Abstract
Anxiety disorders are common, serious and a growing health problem worldwide. However, the causative factors, aetiology and underlying mechanisms of anxiety disorders, as for most psychiatric disorders, remain relatively poorly understood. Animal models are an important aid in giving insight into the aetiology, neurobiology and, ultimately, the therapy of human anxiety disorders. The approach, however, is challenged with a number of complexities. In particular, the heterogeneous nature of anxiety disorders in humans coupled with the associated multifaceted and descriptive diagnostic criteria, creates challenges in both animal modelling and in clinical research. In this paper, we describe some of the more widely used approaches for assessing the anxiolytic activity of known and potential therapeutic agents. These include ethological, conflict-based, hyponeophagia, vocalization-based, physiological and cognitive-based paradigms. Developments in the characterization of translational models are also summarized, as are the challenges facing researchers in their drug discovery efforts in developing new anxiolytic drugs, not least the ever-shifting clinical conceptualization of anxiety disorders. In conclusion, to date, although animal models of anxiety have relatively good validity, anxiolytic drugs with novel mechanisms have been slow to emerge. It is clear that a better alignment of the interactions between basic and clinical scientists is needed if this is to change.
LINKED ARTICLES
This article is part of a themed issue on Translational Neuropharmacology. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.164.issue-4
Keywords: animal models, anxiety, animal tests, predictive validity, preclinical, anxiolytic drugs, novel anxiolytics, elevated plus maze, light–dark box, approach avoidance test
Introduction
‘Now is the age of anxiety’.
WH Auden
Despite the passage of more than 60 years since the publication of Auden's Pulitzer Prize-winning text, it can be argued that both at a global and local level that it is the first part of the 21st century that represents the age of anxiety (Auden, 1947). Anxiety disorders are currently the most prevalent psychiatric diseases in Europe and in the USA, and as such represent a grave and ever-increasing strain on healthcare resources (Kessler et al., 2005b; Alonso and Lépine, 2007; Kessler, 2007; Nutt et al., 2007). Separate large-scale epidemiological studies in both Europe (European Study of the Epidemiology of Mental Disorders) (Alonso et al., 2004) and the USA (National Comorbidity Survey – Replication) (Kessler and Merikangas, 2004) have demonstrated that anxiety disorders have the highest lifetime prevalence estimates (13.6–28.8%) and the earliest age of onset (11 years) of psychiatric disorders (Kessler et al., 2005a,b; Kessler, 2007). Patients suffering from anxiety disorders also frequently present with other comorbid diseases, including not only psychiatric disorders such as depression (Merikangas, 2003; Kessler et al., 2005b), but also medical conditions including functional gastrointestinal disease, asthma, cardiovascular disease, cancer and chronic pain, hypertension and migraine (Härter et al., 2003; Roy-Byrne et al., 2008). As such, anxiety disorders represent a huge burden in terms of both their social impact and their economic cost (Kessler, 2007; Nutt et al., 2007). Our understanding of the pathological processes, aetiology and causative factors underlying anxiety disorders is still unfortunately in its infancy and must be developed if we are to diagnose and treat anxiety disorders more effectively (Wong and Licinio, 2004; Cryan and Holmes, 2005).
In parallel to this, there is a growing realization that the cost of phase II and phase III clinical trials in pharmaceutical drug development is enormous and growing annually (DiMasi et al., 2003), with the cost of central nervous system drug development being higher than that of any other major therapeutic area (Frantz, 2004). Furthermore, clinical trials in psychiatry are burdened, as in many medical disease trials, with very high rates of placebo response (Lakoff, 2002). As a result, before embarking on costly trials, pharmaceutical companies and research-funding agencies increasingly seek assurance that any specific biological target is indeed relevant to the disease (Gomez-Mancilla et al., 2005). Accordingly, there is a growing emphasis on first obtaining proof that a new chemical entity designed to alter the function of a specific target will do so in a predictable and safe manner. Central to this approach, as with all diseases, is the availability of valid preclinical animal models for evaluating the potential utility of novel pharmacotherapeutics. However, as a field, psychiatry has proven to be among the least penetrable clinical disciplines for productively marrying knowledge of human pathology with animal behaviour to develop satisfactory in vivo animal models for evaluating novel treatment approaches. In this review, we highlight the contribution of animal models to the current and future development of anxiolytic drugs.
Anxiety disorders
The anxiety response is an important mechanism by which we adapt and respond to real dangers. Dysregulation of this healthy response resulting in ‘marked, persistent, and excessive or unreasonable fear’ (American Psychiatric Association, 2000), culminating in a significant interference in normal life can be described as an anxiety disorder. From a clinical perspective, anxiety disorders are described by Diagnostic and Statistical Manual IV in terms of subtypes distinguished by the nature of the anxiety-provoking stimulus. Most common among these anxiety disorder subtypes are generalized anxiety disorder (GAD), panic disorder (diagnosed with or without agoraphobia), specific phobia, social phobia, obsessive–compulsive disorder and post-traumatic stress disorder (PTSD). It should be noted that the Diagnostic and Statistical Manual V, due for publication in May 2013, proposes to expand and modify classification, as well as reclassify obsessive–compulsive disorder in a different diagnostic category (Holden, 2010; Miller and Holden, 2010).
While these subdisorders are to a degree epidemiologically comorbid, they display differential responsiveness to the spectrum of anxiolytic drugs currently in clinical use. This suggests that divergent etiological factors may underlie the different disorders. Rating scales, such as the Hamilton rating scale for anxiety and the clinical global impression scale, are used by clinicians both as tools to quantify symptom severity and as measures of treatment efficacy. These disorders furthermore display distinct neurobiological and neuroendocrine characteristics, indicative of differing underlying pathology (Sramek et al., 2002).
Current drug treatment of anxiety
For millennia, humans have sought out chemical agents to modify the effects of stress and feelings of discomfort, tension, anxiety and dysphoria; the oldest of these being ethanol. In the 19th century, alkaloids, bromide salts and choral hydrate were used for their sedative hypnotic medicine. A major breakthrough came with the introduction of barbiturates into the clinical practice in the early part of the 20th century (López-Muñoz et al., 2005). They induce their effects by facilitating the Cl- channel of the GABAA receptor to open, even in the absence of GABA tone. Animal models, especially canine-based paradigms, were particularly useful in identifying the sedative and anticonvulsant properties of such drugs, although self-testing was also very popular in the early days of modern psychopharmacology. While barbiturates were popular as major tranquillizers, their side effects, including sedation and behavioural changes, tolerance, and dependence issues coupled with the fact that their therapeutic dose limit is dangerously close to its toxic level has led to the pharmaceutical industry to seek out safer alternatives (López-Muñoz et al., 2005).
It was in this context that the development of benzodiazepines emerged and revolutionized the treatment of anxiety disorders. The first clinically available benzodiazepine was chlordiazepoxide, which was synthesized by Sternbach in the 1950s at the Hoffman La Roche Pharmaceutical Company (Sternbach, 1979). At a molecular level, benzodiazepines elicit their effects by allosterically activating the GABAA receptor channel at a site distinct from GABA itself, and thus only induce effects in synapses where GABA is present. Key behavioural studies by Randall and colleagues (Hanson, 2005) indicated that chlordiazepoxide might have a distinct pharmacological profile compared with that of barbiturates and other psychoactive drugs such as the antipsychotic chlorpromazine and anti-hypertensive reserpine. These initial tests were carried out in mice and cats, and included the mouse-inclined screen test indicative of muscle relaxation and sedation, a foot shock test showing ‘taming effects’, the anaesthetized cat model of muscle relaxation, seizure-based pentylenetetrazol (PTZ) and electroshock tests (Randall, 1960). Later, more sophisticated tests included Skinner box based Sidman avoidance task (Sidman, 1953; Boren et al., 1959) in rats and monkeys, which provided a sensitive and reliable measure of depressant action on behaviour. Thus, the tests used to illuminate the anxiolytic activity of the first generation of chemically designed anxiolytics were somewhat crude and not selective for anxiety per se. Yet, they highlight the crucial role of animal testing in anxiolytics development. It is somewhat ironic that as the tests employed became more sophisticated (See Table 1) the development of anxiolytic drugs has not greatly increased (Figure 1).
Table 1.
