack years but quit smoking 20 years ago, drank alcohol occasionally and was slightly overweight with a BMI of 27.8. He was a sporty man, cycling a lot without any shortness of breath but his walking distance was reduced to 100 yards due to back and leg pain. No evidence of ischemic heart disease or COPD was found in the cardiac and pulmonary work up. The tibialis posterior and the dorsalis pedis pulses were palpable on both lower extremities. The pre-operative X-ray showed a degenerative lumbar spine with a grade 1 listhesis due to a pars defect at the L3/4 level and height loss in the adjacent intervertebral disc with a total lumbar lordosis of 68.6° and a slight coronal imbalance. Two small osteophytes are present anteriorly at the L4 and L5 vertebral body and light to moderate calcification of the aorta is seen but no severe plaque formation is reported (Fig. 1). The performed MRI scan revealed normal lumbar vertebral alignment and a broad based moderate posterior disc bulge indenting the theca and narrowing the lateral recess with Modic Type II endplate changes at the L3/4 level. The L4/5 level had a broad based moderate posterior disc bulge as well with no apparent compromise to the exiting or descending nerve roots. Again, Modic Type II changes are present at the endplates (Fig. 2).
Fig. 1.
Preoperative lateral and AP X-ray. Enlargement with vessel calcification and osteophytes formation on the right
Fig. 2.
Preoperative MRI scan demonstrating disc changes and encroachment of the nerve root
Diagnostic imaging section (Figs. 1, 2)
Description about the condition, incidence, epidemiology, diagnosis, pathology, differential diagnosis
The perioperative complication rate in anterior surgery ranges from 5 to 14% in total [1, 2]. Vascular injuries are however, relatively rare complications ranging from 1.9 to 18% in the literature, with the highest rates being reported in the early 1990s [3–10]. General risk factors for an increased vascular injury risk are: current or previous osteomyelitis or discogenic infection, spondylolisthesis, osteophyte formation which is deemed to be an indicator for inflammatory reaction in the vessel-surrounding area, previous anterior surgery, transitional lumbo-sacral vertebra and cage migration. Interestingly, atherosclerosis as such is not a direct contraindication for anterior surgery [11] but L4/5 intervertebral exposure seems to increase the risk of vascular injuries, putting the left common iliac artery at particular risk [6, 11, 12]. The majority of vascular complications concern venous laceration mostly present after the retraction of great vessels [6]. The incidence of arterial thrombosis is reported to be very low (0.45–0.9%). The consequences however, are dramatic and occur even in patients without any calcification on pre-operative imaging, with females appearing to be more at risk [3, 11, 13]. In 19 published cases, severe complications like rhabdomyolysis and compartment syndrome with fasciotomy are described, leading, in the worst cases, to the loss of patients if diagnosis of an embolic event is postponed [11, 13–18]. Eighteen patients had thrombosis of the left common iliac artery, three of them a second embolism more distally (2 common femoral and 1 popliteal artery thrombosis) indicating the need of a special surveillance for adequate perfusion at the distal left lower extremity to rule out peripheral occlusions. Clinical signs of acute arterial embolism like paleness, coldness or mottle of the leg may have delayed the occurence causing prolonged treatment, and are therefore insufficient in detecting arterial complications.
Vasospasm due to arterial irritation after retraction may mimic the same symptoms as an acute thrombosis but circulation is most likely re-established after observation. However, in severe cases of vasospasm, the surgical exploration of the artery is indicated [14].
Rationale for treatment and evidence based literature
Anterior interbody fusion offers better reconstruction of lordosis and less paravertebral muscle dissection for range of motion prevention compared to posterior constructs [19]. The anterior access reduces the risk of neurological complications compared to posterior access in the lumbar spine and post-operative wound problems like wound pain or infections are uncommon. The cage insertion offers indirect decompression as an alternative to a classical open posterior decompression, with simultaneous fusion as one stage surgery. Addressing the anterior column support in lumbar reconstruction seems to be advantageous compared to posterior fusion in terms of clinical outcome even if radiological parameters like lordosis do not show significant differences [20]. However, the rationale for this phenomenon is still unclear.
Because thrombosis of the left iliac artery occurs even in patients without any risk factors [3] monitoring with pulse oximetry is used in every single anterior access case as a routine precautious measurement in the institution. This offers an easy method to detect thrombosis and embolism during the surgery, offering immediate treatment possibilities like thrombectomy.
