Abstract
Purpose
The purpose of the study was to develop a surgical classification system for primary malignant sacral tumors.
Methods
The sacrum is divided into three regions 1, 2 and 3 by the S1–S2 and S2–S3 junctions. En bloc resections were classified into five types: type I involves regions 1, or 1 and 2, or regions 1, 2 and 3, type II involves regions 2 and 3, and type III involves only region 3. Type IV includes sagittal hemisacrectomy and resection of a portion of the adjacent ilium. Type V includes the sacrum and the fifth lumbar vertebra. 117 patient cases (68 females and 49 males) were reviewed.
Results
There were two perioperative deaths. Of the 35 patients who should have undergone type I resection, local recurrence (LR) occurred in four of the 14 patients who underwent type I resection with free margins without tumor rupture. The other 21 patients underwent piecemeal resection, and LR occurred in 15 (P = 0.013). 35 patients underwent type II resection. Free margin without tumor rupture was accomplished in 26 and LR occurred in 6. Tumor rupture (TR) occurred in the other 9 and LR occurred in seven (Yates’ P = 0.012). All 33 patients underwent type III resection with free margins without tumor rupture. LR occurred in five. 11 patients had type IV resection. Free margin without tumor rupture was accomplished in seven and LR occurred in three. TR occurred in the other four, and LR occurred in two (Yates’ P = 0.689). One patient underwent type V resection with free margin without tumor rupture and LR occurred. Postoperatively, less than 1/3 needed long-term urethral catheterization. No patients received colostomy for postoperative fecal incontinence. All the patients were able to ambulate.
Conclusion
Our classification system and the corresponding surgical approaches are helpful in dealing with primary malignant sacral tumors. Better oncologic results could be expected if free margin without tumor rupture was accomplished.
Keywords: Sacral tumor, Classification, Surgical approach, En bloc resection
Introduction
Sacral tumors are rare. Among primary sacral tumors, giant-cell tumors are more frequently seen, second only to chordomas, and high-grade malignancies are even more uncommon. Among the secondary sacral tumors, metastatic ones account for most of the cases, and are seen more often than multiple myelomas [13]. Malignant tumors involving the sacrum are so uncommon that it is difficult to accumulate a large series from one institution for the purpose of building a classification system. Sacrectomy with adequate margins is challenging because of the complexity of the surgical approach and morbidities, and the local recurrence rate is fairly high [1, 2, 8, 11, 19].
To improve the surgical strategy for primary malignant sacral tumors, we retrospectively reviewed those cases with primary malignant sacral tumors in our center. The aim was to build up a surgical classification system for primary malignant sacral tumors, based mainly on the extent of sacral involvement. Different types of en bloc resection and complications are described accordingly.
Materials and methods
Surgical classification of different types of en bloc resection for primary malignant sacral tumors
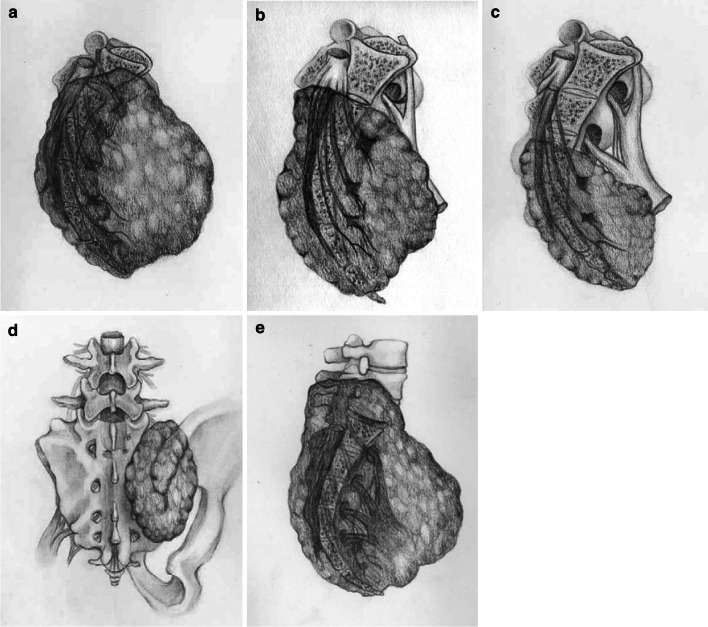
The sacrum is divided into three regions of upper sacrum (1), middle sacrum (2), and lower sacrum (3) by S1–S2 and S2–S3 junctions. Based on the tumor extension, en bloc resections of the primary sacral tumors are classified into five types, as illustrated in Fig. 1. Type I involves regions 1, or 1 and 2, or regions 1, 2 and 3, type II involves regions 2 and 3, and type III involves only region 3 [4]. Type IV is mainly sagittal hemisacrectomy. Usually, a portion of the adjacent ilium has to be resected en bloc with the tumor mass. When the fifth lumbar vertebra is involved in a sacral tumor and has to be resected, it is type V.