Tests of anxiety in animals
| Test | Brief description | Principles | Animals | Clinically available active drugs | References |
|---|---|---|---|---|---|
| Ethological tests | |||||
| Elevated plus maze | Animals are placed on an elevated plus-shaped maze consisting of two open arms and two enclosed arms connected by a central connecting square. | The open arms of the maze are considered to be more aversive than the closed arms. Anxiolytic drug treatment results in increased entries to and time spent in the open arms. | Mice, rats, gerbils | Benzodiazepines, ondansetron, barbituates, ethanol, prazosin, clonidine, 5-HT1A receptor agonists | (Handley and Mithani, 1984; Lister, 1987; Wilks and File, 1988; Moser et al., 1990; Filip et al., 1992; Hogg, 1996; Braun et al., 2011) |
| Elevated zero maze | Animals are placed on an elevated circular maze consisting of four segments: two exposed segments and two walled segments of equal length. | The open segments of the maze are considered to be more aversive than the closed segments. Anxiolytic drug treatment results in increased entries to and time spent in the open segments. | Mice | Benzodiazepines, citalopram (repeated dosing) | (Shepherd et al., 1994; Mombereau et al., 2007; Braun et al., 2011) |
| Light–dark box | Animals are placed in an apparatus consisting of two compartments: an illuminated ‘light’ compartment and a dark compartment. The compartments are connected by a small opening at floor level. Animals are allowed to freely explore the apparatus. | The light compartment is believed to be more aversive to the mouse than the dark compartment. Anxiolytic drug administration increases the number of entries to the light compartment, time spent in the light compartment and reduces freezing behaviour. | Mice, rats | Benzodiazepines, buspirone, ondansetron, paroxetine, dothiepine, moclobemide, clozapine | (Crawley and Goodwin, 1980; Jones et al., 1988; Onaivi and Martin, 1989; Bourin et al., 1996; De Angelis and Furlan, 2000; Hascoët et al., 2000a; Bourin and Hascoët, 2003) |
| Open field | Mice are placed in a brightly lit arena and allowed to freely explore. | The brightly lit exposed arena is a highly anxiogenic environment. Anxiety levels are assessed by a variety of ethological parameters, as well as by the amount of time the animal spends in the more anxiety-provoking central compartment. | Mice, rats | Benzodiazepines, phenobarbitol, acute vigabatrin, zolpidem, buspirone, acute fluoxetine, ondansetron, propanolol | (Crawley, 1981; Lucki et al., 1989; Stefański et al., 1992; Sherif and Oreland, 1995; de Angelis, 1996; Schmitt and Hiemke, 1998; Prut and Belzung, 2003) |
| Defensive marble burying/shock probe burying test | Animals are placed in a novel cage containing bedding and a number of novel marbles or an electrical shock probe. | Burying of the marbles is interpreted as anxiety-like behaviour. Anxiolytic drug treatment reduces the number of marbles buried. | Mice/rats | SSRIs, benzodiazepines, buspirone, chronic desipramine, chlorpromazine, imipramine, haloperidol, morphine, pentobarbitol, progesterone, nitrous oxide, SSRIs | (Treit et al., 1981; Craft et al., 1988; Njung'e and Handley, 1991; Czech and Quock, 1993; Picazo and Fernández-Guasti, 1995; De Boer and Koolhaas, 2003; Joel, 2006) |
| Ultrasonic vocalizations | Mouse pups are separated from their mothers and the frequency of ultrasonic distress calls is recorded | A reduction in the number of USVs emitted from separated pups is regarded as an anxiolytic effect. | Mice, rats, guinea pigs | Benzodiazepines, 5-HT1A receptor agonists, SSRIs | (Nastiti et al., 1991a,b; Groenink et al., 2008; Scattoni et al., 2009) |
| Staircase test | Animals are placed in an enclosed staircase, with the number of steps climbed and the number of rearing behaviours measured. | The climbing of steps in this test is regarded as a measure of exploratory behaviour, with the number of rearing behaviours being regarded as an anxiety-like behaviour. | Mice, rats | benzodiazepines, barbituates | (Molinengo and Ricci-Gamalero, 1970; Hughes, 1972; Cunha and Masur, 1978; Simiand et al., 1984) |
| Social interaction test | Animals are introduced to a novel animal of the same species, and social behaviour is monitored. | Anxiolytic drugs increase the levels of social interaction with the novel mouse. | Mice | Benzodiazepines | (File, 1980) |
| Suok ropewalking/elevated alley test | Animals are placed in the centre of an elevated beam or alley and allowed to freely explore the apparatus. Can be modified to the light–dark Suok test by illuminating one half of the beam and leaving the other half in darkness. | The elevated beam induces anxiety by virtue of its novelty and elevation. Increased horizontal exploration in this test is regarded as a decrease in anxious behaviour. In the light–dark test, increased time spent in the illuminated area is regarded as a decrease in anxiety. The Suok test is also able to detect non-specific drug effects (e.g. ataxia) and is specific use in examining anxiety–vestibular system interactions. | Mice, rats | Benzodiazepines, ethanol | (Kalueff and Tuohimaa, 2005; Kalueff et al., 2007; 2008) |
| T-maze | The elevated T-maze consists of an elevated platform consisting of two opposing exposed arms and one enclosed arm perpendicular to the open arms. Animals are placed at the distal end of the enclosed arm and are allowed to explore the apparatus. After several trials, the animal is placed on the distal end of an open arm and allowed to explore the apparatus. | Learned fear (inhibitory avoidance) can be assessed by measuring the time taken for the animal to leave the enclosed arm subsequent to initial exposure. Anxiolytic agents decrease the amount of time taken by the animal to leave the open arm. Unconditioned fear is measured using the latency of the animal to escape into the closed arm from the open arm. | Mice, rats | Benzodiazepines, buspirone (inhibitory avoidance only) | (Graeff et al., 1998; Carvalho-Netto, 2004) |
| Seed-finding test | Hamsters are fasted overnight and are then removed from their home cages to a novel environment. Sunflower seeds are then placed under the bedding of the home cage. | Latency to find the seed when returned to the cage is taken as a marker of anxious behaviour. | Hamster | Chlordiazepoxide, fluoxetine, buspirone | (King et al., 2002) |
| Anxiety/defence test battery, MDTB | Rats or mice are confronted with a dead or heavily anaesthetized predator (rat) and the defensive behaviour exhibited by the mouse is measured. | Risk assessment, flight responses and defensive attack behaviours are measured, as well as the development of context-dependant defensive behaviours (i.e. in the absence of threat are ethologically examined. | Mice, rats, non-human primates | Benzodiazepines, 5-HT1A receptor agonists, imipramine, chronic SSRIs, chronic TCAs | (Griebel et al., 1995a,b; 1999; Barros et al., 2008) |
| (Modified) hole board test | Rats or mice are placed in an open field containing an opaque holed board. The board contains several holes with removable plastic covers. | The number of entries onto the board and duration spent on the board are measures of anxiolyitc activity. Exploratory behaviours (no. of holes visited) and locomotor activity, as well as a number of ethological parameters can be measured. | Mice, rats | Benzodiazepines, chronic tiagabine, chronic paroxetine | (Ohl et al., 2001a,b; Sillaber et al., 2008; Thoeringer et al., 2010) |
| Conflict tests | |||||
| Geller–Seifter task | Food-deprived animals are trained to activate a lever to receive food (unpunished period). Once trained, the delivery of the food reward coincides with an electrical shock (punished period). | The paradigm generates a conflict between hunger-stimulated approach behaviour and fear of the shock. Anxiolytic drugs increase approach behaviour during the punished period. | Rats | Benzodiazepines, barbituates, clozapine, haloperidol | (Geller and Seifter, 1960; 1962; Geller et al., 1962; Wiley et al., 1993; Millan and Brocco, 2003; Paterson and Hanania, 2010) |
| Vogel punished drinking | Water-deprived rats are given access to water. Drinking behaviour is ‘punished’ with electrical shocks. | The paradigm generates a conflict between thirst-stimulated approach behaviour and fear of the shock. Increases in ‘punished’ water consumption is viewed as an anxiolytic effect. | Rats | Diazepam, zolpidem, phenobarbital, propofol, baclofen, buspirone, ondansetron | (Vogel et al., 1971; Depoortere et al., 1986; Shibata et al., 1989; Filip et al., 1992; Matsuo et al., 1997; Dekeyne et al., 2000; Millan and Brocco, 2003) |