Procedure
The patient was placed supine on the table and pulse oximetry was installed on one of the left toes after pedal pulse control. Perioperative imaging indicating the right levels and skin marking are followed. After aseptic preparation and draping, a standard midline skin incision was performed with preparation to the retroperitoneum while the peritoneum itself was not injured. The peritoneal sac and the soft tissues were retracted for exposure of the great vessels which are carefully dissected with small dissection swabs from the spine at the L3/4 and L4/5 level without any obvious direct injury. The retractors were then repositioned, drawing back the vessels to expose the intervertebral discs. Pulse oximetry was lost after retraction and a stop clock was started, indicating the ischemic time. The anterior ligament, the disc itself and the protruding posterior disc parts were consecutively removed, beginning at the L3/4 level. After cartilage clearance of both endplates and measurement of a convenient template, a BMP2 packed cage was inserted in the intravertebral space and secured with four screws. This procedure was repeated at the lower level in the same manner. After 55 min, the vessels were released but pulse oximetry did not return. The palpation revealed a significant pulse decrease between the aorta, left common iliac artery and common femoral artery. The abdominal access was not closed in case of further vascular intervention and after 5 min a Doppler investigation was performed to rule out any vaso-spastic components. Doppler signals could not be found at the pedal, popliteal artery and common femoral artery indicating acute thrombosis of the left common iliac artery. His leg became cold and mottled so the decision was made to explore the vascular situation at the level of iliac bifurcation (Fig. 3). Arterial clamping followed systemic anticoagulation with 5,000 units of Heparin. After longitudinal incision, a ruptured plaque with fresh thrombotic material was visible (Fig. 4). A 5-French Fogarty catheter was inserted first in the internal iliac artery, delivering several thrombi in five applications. The procedure was repeated on the external iliac artery with removal of several thrombi which can be missed if only the exposed abdominal vessels are palpated.
Fig. 3.
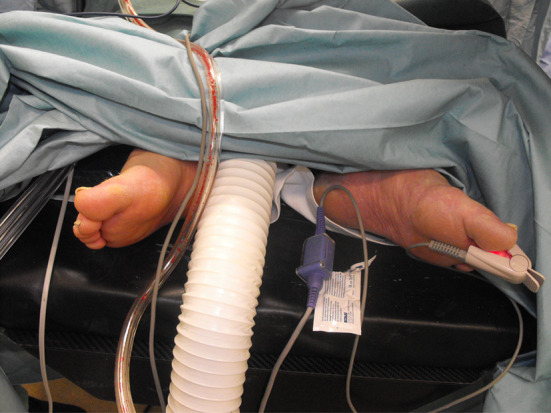
Intraoperative mottled left foot and placement of pulse oximetry
Fig. 4.
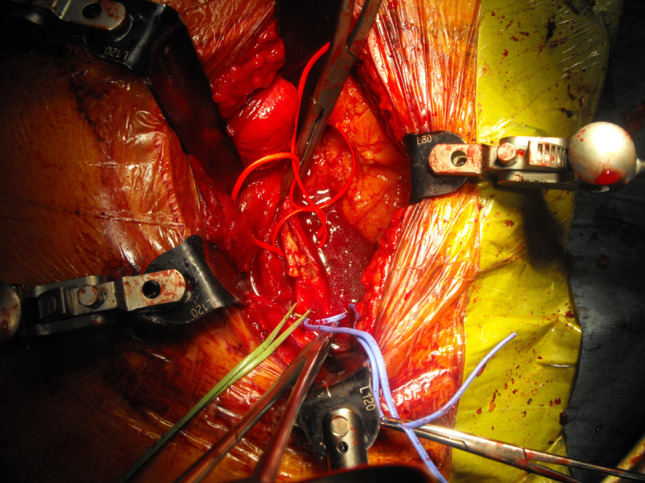
View of ruptured plaque after opening of clamped left common artery secured by additional vessel loops
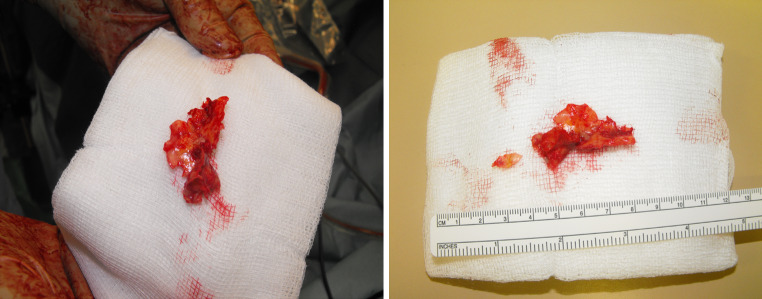
The ruptured plaque was removed via artherectomy and both iliac vessels were flushed with 10 ml heparinised saline (Fig. 5). For closure, a 5 × 5 bovine pericardial patch was fitted and sutured consecutively to the artery with 5-0 Prolene (Fig. 6). The common iliac artery was vented before opening leg circulation and after recirculation, a good pulse was palpable at the common iliac artery and pulse oximetry returned. Doppler signals could be found at the tibialis posterior as well as the dorsalis pedis artery. Surgery could be finished without further complications and pulse oximetry control showed 100% oxygen saturation. The patient was brought to post-anaesthetic recovery and pulse status was controlled with pulse oximetry and palpation.