Fig. 1.
Surgical classification of different types of en bloc resection for primary malignant sacral tumors. a Type I resection, including regions 1 or 1 and 2, or regions 1, 2, and 3. b Type II resection, including regions 2 and 3. c Type III resection, including only region 3. d Type IV includes sagittal hemisacrectomy, and for en bloc resection of the tumor, a portion of the adjacent ilium being resected if it was affected by the tumor. e Type V resection. When a huge sacral tumors invades the fifth lumbar vertebra, the latter should be resected with the tumor
Surgical procedures designed for the different types of en bloc resection
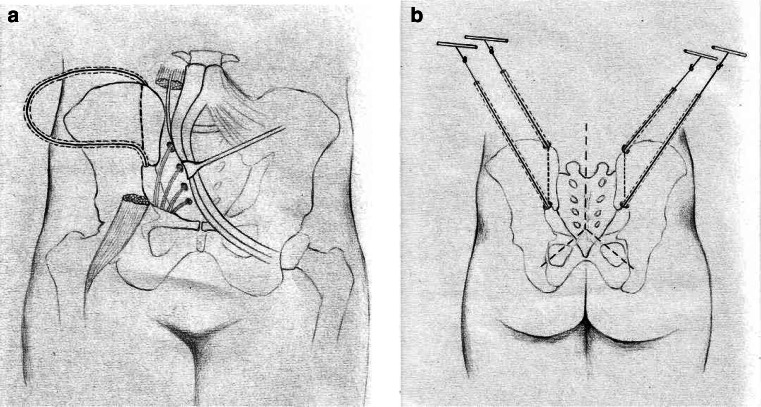
Type I (total sacrectomy) synchronously utilizes an abdominal approach and a posterior approach. The patient is placed in the supine position. The abdominal approach utilizes bilateral incisions that start in the lower half of the area between the lowest rib and the superior iliac crest in the anterior axillary line and extend approximately to the edge of the rectus sheath, just above the symphysis. The retroperitoneal fat is identified and the extraperitoneal space is entered laterally. The psoas muscles and the L4–L5 disc are identified. The ureters and lumbosacral trunks are protected. The iliolumbar vessels, internal iliac arteries, and central sacral vessels are ligated. The internal iliac veins from the tumor are mobilized. The anterior aspect of the sacrum is exposed by sharp dissection and all the vessels that supply the tumor are ligated and transected. Occasionally, temporarily blocking the blood flow with an intra-aortic balloon is helpful for the dissection. The L5–S1 disc is resected. The sacroiliac joint and the wing of the ilium are exposed bilaterally and the oncologically safe plane of dissection in the ilium is confirmed (when the ilium is not affected by the tumor, the dissection plane may pass through the sacroiliac joint). Along the dissection plane, the upper pelvic brim and the intrapelvic portion of the sciatic notch are exposed. To introduce two Gigli saws to the front of the ilia from the posterior approach, two 18# silicon drainage tubes with a transfixion pin at each end are utilized in the anterior procedure. Along the dissection plane, from the inner side of the upper pelvic brim and the sciatic notch, respectively, all layers of the soft tissues, including the skin, are penetrated by the pins. Then the two ends of both tubes are pulled out of the lumbosacral skin. The pins are removed, leaving the tube in place, as shown in Fig. 2. The anterior wound is closed. The patient is turned to the prone position. The posterior approach starts with a reversed Y-shaped incision on the lower back. The sacrotuberous, sacrospinous, and anal coccygeal ligaments are exposed and cut. The rectum is dissected from the front of the sacrum and gauze is packed into the presacral space to protect the rectum. After the L5 laminectomy, bilateral L5 nerves are identified and protected. The dural sac at L5–S1 is then ligated and cut. Around the dissection plane, the posterior aspect of the ilia is exposed. Two Gigli saws are introduced bilaterally through the silicon tubes, the tubes are removed, and osteotomy of the ilia around the sacroiliac joint is completed with the Gigli saws. The total sacrum is resected en bloc by elevating the sacrum from the distal to the proximal end after cutting the sacral nerves.