| Four-plate test | Apparatus consists of a cage with a floor comprised of four metal plates. The animal is allowed to freely explore the novel apparatus, but crossing between floor plates results in a mild electrical shock. | In the four-plate test, the aversive shock can only be avoided by immobility. The number of crossings between quadrants in this paradigm is inversely related to the levels of passive avoidance behaviour. | Mice | Benzodiazepines, SSRIs, SNRIs, tricyclic antidepressants, trazodone | (Aron et al., 1971; Hascoët et al., 2000b) |
| Hyponeophagia tests | |||||
| Novelty-suppressed feeding | Food-deprived animals are introduced to a novel anxiogenic environment where food is presented. | Thus, paradigm generated a conflict between the anxiogenic stimulus and hunger-induced approach behaviour. Anxiolytic drugs reduce the latency of the animal to approach the food. | Mice, rats | TCAs (chronic), benzodiazepines, SSRIs | (Bodnoff et al., 1988; 1989) |
| Novelty-induced hypophagia | Animals are trained to consume a desirable food (e.g. sweetened milk before being presented with the milk in a novel environment. | Hesitation on the part of the animal to consume the highly palatable food in the novel environment is regarded as a measure of both anxiety and of anhedonia. | Mice, rats | Benzodiazepines, buspirone (chronic), SSRIs (chronic), TCAs (chronic), phenobarbitol, valproic acid, propanolol, ondansetron | (Rex et al., 1998; Merali et al., 2003; Santarelli et al., 2003; Dulawa and Hen, 2005) |
| Cognitive-based tests | |||||
| Pavlovian fear conditioning | Animals are trained to associate a particular contextual or cue-related stimulus (CS) with an unpleasant stimuli such as an electric foot shock, acoustic stimulus or an aversive odour (US). The animal then learns to display an observable startle or freezing response to the CS independent of the presence of the US. | Increased levels of freezing behaviour and enhanced startle responses when the CS is presented in the absence of the US are used as measures of fearful behaviour. | Mice, rats | Benzodiazepines, Barbituates, Opiates, Buspirone, Ethanol, acute SSRIs | (Davis, 1990; Fendt and Fanselow, 1999; Borsini et al., 2002) |
| Conditioned emotional response | Animals are trained to associate a specific operant, feeding or drinking behaviour (CS) with unpleasant stimuli (US). | Association of the US behaviour results with the CS results in a decrease in the US behaviour. Anxiolytic drugs reduce this effect. | Mice, rats | Benzodiazepines barbituates, opiates | (Davis, 1990; Goddyn et al., 2008) |
| Conditioned taste aversion | Consumption of sweetened milk is paired with the injection of the malaise inducing compound lithium chloride (LiCl). Animals are then offered a free choice between consumption of water and the same sweetened milk. | Association of the sweetened milk with the LiCl-induced malaise results in a decrease in consumption of the sweetened milk. Anxiolytic drugs reduce this effect. | Rats | Benzodiazepines | (Ervin and Cooper, 1988; Yasoshima and Yamamoto, 2005) |
| Physiological tests | |||||
| Stress-induced hyperthermia | Mice are singly housed overnight prior to rectal temperature measurement. Temperature is measured twice at an interval of 10–15 min. | Rectal temperature measurement is used as a stressor in this procedure. The increase in temperature seen between the first and second measurements is taken regarded as a physiological anxiety response. Drugs regarded as having an anxiolytic effect in this test reduce the magnitude of this temperature increase. | Mice, rats. | Benzodiazepines, alcohol (higher doses are hypothermic), zolpidem, buspirone flesinoxan, chronic SSRI treatment | (Van Bogaert et al., 2006; Bouwknecht et al., 2007; Conley and Hutson, 2007; Vinkers et al., 2008) |
| Autonomic telemetry | Electrocardiogram transmitters are surgically attached to the mouse, allowing remote monitoring of heart rate, body temperature and locomotor activity | The autonomic response to stressful stimuli, such as heart rate, blood pressure, body temperature and locomotor activity, can be measured and recorded in freely behaving animals. | Mice, rats | Diazepam | (van Bogaert et al., 2006) |
Figure 1.
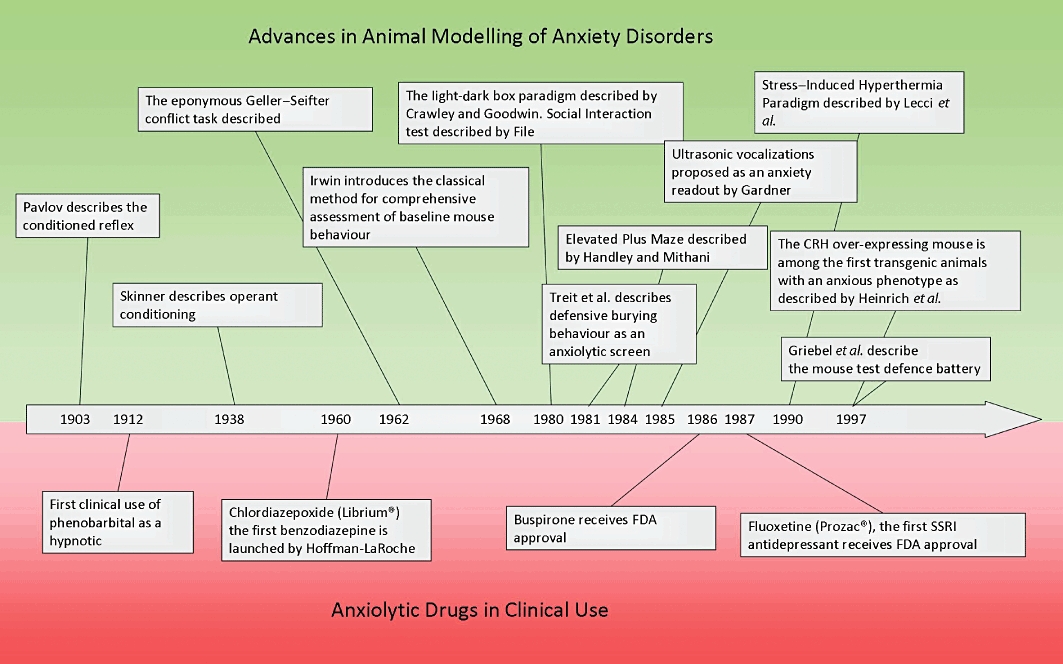
Advances made in the modelling of anxiety disorders in humans compared to the introduction of novel anxiolytic drug classes across the past century. Clinical drug development clearly lags behind the development of novel techniques to model anxiety in animals. A novel class of anxiolytic drug has not entered the market since the approval of fluoxetine, the first SSRI, in 1987, despite numerous advances in the preclinical modelling of anxiety disorders.
Despite the advantages of benzodiazepines over previous drugs, their long-term use is hampered by dependence liability, tolerance, and cognitive and other behavioural side effects. This once again led to a major research effort to try and develop novel non-GABAergic based therapies.
The realization that the serotonergic system plays a role in anxiety has been known for over 50 years since Aprison and Ferster showed that the 5 hydroxytryptamine (serotonin) (5-HT) precursor 5-hydroxytryptophan increased responding in a pigeon conflict model (Aprison and Ferster, 1961). This view mainly arose from some observed activity of 5-HT antagonists in operant conflict paradigms in rats (Robichaud and Sledge, 1969), as well as from an association between reduction in turnover of 5-HT and the anxiolytic effects of benzodiazepines (Goldberg et al., 1967). This research culminated in the development of 5-HT-based therapies for anxiety disorders throughout the 1970s and early 1980s (Taylor and Moon, 1991), chief among them was the azapirone chemical class of which buspirone was the most successful. Buspirone acts as a partial 5-HT1A receptor agonist, and its use confirmed that it was possible to develop novel anxiolytic drugs that lacked the side effects of GABA-based drugs. Also, it opened up the possibility the modulation of the serotonergic system may have clinical benefit in anxiety disorders. Today, buspirone has a somewhat limited use, although it is generally well tolerated with few side effects, its efficacy, is less and onset of action slower than previous drugs such as the benzodiazepines.
The clinical realization that anxiety and depression are co-morbid has led to the clinical observation that selective serotonin re-uptake inhibitors (SSRIs) are effective in treating anxiety disorders following on from observations regarding the efficacy of tricyclic antidepressants in anxiety (Rickels et al., 1974; 1993). Indeed, today SSRIs are first-line therapy for many anxiety disorders (Hoffman and Mathew, 2008). The development of SSRIs for depression and subsequently anxiety was firmly driven by mechanistic studies focusing on the modulation of monoamine neurotransmission in vitro and in vivo with little input of behavioural models initially (Wong et al., 2005). This is a clear and sobering example where animal models had little to do with the clinical introduction of these treatments for anxiety. Indeed, the reliance on traditional animal models of anxiety shows little positive effects of SSRIs, and indeed anxiogenic effects are often observed (Sánchez and Meier, 1997; Borsini et al., 2002). It should, however, be borne in mind that a transient period of increased anxiety is often reported in patients initiated onto SSRI therapy (Vaswani et al., 2003; Baldwin et al., 2010). This has led to much criticism of the models used. Likewise, there has been a growing discussion focused on whether anxiety and depression should be isolated from a drug development perspective (Shorter and Tyrer, 2003). Moreover, given the relative success of SSRIs, it is becoming clear that many pharmaceutical companies are compelled to develop a ‘one pill fits all’ approach to anxiety and mood disorders. This propelled research in the area of neuropeptides such as corticotrophin-releasing factor receptor antagonists, neurokinin 1 (NK1) receptor antagonists and melanocortin antagonists, which to date have yet to fulfil its initial promise (Takahashi, 2001; Shimazaki et al., 2006; Ebner et al., 2009). Recent drug discovery efforts have additionally focused on ligands acting at G-protein-coupled receptors for the non-monoaminergic neurotransmitters GABA and glutamate (Chojnacka-Wójcik et al., 2001; Cryan and Kaupmann, 2005). The current status of several promising putative drug classes for anxiety is given in Table 2.