Fig. 5.
Removed plaque
Fig. 6.
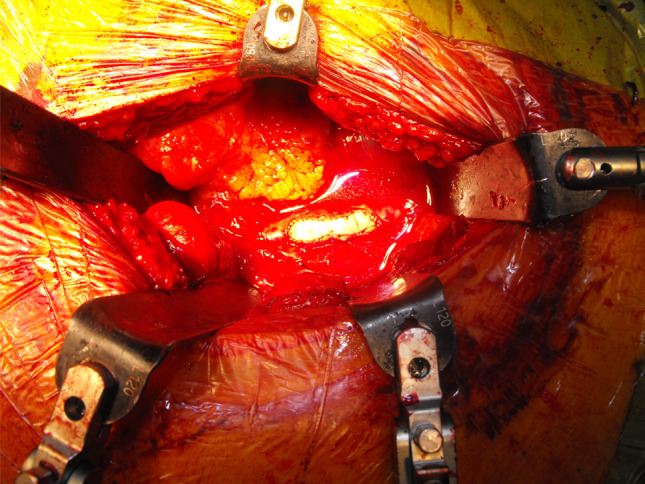
Patch closure of the left common iliac artery with bovine pericardial patch
Procedure imaging section (Figs. 3–7)
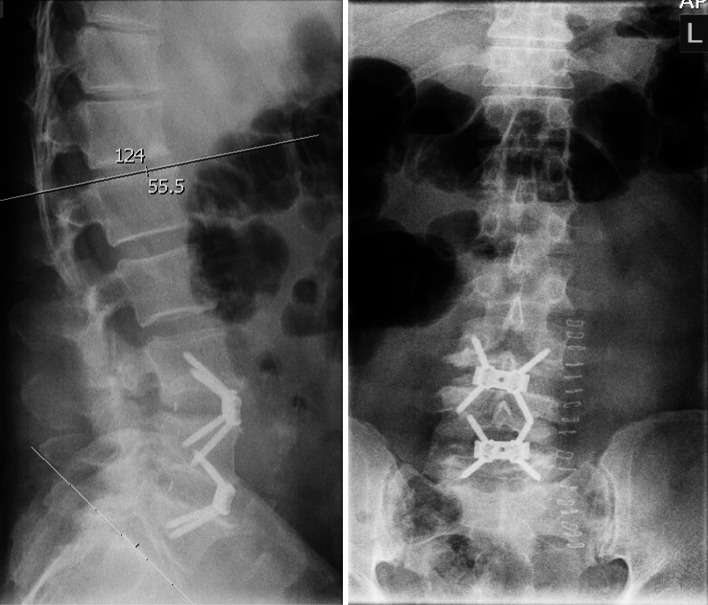
Fig. 7.
Postoperative X-ray control with restoration of lordosis
Outcome with documentation
The in-hospital stay was uneventful and he could be discharged after 10 days without the pulse deficit and sufficient perfusion of the left leg. A postoperative X-ray showed a satisfactory restoration of his total lumbar lordosis (now 55.5°) and his back and sciatic symptoms subjectively improved (Fig. 7).