Fig. 2.
In order to introduce two Gigli saws to the front of the ilia from the posterior approach, two 18# silicon drainage tubes with a transfixion pin at each end are utilized in the anterior procedure. Along the dissection plane, from the inner side of the upper pelvic brim and the sciatic notch, respectively, all layers of the soft tissues, including the skin, are penetrated by the pins. Then the two ends of both tubes are pulled out of the lumbosacral skin. The pins are removed, leaving the tube in place (a). Two Gigli saws are introduced bilaterally through the silicon tubes, the tubes are removed, and osteotomy of the ilia around the sacroiliac joint is completed with the Gigli saws (b)
Type II uses only a posterior approach. The incision, dissection of the ligaments attached to the sacrum, and mobilization of the rectum off the sacrum are similar to the procedure used for type I. The sacral canal is then exposed at and above the S1 level, and bilateral S1 nerve roots are protected if possible. The dural sac is subsequently cut off and ligated caudally to the S1 nerve roots. The body of the sacrum at the S1 level and the bilateral ilia are cut by milling bur and the specimen can be removed en bloc.
Type III uses similar procedures to type II, except that the bilateral ilia do not need to be resected; the sacrum is cut at the S2 level and the S2 nerve root can be saved in most circumstances.
Type IV is performed in a lateral decubitus position. Ilioinguinal incision is adopted and elongated posteriorly along the iliac crest to the midline of the back. By a retroperitoneal approach, the ureter and the rectum are mobilized and protected. The ipsilateral internal iliac artery and the middle sacral vessels are ligated. The anterior aspect of the tumor is dissected from the noninvolved anterior and lateral tissues. All the ipsilateral sacral nerve roots are ligated and cut. The anterior bony surface of the sacrum is exposed along the midline and the anterior aspect of the ilium is exposed along the planned cut line. The ipsilateral half of the L5–S1 disc is removed. A posterior vertical midline incision is used to expose the posterior aspect of the sacrum and the adjacent ilium. The ipsilateral muscles and ligaments attached to the sacrum are dissected and all the contralateral nerve roots that can be preserved after laminectomy are isolated. The ipsilateral sacral nerve roots are ligated and cut off. The sacrum is cut along the midline and the ilium is cut along the planned safe dissection plane by milling burr or Gigli saw and the specimen can be removed en bloc.
Type V uses the same procedures as type I. Besides the resection extent of type I, the resection extension of this type also includes the fifth lumbar vertebra.
Patient population
Between July 2001 and June 2010, 117 patients (68 females and 49 males) with primary malignant sacral tumors were treated at the Musculoskeletal Tumor Center, People’s Hospital, Peking University. The mean patient age was 49.1 years (range 11–75 years). We excluded those patients who had previously undergone an operation. Besides a history and neurological examination, the preoperative evaluation included plain radiography, nuclear magnetic resonance imaging, and computerized tomography scanning. Postoperative ambulatory status and the sphincter function were evaluated at each follow-up.