Table 2.
Novel anxiolytic targets
| Brain Target | Drug Action | Sample Compound | Behavioural Effects in animal models | Comments | References |
|---|---|---|---|---|---|
| GABA | |||||
| GABAA receptors | α2 subunit specific agonists | MRK-409 | Anxiolytic effects in rat elevated plus maze, conditioned startle response and punished drinking paradigms, as well as conditioned emotional response paradigm in primates | The safety and tolerability of MRK-409 were examined in humans and it was discovered to possess sedative properties, not observed in preclinical studies. Development was abandoned. | (Atack et al., 2011) |
| GABAB receptors | Positive allosteric modulators | GS39783 | Anxiolytic effects in the light–dark box, elevated plus maze, elevated zero maze and stress-induced hyperthermia paradigm | (Cryan et al., 2004; Cryan and Kaupmann, 2005) | |
| Glutamate | |||||
| NMDA receptor | Partial agonists | d-cycloserine | Facilitates fear extinction in rodents | Effective in reducing conditioned fear responses in acrophobic patients | (Ressler et al., 2004; Amaral and Roesler, 2008) |
| mGluR2/3 | Agonists | LY354740, LY314582, APDC | Block expression of fear potentiated startle in rats, anxiolytic effects in the EPM in mice and rats, anxiolytic effects in mouse and rat conflict tests, attenuates lactate-induced panic in rats, anxiolytic in the SIH paradigm reduces startle response following drug of abuse withdrawal in rats. | LY544344, a prodrug of LY354740 with improved oral bioavailabilty, has shown efficacy and tolerability in human GAD patients. Discontinued. | (Monn et al., 1997; Helton et al., 1998; Benvenga et al., 1999; Kłodzińska et al., 1999; Spooren et al., 2002; Dunayevich et al., 2008) |
| mGluR2 | Positive allosteric modulators | 4-MPTTS, CBiPES, ADX71149 | Block expression of fear-potentiated startle in rats and has an anxiolytic effect in the rat SIH paradigm | ADX71149 is in phase 2 clinical trials | (Johnson et al., 2005, Addex company website) |
| mGluR4 | Agonists | LSP1-2111 | Anxiolytic effects in the EPM and reduces the SIH response in mice. Reduction in the SIH response in diminished in 5HT-depleted animals. | (Wierońska et al., 2010) | |
| mGluR5 | Antagonists | MPEP, MTEP | MPEP has anxiolytic effects in the Geller–Seifter test, the Vogel punished drinking test, conditioned emotional response paradigm, as well as in the social interaction test, defensive marble burying, the elevated plus maze and the SIH paradigm | (Kuhn et al., 2002; Spooren and Gasparini, 2004; Ballard et al., 2005; Varty et al., 2005) | |
| mGluR7 | Agonists | AMN082 | Anxiolytic effects in the four-plate test and reduces the SIH response in mice. Facilitates extinction of amygdala-dependent fear conditioning and inhibits acquisition of Pavlovian fear conditioning. | (Fendt et al., 2008; Stachowicz et al., 2008) | |
| mGluR8 | Agonist/positive allosteric modulator | DCPG (agonist) AZ12216052 (Positive allosteric modulator) | Anxiolytic effects in the elevated zero maze and acoustic startle paradigm (positive modulator only) | (Duvoisin et al., 2010) | |
| Serotonin | |||||
| 5-HT7 receptor | Antagonist | SB269970 | Anxiolytic in the elevated plus maze, Vogel punished drinking and four-plate test | (Wesolowska et al., 2006) | |
| 5-HT1A receptor | Antagonists | WAY100635, p-MPP, ISL88.0338 | Anxiolytic in the mouse defensive test battery | (Griebel et al., 1999) | |
| 5-HT2C receptor | Antagonists | S32006 | Anxiolytic in the defensive marble-burying paradigm, Vogel punished drinking and social interaction tests | (Dekeyne et al., 2008; Wacker and Miller, 2008) | |
| Neuropeptide systems | |||||
| CRH1 receptor | Antagonists | Antalarmin, pexacerfont, GSK561679, CP154-526 | Anxiolytic effects across a wide range of behavioural tests and between a variety of species. | Pexacerfont failed in to improve anxiety symptoms in generalized anxiety disorder in a randomized control trial. GSK561679 is currently undergoing clinical trials for anxiety disorders. | (Kehne & De Lombaert, 2002; Takahashi, 2001; Coric et al., 2010) |
| CRH2 receptor | Antagonists | Anti-savagine-30 | Anxiolytic effects in the elevated plus maze, conditioned freezing and defensive behaviour. | (Takahashi, 2001) | |
| NK1 receptor | Antagonists | Vestipitant | Anxiolytic effects on fear-induced foot tapping and social interaction in gerbils, USVs in guinea pigs, punished drinking and fear induced USVs in rats and marble-burying behaviour in mice. | Vestipitant is currently in phase II clinical trials in combination with paroxetine for social anxiety disorder. | (Ebner and Singewald, 2006; Brocco et al., 2008; Ebner et al., 2009) (http://www.clinicaltrials,gov) |
| Translocator protein (18 kD) | Neurosteroid production enhancement | XBD173 | Anxiolytic effects in the rat social interaction test and elevated plus maze. Inhibits sodium lactate and CCK-4 induced panic in rats. | Inhibits CCK-4 induced anxiety in healthy human volunteers. | (Rupprecht et al., 2009; 2010) |
| Other neurotransmitter systems | |||||
| MCH1 | Antagonists | SNAP −7941, TPI 1361–17 | Increases social interaction in the social interaction test. TPI 1361–17 has an anxiolytic effect in the EPM and light–dark box. | (Doggrell, 2003; Shimazaki et al., 2006; Lee et al., 2010) |
It is clear that while there are certain overlapping factors contributing to the natural history of anxiety and depression, the symptomatic manifestation and treatment of each can be very different; benzodiazepines, for example, have limited efficacy in depression and yet represent a very effective intervention in anxiety disorders, whereas SSRI antidepressants are useful in both disorders. Thus, understanding the neural circuits of both these disorders is crucial to devising novel interventions. Animal models will be critical for such approaches, although it must be remembered that animal models can only be as valid as the clinical knowledge that their translational validity is based on and that a better clinical understanding of the diverging nature of the two disorders is still of the upmost importance.
Endophenotypes
A growing recognition of the complex and heterogeneous nature of anxiety has resulted in an effort to re-evaluate the diagnosis and treatment of anxiety disorders, and develop a novel approach where individual behavioural, physiological and neurochemical end points are specifically considered as opposed to a syndrome-based approach (Geyer and Markou, 2002; Gottesman and Gould, 2003; Hasler et al., 2004). Considered from a genetic perspective, the clinical deconstruction of anxiety can be described in terms of endophenotypes. These are cognitive, psychological, anatomical or biochemical traits which are hereditary and represent reliable markers of both the disease state and disease risk (Hasler et al., 2004). The endophenotypes present in anxiety disorders may allow for a more effective analysis of the neurobiological and genetic factors that contribute to their development in humans, as well as representing facets of disease more amenable to the development of valid animal models (Gottesman and Gould, 2003; Hasler et al., 2004). The behavioural endophenotypes of anxiety disorders such as autonomic hyper arousal, impaired extinction of traumatic memories, sleep disturbances and avoidance of difficulty to escape areas can all be readily modelled in existing behavioural paradigms (Cryan and Holmes, 2005).