Conflict of interest
No benefits or funds in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributor Information
M. A. König, Phone: +44-115-9249924, FAX: +44-115-9709991, Email: matthias.konig@nuh.nhs.uk
B. M. Boszczyk, Email: Bronek.Boszczyk@nuh.nhs.uk
References
- 1.Mayer HM. The ALIF concept. Eur Spine J. 2000;9:35–43. doi: 10.1007/PL00010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complication. Spine J. 2002;2(3):216–223. doi: 10.1016/S1529-9430(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 3.Brau SA, Delamarter RB, Schiffman ML, Williams LA, Watkins RG. Vascular injury during anterior lumbar surgery. Spine J. 2004;4(4):409–412. doi: 10.1016/j.spinee.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Garg J, Woo K, Hirsch J, Bruffey JD, Dilley RB. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51(4):946–950. doi: 10.1016/j.jvs.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP., Jr Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine. 2007;32(24):2751–2758. doi: 10.1097/BRS.0b013e31815a996e. [DOI] [PubMed] [Google Scholar]
- 6.Wood KB, Devine J, Fischer D, Dettori JR, Janssen M. Vascular injury in elective anterior lumbosacral surgery. Spine. 2010;35(9 Suppl):S66–S75. doi: 10.1097/BRS.0b013e3181d83411. [DOI] [PubMed] [Google Scholar]
- 7.Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 Suppl):60–64. doi: 10.3171/spi.1999.91.1.0060. [DOI] [PubMed] [Google Scholar]
- 8.Oskouian RJ, Jr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96(1 Suppl):1–5. doi: 10.3171/jns.2002.96.1.0001. [DOI] [PubMed] [Google Scholar]
- 9.Hamdan AD, Malek JY, Schermerhorn ML, Aulivola B, Blattman SB, Pomposelli FB., Jr Vascular injury during anterior exposure of the spine. J Vasc Surg. 2008;48(3):650–654. doi: 10.1016/j.jvs.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Bingol H, Cingoz F, Yilmaz AT, Yasar M, Tatar H. Vascular complications related to lumbar disc surgery. J Neurosurg. 2004;100(3 Suppl Spine):249–253. doi: 10.3171/spi.2004.100.3.0249. [DOI] [PubMed] [Google Scholar]
- 11.Brau SA, Delamarter RB, Schiffman ML, Williams LA, Watkins RG. Left iliac artery thrombosis during anterior lumbar surgery. Ann Vasc Surg. 2004;18(1):48–51. doi: 10.1007/s10016-003-0104-0. [DOI] [PubMed] [Google Scholar]
- 12.Brau SA, Spoonamore MJ, Snyder L, Gilbert C, Rhonda G, Williams LA, Watkins RG. Nerve monitoring changes related to iliac artery compression during anterior lumbar spine surgery. Spine J. 2003;3(5):351–355. doi: 10.1016/S1529-9430(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni SS, Lowery GL, Ross RE, Ravi Sankar K, Lykomitros V. Arterial complications following anterior lumbar interbody fusion: report of eight cases. Eur Spine J. 2003;12(1):48–54. doi: 10.1007/s00586-002-0460-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Choi KC, Jung B, Lee SH. Thrombosis of the left common iliac artery following lumber interbody fusion: Case report and review of the literature. J Korean Neurosurg Soc. 2009;45(4):249–252. doi: 10.3340/jkns.2009.45.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsicano J, Mirovsky Y, Remer S, Bloom N, Neuwirth M. Thrombotic occlusion of the left common iliac artery after an anterior retroperitoneal approach to the lumbar spine. Spine. 1994;19(3):357–359. doi: 10.1097/00007632-199402000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Raskas DS, Delamarter RB. Occlusion of the left iliac artery after retroperitoneal exposure of the spine. Clin Orthop Relat Res. 1997;338:86–89. doi: 10.1097/00003086-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Hackenberg L, Liljenqvist U, Halm H, Winkelmann W. Occlusion of the left common iliac artery and consecutive thromboembolism of the left popliteal artery following anterior lumbar interbody fusion. J Spinal Disord. 2001;14(4):365–368. doi: 10.1097/00002517-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Khazim R, Boos N, Webb JK. Progressive thrombotic occlusion of the left common iliac artery after anterior lumbar interbody fusion. Eur Spine J. 1998;7(3):239–241. doi: 10.1007/s005860050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappuccino A, Cornwall GB, Turner AW, Fogel GR, Duong HT, Kim KD, Brodke DS. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine. 2010;35(26 Suppl):S361–S367. doi: 10.1097/BRS.0b013e318202308b. [DOI] [PubMed] [Google Scholar]
- 20.Videbaek TS, Bünger CE, Henriksen M, Neils E, Christensen FB. Sagittal spinal balance after lumbar spinal fusion: the impact of anterior column support results from a randomized clinical trial with an 8–13-year radiographic follow-up. Spine. 2011;36(3):183–191. doi: 10.1097/BRS.0b013e3181cc8fce. [DOI] [PubMed] [Google Scholar]