The histological composition of the tumors included chordoma in 76 patients, chondrosarcoma in 12, Ewing’s sarcoma in 8, osteosarcoma in 6, malignant peripheral nerve sheath tumor in 6, malignancy in giant cell tumor in 3, malignant fibrous histocytoma in 3, angiosarcoma in 2, and liposarcoma in 1.
According to our surgical classification system for en bloc resection of sacral tumors, there should have been 36 type I, 36 type II, 33 type III, 11 type IV, and 1 type V en bloc resections. Detailed histological composition of each type is listed in Table 1.
Table 1.
The detailed histological composition of each surgical type
| Resection types/histological diagnoses | I | II | III | IV | V | Total |
|---|---|---|---|---|---|---|
| Chordoma | 7 | 36 | 33 | – | – | 76 |
| Chondrosarcoma | 8 | – | – | 4 | – | 12 |
| Ewing’s sarcoma | 4 | – | – | 3 | 1 | 8 |
| Osteosarcoma | 5 | – | – | 1 | – | 6 |
| Malignant peripheral nerve sheath tumor | 3 | – | – | 3 | – | 6 |
| Malignancy in giant cell tumor | 3 | – | – | – | – | 3 |
| Malignant fibrous histocytoma | 3 | – | – | – | – | 3 |
| Angiosarcoma | 2 | – | – | – | – | 2 |
| Liposarcoma | 1 | – | – | – | – | 1 |
| Total | 36 | 36 | 33 | 11 | 1 | 117 |
Before the year 2007, due to technique reasons, 21 patients who should have undergone type I en bloc resection had received piecemeal resection by posterior approach. En bloc resection was done in all the other patients.
For patients with high-grade sarcomas, chemotherapy and radiotherapy would be given as their counterparts located in limbs.
The chi-square test was used to detect group differences. Yates’ correction was employed when expected frequencies were less than 5. A P value <0.05 was considered to be significant.
Results
Operative time and intraoperative blood loss
For the 15 patients who received type I resection, the average operative time was 9.8 h (range 7.1–13.1 h) and the average intraoperative blood loss was 4,200 ml (range 2,000–6,100 ml). For the other 21 patients who underwent piecemeal resection, the two parameters were 4.63 h (range 2.1–7.7 h) and 5,150 ml (2,100–11,000 ml), respectively.
The average operative time and intraoperative blood loss for type II resection (36 patients) was 4.3 h (range 2.1–5.0) and 3,050 ml (range 450–7,000 ml), respectively. For type III resections (33 patients), the two parameters were 3.1 h (range 1.7–5.2 h) and 2,300 ml (300–4,700 ml), respectively. For type IV resections (11 patients), the two parameters were 8.1 h (5.6–11.5 h) and 3,700 ml (1,700–5,500 ml), respectively. One patient received type V resection, which took 13 h and had an intraoperative blood loss of 1,900 ml.
Complications
There were two perioperative deaths in this series. In 2002, one patient with chordoma who underwent type II resection died of multisystem and organ failure caused by hemorrhage shock on the second day after the operation. In 2003, a patient with chondrosarcoma who underwent type I resection died of pulmonary embolism the very night of the operation.
One patient sustained significant intraoperative hemorrhage during tumor resection that mandated temporary packing and reexploration. Three patients in the series had injury to the rectum during the dissection and colostomy was done simultaneously. No postoperative ileus was found in this study.
Thirty-one patients had wound problems. Of these, three (21.4%) patients had type I resections, six (28.6%) should have had type I resections, but had piecemeal resections, 11(31.4%) had type II resections, eight (24.2%) had type III resections, and three (27.3%) had type IV resections. These wound problems were settled successfully by debridement, drainage, and systemic use of antibiotics.