Like other medical disciplines, concerted effort is focused on the generation of novel models of anxiety (i.e. an effort to induce in animals a hyper-anxious state), analogous to the state seen in anxiety disorder patients, which can be detected by increased sensitivity to the anxiety-provoking nature of behavioural tests (Rodgers, 2010). Development of novel genetic animal models has proven invaluable not only in this regard, but in the dissection of neurobiological basis of anxiety behaviour and in indicating potential therapeutic avenues for treatment of anxiety disorders (Jacobson and Cryan, 2010). Because the demonstration of an anxious phenotype in the corticotrophin-releasing-hormone over-expressing mouse (Heinrichs et al., 1997), knock-out and transgenic mice have played a vital role in both understanding the in vivo function of putative drug targets and now represent the definitive target validation strategy. More sophisticated techniques, such as tet-on/off and Cre-lox mediated gene expression systems, as well as siRNA-mediated gene knock down allow temporal and regionally specific control of gene expression in the brain, making transgenic mice an even more useful tool for drug discovery (Gross et al., 2002; Heck et al., 2004; Cryan and Holmes, 2005; Jacobson and Cryan, 2010). Genetic models of anxiety behaviour are listed in Table 3 (Finn et al., 2003). Combining these genetic techniques with endophenotype-based, translationally valid animal models is a central strategy in overcoming the challenges inherent in developing novel treatment strategies for anxiety; however, it is not without its caveats. The genetic models of anxiety listed in Table 3 display anxious phenotypes in some reports, but display phenotypes of reduced or unaltered anxiety in other cases. A prominent example is the GAT1 KO mouse which has been shown to display an anxious phenotype in the open field (Chiu et al., 2005), but also decreased levels of anxiety in several measures of anxiety when generated on a different genetic background (Liu et al., 2007). Background strain can play a highly influential role on the behavioural effects of genetic alterations (Crawley et al., 1997), and is perhaps most notable in genetic models where animals of the 129 strain are used in the generation process. In many cases, anxious behaviour may be more associated with the use of 129 strain mouse during the generation process than the genetic modification itself (Crawley et al., 1997; Võikar et al., 2001; Cook et al., 2002; Eisener-Dorman et al., 2010). This may partly explain the surprisingly extensive list of mutations that result in an anxious phenotype propelling the question as to why so many mutant mice are unhappy (Holmes and Cryan, 2006). Other possibilities include that the test used are conceptually attractive, easy to construct and carry out with minimal requirement for extensive training of either mouse or experimenter (Holmes and Cryan, 2006).
Table 3.
Genetic and Environmental Models of anxiety (See also Finn et al., 2003)
| Model | Description | Anxiety-like behavioural phenotype | Reference |
|---|---|---|---|
| Genetic models | |||
| Selective breeding | |||
| BALB/c mouse | Inbred mouse strain | High Levels of anxious behaviour in the open field, enhanced conditioned fear learning and greater levels of anxious behaviour in the light–dark box. | (Crawley and Davis, 1982; Crawley et al., 1997; Owen et al., 1997; Griebel et al., 2000; Belzung and Griebel, 2001) |
| BTBR T + tf/J mouse | Inbred mouse strain | Anxious phenotype in the elevated plus maze, as well as increased anxiety like behaviour in measures of social interaction | (Pobbe et al., 2010) |
| Wistar-Kyoto (WKY) rat | Outbred Rat Strain | Hypoactivity in the open-field test and are more vulnerable to stress-induced gastric ulceration than control Sprague-Dawley rats, hyper vigilant phenotype. | (Paré, 1994; McAuley et al., 2009) |
| LAB/HAB | Outbred mouse (CD-1) or rat (Wistar) strains selectively bred for high or low anxiety. | Greater level of anxiety behaviour in the elevated plus maze and light–dark box. | (Saloméet al., 2002; Kromer et al., 2005; Landgraf et al., 2007) |
| High DPAT/Low DPAT | Rat strains bred for low or high levels of responsivity to the hypothermic effects of 5-HT1A receptor agonist 8-OH-DPAT | High DPAT rats display enhanced anxiety behaviour in social interaction tests and conflict tasks compared to low DPAT mice. | (Commissaris et al., 2000) |
| Roman high-(RHA/Verh) and low-(RLA/Verh) avoidance rats | Rat strains selectively bred for good (RHA) and poor (RLA) performance in two-way, active avoidance paradigms. | RLA/Verh rats display enhanced anxiety behaviour and enhanced neuroendocrine stress response, a tendency towards a passive response to novel environments and higher levels of conditioned fear, as well as increased anxiety in the open field, light–dark box and elevated plus maze than RHA/Verh rats. | (Steimer et al., 1997; Yilmazer-Hanke et al., 2002; Steimer and Driscoll, 2003; López-Aumatell et al., 2009) |
| Maudsley reactive (MR/Har) and non-reactive (MNRA/Har) rats | Rat strains bred, respectively, for high and low open field-induced defecation | MR/Har rats display a lower level of activity in the open field, a more anxious phenotype in conflict tests which is insensitive to benzodiazepine treatment, reduced exploratory behaviour to novel stimuli, enhanced startle response with reduced within-session habituation to acoustic stimulus, greater levels of stress-induced USVs (maternal separation and air puff) compared to MNRA/Har | (Commissaris et al., 1989; 1992; 1996; Blizard and Adams, 2002) |
| 129 mice | Inbred mouse strain | Impaired Pavlovian fear conditioning. 129/P3 mice fail to habituate to behavioural test of anxiety and display increased vulnerability to chronic mild stress | (Camp et al., 2009; Salomons et al., 2010a,b) |
| Fawn Hooded (FH/Wjd) rat | Inbred rat strain | The FH/Wjd rat displays lower levels of social behaviour in both anxiogenic and neutral environments than both the Wistar and the Sprague-Dawley rat. | (Kantor et al., 2000) |
| Single-gene manipulation models | |||
| COMT knock-out | Mice with targeted deletion of the catechol-O-methyl transferase gene | Increased levels of anxiety behaviour in the light–dark box seen in females only | (Gogos et al., 1998) |
| Adra2a knock-out | Mice with targeted deletion of the α2A adreno receptor gene | Increased anxiety behaviour in the open field, elevated plus maze and the light–dark box | (Schramm et al., 2001; Lähdesmäki et al., 2002) |
| 5-HT1A receptor KO | Mice with targeted deletion of the 5HT1A receptor gene | Increased anxiety behaviour in the open field, elevated plus maze, elevated zero maze, novelty-suppressed feeding and novel object exploration paradigms, as well as increased fear responses to contextual cues in a fear-conditioning paradigm | (Heisler et al., 1998; Ramboz et al., 1998; Gross et al., 2000; Klemenhagen et al., 2006) |
| Early life 5-HT1A receptor KO (P4-21) | Mice where the 5-HT1A receptor expression is conditionally ablated from postnatal days 4–21 | Increased anxiety behaviour in the open field, novelty-suppressed feeding and elevated plus maze in adult life | (Gross et al., 2002) |
| 5-HTT knock-out | Mice with targeted deletion of the serotonin transporter gene | Increased anxiety behaviour in the elevated zero maze, light–dark box test, elevated plus maze, open field and a hyponeophagia paradigm | (Carroll et al., 2007; Line et al., 2011) |
| GAD65 knock-out | Mice with targeted deletion of the glutamic acid decarboxylase 65 isoform gene | Increased anxiety behaviour in the open field and in the elevated zero maze. Selective alterations in the quality of the mouse response in conditioned fear paradigms | (Kash et al., 1999; Stork et al., 2003) |
| GAT1 knock-out | Mice with targeted deletion of the GABA transporter (GAT1) gene | Increased anxiety behaviour in the open field | (Chiu et al., 2005) |
| GABAA receptor γ2 knock-out | Mice with heterozygous deletion of the GABAAγ2 receptor subunit gene | Increased anxiety behaviour in the open field, elevated plus maze, light–dark box and free choice exploration | (Crestani et al., 1999; Chandra et al., 2005) |
| GABAA receptor γ2L knock-out | Mice with targeted deletion of the GABAAγ2L receptor subunit gene | Anxious phenotype in the elevated plus maze | (Homanics et al., 1999) |
| GABAB1 receptor subunit knock-out | Mice with targeted deletion of the GABAB1 receptor subunit gene | Panic-like response in the elevated zero maze, as well as increased anxiety behaviour in the light–dark box and staircase test | (Cryan and Kaupmann, 2005) |
| GABAB2 receptor subunit knock-out | Mice with targeted deletion of the GABAB2 receptor subunit gene | Increased levels of anxiety in the light–dark box | (Mombereau et al., 2005) |
| CRH over-expression | Transgenic mice which over-express the CRH gene | Increased anxiety in the open field, elevated zero maze and elevated plus maze | (Heinrichs et al., 1997; van Gaalen et al., 2002) |
| Early life CRH over-expression | Transgenic mice in which CRH is transiently over-expressed from postnatal days 0–21 | Increased anxiety behaviour in the open field and light–dark box tests in adult life | (Kolber et al., 2010) |
| Urocortin knock-out | Mice with targeted deletion of the urocortin gene | Increased anxiety in the open field and the elevated plus maze | (Vetter et al., 2002) |
| CRH-BP knock-out | Mice with targeted deletion of the corticotrophin-binding protein gene | Increased anxiety phenotype in the open field, elevated plus maze and increases in defensive withdrawal | (Karolyi et al., 1999) |
| APOE knock-out | Mice with selective deletion of the apolipoprotein E gene | Increased levels of anxietty behaviour in the elevated plus maze | (Raber, 2007) |