Resection margin and oncologic results
The mean follow-up time for the 115 surviving patients was 41 months (6–103 months). At the last follow-up, 11 patients had died of metastasis. Of the 35 patients who should have undergone type I resection, en bloc resection with free margin without tumor rupture was accomplished in 14 and local recurrence occurred in four patients (28.6%). The other 21 patients underwent piecemeal resection by posterior approach, and local recurrence occurred in 15 (71.4%) of them. The difference between these two groups was statistically significant (P = 0.013).
Of the 35 patients who underwent type II resection, free resection margin without tumor rupture was done in 26 patients and local recurrence occurred in six (23.1%) patients. Of the other nine patients, tumor rupture occurred during the operation and local recurrence occurred in seven (77.8%). The difference between these two groups was statistically significant (Yates’ P = 0.012). All 33 patients who underwent type III resections had a free margin without tumor rupture, and local recurrence occurred in five (15.2%) patients. Of the 11 patients who had type IV resection, free resection margin without tumor rupture was accomplished in seven patients and local recurrence occurred in three (42.9%) patients. Tumor rupture occurred in the other four patients, and local recurrence occurred in two patients (50.0%). The difference between these two groups was not statistically significant (Yates’ P = 0.689). The only patient who underwent type V resection had a free resection margin without tumor rupture. However, local recurrence occurred in this case.
Functional results
No or only slight sphincter function loss was observed in 17 patients who underwent type III resection, and unilateral or bilateral S3 nerve roots were preserved during the operation. These patients had no fecal incontinence and the urethral catheter was removed successfully within 3 weeks of surgery. All the other patients sustained significant sphincter dysfunction that required urinary catheterization and enema for regular bowel evacuation. However, only 38 patients still required urethral catheterization 6 months after the operation. All the other patients could urinate through increasing their intra-abdominal pressure. In this study, except for the three patients who underwent colostomy in the one-stage operation, no patients received colostomy for postoperative fecal incontinence.
All the patients were able to ambulate postoperatively. At follow-up 3 months after the operation, all could walk without the use of a walking aid, except for one who had a type V resection in which the bilateral L5 nerve roots were sacrificed. He lost ankle dorsal expansion and plantar flexion of both feet and was eventually able to walk with crutches and the use of a pair of ankle–foot orthoses. Unilateral loss or weakness of plantar flexion occurred in 11 patients who underwent type I resection, 13 who underwent type II resection, and all 11 patients who underwent type IV resection. Bilateral loss or weakness of plantar flexion occurred in 17 patients who underwent type I resection and 7 who underwent type II resection.
The data on postoperative sexual ability were incomplete. Most male patients lost penis erection ability.
Discussion
With the exception of Ewing’s sarcoma, the majority of primary malignant sacral tumors are insensitive to radiotherapy and chemotherapy, and en bloc sacrectomy may provide a chance to cure for primary malignant sacral tumors [3, 6, 7, 9, 10, 12, 15–18, 22–27]. However, to resect a primary malignant sacral tumor with adequate margins is extraordinarily challenging [23]. The anterior aspect of the sacrum is covered with enormous blood vessels, which means even a normal sacrum is like a huge “blood sinusoid”. When a tumor is malignant, there are abnormal vessels around the pseudocapsule. Keeping the intraoperative hemorrhage under control is a prerequisite for a successful operation [21]. In the past 10 years, we have successfully developed the technique of aortic balloon occlusion to decrease blood loss during sacral tumor resection. This technique can be safely and easily used together with selective tumor vessel embolism and internal iliac artery ligation. In the past 7 years, there have been no perioperative deaths due to uncontrollable hemorrhage shock [20]. This technique has made en bloc resection of sacral tumors with a safe margin possible.
For most patients with a primary malignant sacral tumor, piecemeal resection would inevitably lead to local recurrence and extensive operating field contamination. This could be fatal, because the chance of these patients receiving an adequate resection margin in the next operation is slim. It is very important to standardize the surgical procedures. Primary malignant sacral tumors are so rare that it took around a decade for one center to accumulate 117 cases. Based on these cases, we proposed a classification system for single-stage en bloc resection of primary malignant sacral tumors according to the extent of the sacrum that should be resected. We classified type I, II, and III en bloc sacral resection based primarily on S1–S2 and S2–S3 junctions. These anatomic landmarks can be easily indentified on preoperative images and in operations. An en bloc sacral resection classification system, which was based on the level of nerve root sacrifice, was proposed in 2005 [9]. We did not use the level of nerve root sacrifice as anatomic landmarks in our classification system, because the nerve roots may adhere to and be displaced by the presacral soft tissue mass of the tumor, and could be sacrificed at various anatomic points. A lateral group of sacral resections for eccentric tumors is classified as type IV in our system. It is not further classified into the low sacral or high sacral groups, because all the low sacral tumors in ours series were chordomas, which are always centrically located. As the Hospital Committee on Ethics does not permit hemicorporectomy, we have no such cases, and have not included it in our classification. In our experience, type V resection in our classification system has the widest resection extension.
For type I, IV, and V resections, a combined anterior and posterior approach is obligatory. Although it was reported that total sacrectomy could be performed simply through a posterior approach, we think this risks dilacerations of the iliac vessels, especially the veins, which are attached to the anterior aspect of L5 and S1 vertebra [14]. Dilacerating iliac vessels can cause uncontrollable hemorrhage and repairing or ligating these vessels from a posterior approach is difficult and sometimes damages the external iliac vessels. The technique of introducing a pair of Gigli saws by the posterior approach through silicon tubes placed in advance by the anterior approach is unique. This allows accurate cuts in the ilia adjacent to the sacroiliac joints and protects the pelvic viscera, nerves, and vessels at the same time.
In other studies, postoperative bowel ileus is a common complication when anterior transabdominal approaches are used [9]. We exposed the retroperitoneal and presacral space extraperitoneally and accordingly, no bowel ileus was observed in our series. We did not use the large semicircular incision proposed by Bertil Stener [19], because we believe our anterior incisions can expose the operation field well during surgery.
In this study, we tried not to use the term “wide resection”, because it is very difficult to define the pseudocapsule of most primary malignant sacral tumors [4]. These tumors usually extend into the presacral space. Although the peritoneum and presacral fascia are considered to act as a natural barrier, the resection margin cannot be wide if the rectum is reserved. Further, the sacral canal would be involved in many cases, and this would also make a wide margin impossible. Based on these specific anatomical characteristics of primary malignant sacral tumors, we prefer to classify the resection margins into three types, en bloc resection with free margin without tumor rupture, en bloc resection with tumor rupture, and piecemeal resection.
The findings of other studies concur that en bloc resection with free margin without tumor rupture has the lowest local recurrence rate [2, 5, 17, 27]. For type I resections with free margin without tumor rupture, the local recurrence rate was 28.6%, while for those who underwent piecemeal resection, it was 71.4% (P = 0.013). For type II resection with free resection margin without tumor rupture, the local recurrence rate was 23.1%, while for those whose tumor ruptured, it was 77.8% (P = 0.012). All type III resections had a free margin without tumor rupture, and the local recurrence rate was only 15.2%. Due to the limited number of cases, the difference in local recurrence between the groups of type IV resection was not statistically significant.
As rectal fascia and sacral periosteum are barriers to tumor invasion and infiltration, the rectal wall is seldom involved. However, the presacral soft tissue can be extensive, and can displace all the vital pelvic structures and cause extensive adhesions. It can sometimes invade the surrounding soft tissue structures such as the lumbosacral canal, and the gluteus maximus, sacrospinalis, and especially, piriformis muscles. The postoperative local recurrence rate could therefore still be quite high even with en bloc resection. We agree that infiltration of the musculature adjacent to the sacrum and/or involvement of the sacroiliac joints increase the local recurrence rate and parts of the infiltrated posterior pelvic musculature and sacroiliac joints should be resected en bloc with the tumor. [12]. So, MR images should be carefully evaluated to ascertain the extent of the disease and plan surgery preoperatively. However, infiltration of these adjacent musculatures and/or sacroiliac joints was not included in our classification system, because the latter is focused on en bloc resection for primary sacral tumor and the corresponding surgical approaches. It does not seem to influence the approach chosen preoperatively.