| Otsuka Long Evans Tokushima Fatty (OLETF) rat | OLETF rat constitutively lacks the CCK1 receptor | High levels of anxiety behaviour in the elevated plus maze, light–dark box and open field tests | (Kobayashi et al., 1996; Yamamoto et al., 2000) |
| mGluR5 receptor knock-out | Mice with a targeted deletion of the mGlu5 receptor gene | Increased anxiety behaviour in the elevated plus maze | (Wu et al., 2007) |
| Desert hedgehog knock-out | Mice with a targeted deletion of the desert hedgehog gene | Enhanced anxiety behaviour in the Vogel-punished drinking test | (Umehara et al., 2006) |
| TSC-DN mice | Mice dominant negative for the tuberous sclerosis-associated gene TSC-DN | Increased anxety behaviour in the elevated plus maze, as well as in the open field | (Ehninger and Silva, 2010) |
| APP transgenic mice | Transgenic mice expressing mutant human β-amyloid precursor protein | Increased anxiety behaviour in the open field and light–dark box as well as greater levels of freezing in a conditioned fear paradigm | (España et al., 2010) |
| 3xTG-AD transgenic mice | Transgenic mice expressing human β-amyloid precursor protein, tau and PS1 | Increased anxiety behaviour in the open field and light–dark box, as well as greater levels of freezing in a conditioned fear paradigm | (España et al., 2010) |
| TgActbetaE mice | Transgenic mice over expressing activin E | Increased anxiety behaviour in the open field test and the elevated plus maze | (Sekiyama et al., 2009) |
| α-CaMKII transgenic mice | Mice which over-express the Ca2+/calmodulin-dependant protein kinase (α-CaMKII) | Increased anxiety behaviour in open field, elevated zero maze, light–dark transition and social interaction tests | (Hasegawa et al., 2009) |
| TgNTRK3 mice | Mice over-expressing the full length neurotrophin receptor TrkC | Increased anxiety behaviour in the elevated plus maze and elevated zero maze, as well as a panic reaction in the mouse defensive test battery | (Dierssen et al., 2006) |
| TGR(ASrAOGEN)680 Rat | A transgenic rat expressing anti-sense RNA to angiotensinogen in the brain | Increased anxiety behaviour in the elevated plus maze, light–dark box and open field | (Voigt et al., 2005) |
| Hdc knock-out mice | Mice with a targeted deletion of the histidine decarboxylase receptor | Increased anxiety behaviour in the elevated plus maze, light–dark box and open field seen in females | (Acevedo et al., 2006) |
| SF1 knock-out mice | Mice with a targeted deletion of the steroidogenic factor 1 gene specifically in the CNS | Increased anxiety behaviour in the elevated plus maze, the light–dark box, the open field and the defensive marble burying paradigm | (Zhao et al., 2008) |
| FMR1 knock-out mice | Mice with targeted deletion of the fragile-X-mental retardation gene 1 | Increased anxiety behaviour in the mirror chamber test and in the social interaction test | (Spencer et al., 2005) |
| Environmental models | |||
| Maternal separation | Mouse or rat pups are separated from their mothers for brief periods during early life | Maternally separated rats display increased anxiety behaviour in the elevated plus maze in adulthood and a heightened neuroendocrine response to stress. | (Plotsky and Meaney, 1993; Wigger and Neumann, 1999) |
| Maternal separation with early weaning (MSEW) | Mouse pups undergo maternal separation coupled with early weaning | Mice that have undergone MSEW display an anxious phenotype in the open field and elevated plus maze tests | (George et al., 2010) |
| Social isolation (SI) rearing | Mice pups are singly housed from weaning (3 weeks) until adult hood | SI mice have increased levels of anxious behaviour in the elevated plus maze and novel object recognition test. The SI mice additionally have a depression-like phenotype and display heightened levels of aggression | (Koike et al., 2009) |
| Chemical models | |||
| Pentylenetetrazole (PTZ) induced anxiety | Administration of the GABAA antagonist PTZ is a highly anxiogenic stimulus. Rats can be trained to discriminate PTZ administration from saline. | PTZ induces an anxiogenic effects in the elevated plus maze, as well as in conflict test. Animals trained to discriminate between PTZ and saline display PTZ appropriated responses to predator stress, alarm pheromones and to social defeat. | (Jung et al., 2002) |
| Sodium lactate-induced anxiety | Administration of IV sodium lactate produces a panic response in human volunteers and increases in anxious behaviour in rats | Sodium lactate infusion reduces levels of social interaction and increases levels of anxious behaviour in the elevated plus maze | (Johnson et al., 2008; Shekhar et al., 1996; 2010) |
| m-chlorophenylpiperazine (mCPP)-induced anxiety | Administration of the non-selective serotonin antagonist mCPP is a highly anxiogenic stimulus. Rats can be trained to discriminate mCPP administration from saline. | mCPP administration increases anxious behaviour in the elevated plus maze, elevated t maze, elevated zero maze, Geller-Seifter test, social interaction test and shock induced vocalizations | (Gatch, 2003) |
| CCK induced anxiety | Administration of cholecystokinin evokes a panic like response in rodents | CCK-8 produces a spontaneous freezing response and evokes anxious behaviour in the elevated T-maze and the elevated plus maze. CCK-4 produces a panic like reaction when injected into the dorsal periaquiductal grey area. | (Mongeau and Marsden, 1997; Netto and Guimarães, 2004; Zanoveli et al., 2004; Rupprecht et al., 2009) |
Manipulation of the early life experience of an animal represents an important avenue by which anxiety can be experimentally provoked in a translationally valid manner. Studies of the human population have revealed that adult behaviour is strongly influenced by an interaction of both early life environment and genetic background (Caspi et al., 2002; 2003). In an attempt to study this aspect of development, several experimental protocols have been used to induce anxiety behaviour by modifying early life environment (Plotsky and Meaney, 1993; Wigger and Neumann, 1999; Koike et al., 2009; George et al., 2010), neurochemical function (Ansorge et al., 2004; Depino et al., 2008), as well as altering early life gene expression (Gross et al., 2002; Kolber et al., 2010). These interventions are detailed in Table 3. Similarly to genetic models, the robustness of these environmentally induced models of anxiety varies extensively. In particular, while maternal separation has been shown to generate an anxious phenotype (Plotsky and Meaney, 1993; Wigger and Neumann, 1999) in some reports, this is not the case (Lehmann and Feldon, 2000; Millstein and Holmes, 2007; Savignac et al., 2011). The results achieved here are heavily dependant on procedural factors such as the length of separation and on subject factors such as gender (O'Mahony et al., 2010).
Basic concepts in animal modelling of anxiety disorders
Many of the symptoms of anxiety disorders are dependent on the processing of complex psychological and cognitive concepts that clearly cannot be measured in animals, such as ‘fear of losing control or going crazy’ or a ‘sense of a foreshortened future’. It is thus clear from the clinical presentation of anxiety disorders that they can never be fully emulated as a syndrome in animals (Cryan and Holmes, 2005; Arguello and Gogos, 2006; Crawley, 2007). If, however, we consider the substantial conservation of genetic, neurochemical and neuroanatomical features seen across mammals (Jones, 2002; Tecott, 2003; Arguello and Gogos, 2006), as well as Darwin's observations regarding the conservation of many fundamental, behavioural and pharmacological responses between species (Darwin, 1871; 1872), theoretically, by studying the neural and genetic determinants of animal behavioural response, we can, by inference, develop our understanding of the neural and genetic basis of human behaviour under both normal and pathological states (Geyer and Markou, 2002; Cryan and Holmes, 2005; Crawley, 2007). A necessary extension of this theory is that the validity of any animal model of psychiatric disease is determined by the robustness of the diagnostic techniques used to describe the disease state in the clinic. Translational interspecies comparisons are dependent on combined advances in the fields of both human diagnostics and animal modelling, as well as developments in our understanding of behavioural, genetic and neurobiological function in healthy humans and animals (Geyer and Markou, 2002; Markou et al., 2009). Likewise, novel reverse translational approaches, such as measuring human exploratory behaviour (Perry et al., 2009), may provide novel ways to model anxiety disorder endophenotypes in animals.
To determine the validity of an experimental model of a neuropsychiatric endophenotype, standardized criteria such as those proposed by McKinney and Bunney (1969) for depression, and which are equally applicable to anxiety disorders, can be used. These authors suggest that animal models should bear a reasonable analogy to the human disorder in either manifestation or symptoms, induce a behavioural change that can be objectively monitored, display sensitivity to effective clinical treatments and display inter-researcher reproducibility in order to be considered valid (McKinney and Bunney, 1969). Current thinking on the validity of animal models acknowledges the existence of several types of validity, including face validity (similar symptom manifestation to the clinical condition), construct validity (similar underlying biology), predictive validity (responsiveness to clinically effective therapeutic agents), etiological validity (induced by similar stimuli as the clinical condition), convergent validity (convergent measures with other construct based models) and divergent validity (divergent measures from other construct-based models) while maintaining that the reliability and predictive validity are the most important criteria in determining the overall validity of the system (Geyer and Markou, 2002).