For anatomic reasons, postoperative impairment of bladder, bowel, sexual, and ambulatory functions is inevitable. However, the problems were not as severe as expected. All the patients in this study had postoperative ambulatory ability. Only the patient who underwent type V resection needed crutches and ankle–foot orthoses. No or only slight sphincter function loss was observed in patients whose unilateral or bilateral S3 nerve roots were preserved. For those patients who had significant sphincter dysfunction, less than 40% patents required urethral catheterization 6 months after the operation and none of the patients needed colostomy for postoperative fecal incontinence. When we compare all these complications to the local recurrence of the tumor, we believe sacrificing the nerve roots to acquire en bloc resection with a free margin is worthwhile.
As the local recurrence of the tumor is mainly associated with the surgical method, and the metastasis of the tumor is mainly associated with the tumor’s intrinsic biological behavior, we focused our study on the correlation between the surgical classification and surgical approaches to properly resect the lesions, rather than involving other concerns such as tumor metastasis or survival rate. The reconstruction of bony defects and wound complications are not the focus of the article, but may be discussed in other articles.
Conflict of interest
The authors made no disclosures.
References
- 1.Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–1212. doi: 10.1002/1097-0142(20010401)91:7<1201::AID-CNCR1120>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. doi: 10.1002/(SICI)1097-0142(20000501)88:9<2122::AID-CNCR19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Berven S, Zurakowski D, Mankin HJ, Gebhardt MC, Springfield DS, Hornicek FJ. Clinical outcome in chordoma: utility of flow cytometry in DNA determination. Spine (Phila Pa 1976) 2002;27(4):374–379. doi: 10.1097/00007632-200202150-00010. [DOI] [PubMed] [Google Scholar]
- 4.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 5.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine. 1999;24:1639–1645. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Doita M, Harada T, Iguchi T, Sumi M, Sha H, Yoshiya S, Kurosaka M. Total sacrectomy and reconstruction for sacral tumors. Spine. 2003;28:296–301. doi: 10.1097/01.BRS.0000083230.12704.E3. [DOI] [PubMed] [Google Scholar]
- 7.Evans RG, Nesbit ME, Gehan EA, Garnsey LA, Burgert O, Jr, Vietti TJ, Cangir A, Tefft M, Thomas P, Askin FB, et al. Multimodal therapy for the management of localized Ewing’s sarcoma of pelvic and sacral bones: a report from the second intergroup study. J Clin Oncol. 1991;9:1173–1180. doi: 10.1200/JCO.1991.9.7.1173. [DOI] [PubMed] [Google Scholar]
- 8.Fourney DR, Gokaslan ZL. Current management of sacral chordoma. Neurosurg Focus. 2003;15(2):E9. doi: 10.3171/foc.2003.15.2.9. [DOI] [PubMed] [Google Scholar]
- 9.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, Suki D, Gallia GL, Garonzik I, Gokaslan ZL. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 10.Gokaslan ZL, Romsdahl MM, Kroll SS, Walsh GL, Gillis TA, Wildrick DM, Leavens ME. Total sacrectomy and Galveston L-rod reconstruction for malignant neoplasms. J Neurosurg. 1997;87:781–787. doi: 10.3171/jns.1997.87.5.0781. [DOI] [PubMed] [Google Scholar]