In the context of anxiety, it has been argued by Treit et al. (2010) that the validity of behavioural tests of anxiety should be based on three principles arising from the evolutionarily conserved roles the fear response plays in normal survival behaviour. Firstly, a correspondence between the behavioural fear expressions in the animal model biochemical or physiological correlates of these behaviours, and the expression of isomorphic behavioural responses in humans. Secondly, if no isomorphism is present, biological function should be conserved between the anxiety-like behaviour in the animal model and the human fear response. And thirdly, conservation of the neural mechanisms, engaged during the fear response, that underlie anxiety-related behaviour in both animals and humans (Treit et al., 2010).
In preclinical psychiatry research, there remains some confusion on the distinction between an animal model versus a test (see Cryan and Slattery, 2007). When describing preclinical anxiety research, it is important to try and draw a distinction between animal models of anxiety and experimental tests of anxiety (Rodgers, 2010). In general, when the term ‘animal model of anxiety’ is used, it refers to an animal that exhibits a phenotype behaviourally relevant to clinical anxiety disorders. When we use the term ‘test of anxiety’, we refer to a behavioural paradigm that induces a quantifiable fear-related behaviour related to the normal adaptive fear response (Young and Liberzon, 2002; Rodgers, 2010). We can thus say that a model comprises both an independent variable (i.e. the inducing manipulation) and a dependent variable (i.e. the behavioural/neurochemical readout) (Geyer and Markou, 2000), whereas a test simply comprises a dependent variable. Thorough clinical understanding of the underlying pathophysiology of anxiety disorders is vital to determining appropriate independent variables in preclinical research. Identification of appropriate anxiety endophenotypes has been useful in this regard (Cryan and Slattery, 2007). Tests of anxiety are often described as ‘models of anxiety’ based on the translationally questionable premise that anxiety disorders represent an exaggerated activation of the normal fear response, when in fact they more accurately represent models of particular behavioural endophenotypes present in anxiety disorders and indeed models of anxiolytic drug activity (Young and Liberzon, 2002; Cryan and Holmes, 2005; Holmes and Cryan, 2006). Rodgers (2010) points to the fact that the distinction between animal test and animal model in anxiety research highlights the crucial difference in the knowledge we can garner from their use in understanding the neural circuitry of anxiety. Studying the induction of fear in an animal test in a normal animal can provide insight into the neurobiology of the adaptive fear response, but may not necessarily be appropriate for investigating the dysregulated fear responses observed in anxiety disorder patients (Rodgers, 2010). It is thus important to remember that symptoms could conceivably arise from pathological processes upstream of the fear response and not from an abnormal fear response per se. Knowledge of the dysregulated anxiety response in humans is thus best derived from animals with a translationally relevant dysregulation of their anxiety response, evidenced by greater levels of anxiety in etiologically valid behavioural tests. This may explain why, although our knowledge of the basic fear response has become highly developed over the past number of decades, the pathophysiology of anxiety disorders remains impenetrable (Rodgers, 2010).
Human models of anxiety: translating and adapting
All the abovementioned approaches share in common the precept that the validity of an animal model of anxiety is dependent on solid understanding of the ethological manifestation of anxiety in humans. Vital to this is the pharmacological validation of several fear/anxiety/stress-provoking paradigms that can be used to mimic in humans a state similar to the symptoms experienced by anxiety disorder patients. These include generating classical conditioned fear in humans, generating anxiety via public speaking, measuring attentiveness to threatening cues using the Stroop-word colour task, as well as measuring fear-potentiated startle in humans (Graeff et al., 2003).
The provocation of panic attacks using cholecystokinin (CCK) is a well-characterized method for the study of anxiety in humans (Koszycki et al., 1991), and has proven to be of use in exploring the neurochemical (Zwanzger et al., 2003; Maron et al., 2009), genetic (Maron et al., 2010) and psychological (Tõru et al., 2010) aspects of panic disorder, as well as representing a potentially useful screen for novel anxiolytic drugs (Kellner et al., 2005; Kronenberg et al., 2005; Eser et al., 2007). Anxiety in humans can also be generated experimentally using chemical agents such as caffeine (Nardi et al., 2007), m-chlorophenylpiperazine (mCPP) (Kahn et al., 1990), yohimbine (Charney et al., 1984), CO2 inhalation (Nardi et al., 2007), sodium lactate (Liebowitz et al., 1984; 1985) and isoproterenol (Pohl et al., 1987; Balon et al., 1988; Yeragani et al., 2007). A detailed summary of these techniques is described in Table 4. The pharmacological validation of these techniques, however, lags far well behind developments in animal modelling of anxiety. It is vital that a greater investment is made into fully validating such paradigms both in terms of predictive and face validity. This is one area where the pharmaceutical industries must combine with clinical and basic scientists to really invest substantially in such research (Conn and Roth, 2008). Moreover, with anxiety, we have a clear advantage over depression drug development with the very fact that fear can be relatively easily induced. Moreover, advances in neuroimaging and neurophysiology are unravelling clear circuits that are involved in various anxiety disorders (Schiller and Delgado, 2010; Schiller et al., 2010). The ability of pharmaceutical agents or psychological methods to reverse the patterns of neuronal function associated with anxiety disorders is also at a very limited state, but is a very attractive avenue for future innovation (Murphy, 2010). Moreover, developing this approach to modelling clinical anxiety in humans will greatly facilitate early proof of concept clinical trials.
Table 4.
Modelling anxiety in humans
| Test | Description | Readouts | Behavioural findings | References |
|---|---|---|---|---|
| Chemical | ||||
| Respiratory challenge tests (35% CO2 induced panic/hyperventilation induced panic/breath holding-induced panic) | Inhalation of 35% CO2, hyperventilation and extended breath holding can induce panic attacks in vulnerable patients | Self-reporting of panic type symptoms | Patients suffering from respiratory type panic disorders are more vulnerable to panic attacks in response to these tests. | (Nardi et al., 2006) |
| Caffeine challenge | Consumption of high doses of caffeine can induce panic attacks in vulnerable patients. | Self-reporting of panic type symptoms | GAD patients, PD patients, performance social anxiety disorder patients are particularly vulnerable to caffeine-induced panic attacks | (Lara, 2010) |
| Yohimbine Challenge | Administration of the α2 adrenergic receptor antagonist yohimbine induces panic like symptoms in panic disorder patients and healthy controls | Self reported psychological and somatic panic symptoms, physiological parameters, plasma levels of the noradrenaline metabolite 3-methoxy-4-hydroxyphenylglycol | Yohimbine sensitivity correlates strongly with panic attack frequency in panic disorder patients | (Charney et al., 1984) |
| Sodium lactate challenge | Alterations in the pH and pCO2 induced by IV infusion of sodium lactate results in panic-like symptoms | Self-reporting of panic symptoms | Panic disorder patients are more vulnerable to isproterenol-induced panic attacks than healthy controls. The threshold for sodium lactate induced panic attacks is increased by TCA treatment | (Liebowitz et al., 1984; 1985; Yeragani et al., 1988) |
| Isoproterenol | Infusion of the β-adrenergic agonist isoproternol results in panic-like symptoms in vulnerable patients | Self-reporting of panic response, alterations in cardiac function | Panic disorder patients are more vulnerable to isproterenol-induced panic attacks than healthy controls, as well as greater sensitivity to the arrhythmic effects of the drug | (Pohl et al., 1987; Balon et al., 1988; Yeragani et al., 2007) |
| mCPP | Administration of the 5-HT receptor agonist m-chlorophenylpiperazine results in an anxiety response, largely mediated by activation of the 5-HT2C receptor | Self-reporting of anxiety symptoms, neuroendocrine stress markers | mCPP administration results in an exacerbation of symptoms in obsessive–compulsive disorder at low doses | (Kahn et al., 1990; Erzegovesi et al., 2001) |
| CCK | Administration of CCK results in an anxiety response | Self-reporting of anxiety symptoms, neuroendocrine stress markers | Patients with panic disorder are more vulnerable to the panic-inducing effects of CCK. Effects reduced by benzodiazepines and tiagabine in healthy volunteers. | (Koszycki et al., 1991; Bradwejn and Koszycki, 2001; Rupprecht et al., 2009; Zwanzger et al., 2009) |
| Psycho-social | ||||
| Trier social stress Test (TSST) | The TSST consists of an anticipatory period and a test period where subjects must perform free speech and mental arithmetic in front of a panel of observers. | Neuroendocrine, plasma neurotransmitter and other physiological parameters can indicate the stress response to the TSST. | (Kirschbaum et al., 1993; von Dawans et al., 2010) | |