- 11.Huth JF, Dawson EG, Eilber FR. Abdominosacral resection for malignant tumors of the sacrum. Am J Surg. 1984;148:157–161. doi: 10.1016/0002-9610(84)90304-0. [DOI] [PubMed] [Google Scholar]
- 12.Ishii K, Chiba K, Watanabe M, Yabe H, Fujimura Y, Toyama Y. Local recurrence after S2–3 sacrectomy in sacral chordoma. Report of four cases. J Neurosurg. 2002;97(1 Suppl):98–101. doi: 10.3171/spi.2002.97.1.0098. [DOI] [PubMed] [Google Scholar]
- 13.Simon MA, Springfield D. Surgery for bone and soft-tissue tumors. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 444–450. [Google Scholar]
- 14.McLoughlin GS, Sciubba DM, Suk I, Witham T, Bydon A, Gokaslan ZL, Wolinsky JP. En bloc total sacrectomy performed in a single stage through a posterior approach. Neurosurgery. 2008;63(1 Suppl 1):ONS115–ONS120. doi: 10.1227/01.NEU.0000312354.43020.03. [DOI] [PubMed] [Google Scholar]
- 15.Ozdemir MH, Gürkan I, Yildiz Y, Yilmaz C, Saglik Y. Surgical treatment of malignant tumors of the sacrum. Eur Surg Oncol. 1999;25:44–49. doi: 10.1053/ejso.1998.0598. [DOI] [PubMed] [Google Scholar]
- 16.Randall RL, Bruckner J, Lloyd C, Pohlman TH, Conrad EU., 3rd Sacral resection and reconstruction for tumors and tumor-like conditions. Orthopedics. 2005;28:307–313. doi: 10.3928/0147-7447-20050301-17. [DOI] [PubMed] [Google Scholar]
- 17.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–1484. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Shikata J, Yamamuro T, Kotoura Y, Mikawa Y, Iida H, Maetani S. Total sacrectomy and reconstruction for primary tumors. Report of two cases. J Bone Joint Surg Am. 1998;70:122–125. [PubMed] [Google Scholar]
- 19.Stener B, Gunterberg B. High amputation of the sacrum for extirpation of tumors. Principles and technique. Spine. 1978;3:351–366. doi: 10.1097/00007632-197812000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Tang X, Guo W, Yang R, Tang S, Dong S. Use of aortic balloon occlusion to decrease blood loss during sacral tumor resection. J Bone Joint Surg Am. 2010;92(8):1747–1753. doi: 10.2106/JBJS.I.01333. [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Guo W, Yang R, Tang S, Ji T. Risk factors for blood loss during sacral tumor resection. Clin Orthop Relat Res. 2009;467(6):1599–1604. doi: 10.1007/s11999-008-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomita K, Tsuchiya H. Total sacrectomy and reconstruction for huge sacral tumors. Spine. 1990;15:1223–1227. doi: 10.1097/00007632-199011010-00024. [DOI] [PubMed] [Google Scholar]
- 23.Touran T, Frost DB, O’Connell TX. Sacral resection operative technique and outcome. Arch Surg. 1990;125:911–913. doi: 10.1001/archsurg.1990.01410190109017. [DOI] [PubMed] [Google Scholar]
- 24.Wuisman P, Lieshout O, Sugihara S, van Dijk M. Total sacrectomy and reconstruction: oncologic and functional outcome. Clin Orthop Relat Res. 2000;381:192–203. doi: 10.1097/00003086-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Yang RS, Eckardt JJ, Eilber FR, Rosen G, Forscher CA, Dorey FJ, Kelly CM, al-Shaikh R. Surgical indications for Ewing’s sarcoma of the pelvis. Cancer. 1995;76:1388–1397. doi: 10.1002/1097-0142(19951015)76:8<1388::AID-CNCR2820760814>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85(4):878–883. doi: 10.1002/(SICI)1097-0142(19990215)85:4<878::AID-CNCR15>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, Gokaslan ZL. Sacral chordoma: 40 year experience at a major cancer center. Neurosurgery. 1999;44:74–8027. doi: 10.1097/00006123-199901000-00041. [DOI] [PubMed] [Google Scholar]