| Video Recorded Stroop Colour Word Test (VRSCWT) | Subjects are presented with a cognitive task where they are challenged to name the font colour of a series of words. The performance of this test induces a stress response in humans. Attentional bias to emotionally valent and disorder-specific words can be detected. | Physiological parameters, self-reported anxiety levels, attentional bias to specific word stimuli | GAD patients display enhanced emotional bias towards emotional words, whereas social phobia patients display emotional bias to disorder specific (speech related) words. Non-patients with increased levels of self-reported anxiety also display higher emotional bias towards emotionally valent words. | (Tulen et al., 1989; Williams et al., 1996; Leite et al., 1999; Becker et al., 2001; Sass et al., 2010) |
| Dot probe task | Subjects are presented a combination of emotional and neutral facial expressions, and are asked to identify a dot sometimes presented in combination. | Time taken to identify the presentation of the dot in combination with emotional faces can indicate an attentional bias. | Anxiety disorder patients display an attentional bias to emotional facial expressions. Diazepam can reduce this attentional bias in healthy volunteers. | (Bradley et al., 1999; Murphy et al., 2008) |
| Stabilometric analysis of freezing responses | Test subjects are shown a combination of anxiogenic, mutilation and neutral images | Stabilometry is used to detect changes in posture including freezing behaviour and physiological parameters | A freezing-type response is seen in humans confronted by a series of mutilation images. Panic disorder patients display a freezing-like behavioural response in anticipation of seeing the images. | (Azevedo et al., 2005; Lopes et al., 2009) |
| Human behavioural monitor | The test subject is introduced to a novel room containing no chairs, basic furniture and several small items likely to evoke exploration | Locomotor activity, exploratory behaviour and physiological parameters | Both bipolar mania patients and schizophrenia patients display different behavioural patterns to healthy controls. | (Perry et al., 2009) |
| Fear-potentiated startle | Test subjects are presented with a neutral stimulus (CS) in combination with a painful stimulus (US). Startle response is then measured in response to a presentation of the CS alone. | Startle response to is measured both at baseline levels and subsequent to expose to the CS. | PTSD and panic disorder patients display enhanced baseline startle responses, as well as in the offspring of anxiety disorder patients. | (Grillon, 2002; 2008) |
Animal models and tests used in assessing anxiolytic action
By far, the most commonly used species in preclinical anxiety research are the mouse (Mus musculus) and the rat (Rattus norvegicus), although as noted early studies in dogs and pigeons have had their use. Traditionally, rats have been the species of choice for behavioural pharmacology due to the practical considerations of their size and amenity to surgical intervention, as well as superior cognitive ability and superior performance in operant and cognitive tasks. Many commonly used behavioural paradigms were initially developed and validated as screens of anxiolytic activity in the rat before adaptation to use with other species (Cryan and Holmes, 2005). The development of novel genetic modification techniques, developed most extensively in the murine models, has led to a surge in the popularity of the mouse in neuropsychiatric research. The mouse additionally has the advantages as regards ease of breeding, low cost, short generation turnover and smaller size from a drug-dosing perspective (Joyner and Sedivy, 2000; Tarantino and Bucan, 2000; Tecott, 2003; Cryan and Holmes, 2005; Crawley, 2007; Jacobson and Cryan, 2007; Phillips et al., 2007). However, this has brought with it its own logistical problems in terms of difficulty in combining blood collection for pharmacokinetic–pharmacodynamic studies or biomarker analysis. Moreover, the enormous interstrain difference in mouse behaviour across many anxiety tests both under baseline conditions and in response to pharmacological manipulation (Jacobson and Cryan, 2010) can make interpretation of data difficult. The question which invariably arises as to which mouse strain is most like human is not an easy question to try and answer. Thus, it is becoming clear that testing of putative anxiolytic drugs requires testing across multiple strains (and species if possible) to ensure the risk of a false negative. It should be noted also that the manner in which a rodent responds to an anxiety-provoking situation may be qualitatively different to that of humans, but it is becoming clear that many of the same neuronal circuits are recruited (Singewald, 2007). Often, efforts at developing of translational models of anxiety are interpreted as forming completely homologous models in both humans and rodents; while this may be possible in certain domains [e.g. startle response, stress-induced hyperthermia (SIH)], it also may be a very narrow approach and disregards the ethological and species-specific aspects of mouse behaviour (Rodgers et al., 1997). In the next section, we will detail some of the more widely used animal tests for assessing anxiolytic action, which are additionally summarized in Table 1.
Approach–avoidance tests in laboratory animals
Several forms of anxiety test have been employed and validated to measure levels of anxiety in rodents, many of which are designed based on the concept that anxiety disorders represent extreme states of a continuum of anxiety-related behaviour (Cryan and Holmes, 2005). Many tests have an ethological foundation based on the conflict that exists in small rodents, such as rats and mice, between the natural exploratory drive in these animals and aversion to exposed brightly lit environments (Rodgers, 1997). These models emerged over the past 40 years or so and relied on an ethological approach to understanding anxiety as opposed to the pharmacological approaches used in the development of drugs such as the benzodiazepines (see above). Behavioural paradigms based on approach–avoidance conflict include the elevated plus maze (Handley and Mithani, 1984; Pellow et al., 1985; Lister, 1987; Rodgers, 1997; Holmes, 2001; Crawley, 2007), elevated zero maze (Lee and Rodgers, 1990; Shepherd et al., 1994), open-field test, light–dark box test (Crawley, 2007), staircase test (Simiand et al., 1984) and mirrored arena (Rodgers, 1997; Rodgers et al., 1997; Belzung and Griebel, 2001; Crawley, 2007) where avoidance of exposed, brightly lit or elevated areas is measured. The modified hole-board test combines the approach–avoidance aspects of the open field with the addition of board containing several holes which allows for the direct measurement of exploratory behaviour (Ohl et al., 2001a,b). Within these approach avoidance procedures, several species-specific behaviours and postures are quantified and used as behavioural readouts. Reductions in these ‘ethological parameters’ such as head dipping over the edges of elevated apparatuses, rearing and stretch-attend postures regarded as a manifestation of increased anxiety (Shepherd et al., 1994; Rodgers, 1997; Rodgers et al., 1997; Belzung and Griebel, 2001). Ethological analysis is taken to its extreme in the measurement of mouse risk assessment, flight and defensive attack behaviour following threat cue exposure in the mouse defence test battery (MDTB) (Blanchard, 2003). Apprehension and heightened levels of vigilance are frequently a component of anxiety disorders, and measurement of risk assessment behaviour, indexed by relevant ethological parameters, is regarded as a model of this endophenotype (Rodgers, 1997; Blanchard, 2003; Cryan and Holmes, 2005).
Although the constructs underling each of these approach–avoidance tests, it is important to emphasize that the pharmacology and underlying neurobiology are not necessarily identical. To add to the complexity, large species and strain differences occur. Thus, it is very difficult to define which test is the best to model human anxiety responses. This necessitates the use of a battery-style approach for assessing novel pharmacological agents. However, questions always emerge if a compound is showing an anxiolytic effect in more tests, is it going to be more effective in the clinic? The reciprocal experiences of researchers with SSRIs [very little activity (Borsini et al., 2002)] and NK1 receptor antagonists [activity in a number of tests (Varty et al., 2002; Vendruscolo et al., 2003; Heldt et al., 2009)] would suggest not.
Conflict-based anxiety tests in laboratory animals
Conflict-based models have been among the most sensitive to GABAergic manipulation and have been played an important role in assessing anxiolytic potential. Since the time of Sigmund Freud, many theories have been introduced to explain the relationship between anxiety and internal conflict (Sato, 2005), especially in relation to psychodynamic theories. Freud (1966) discussed internal conflict in relation to the three structures of the mind. Anxiety according to this view is caused by the psychic tension among the forces representative of the id, ego and superego. Another commonly discussed theory concerning the relationship between internal conflict and anxiety is Alfred Adler's (1954) theory of inferiority. In his work, Adler discussed the process of how our primary internal conflicts are caused by various feelings of inferiority in great detail. Adler suggests that these feelings of inferiority are also assumed to be one of the common causes of anxiety. Sato (2005) describes conflict situations within a framework that when something is consistent with our desires, we feel comfortable. When something is inconsistent with our desires, we feel anxiety. Therefore, internal conflict can be conceptualized using two constructs: (i) what we desire; and (ii) what has, is or could happen. When what we desire matches what has, is or could happen, we feel comfortable. When what we desire does not match what has, is or could happen, we feel anxiety. As is evident from the way the model is worded, this applies regardless of whether we are dealing with events in the past, present or future.
Therefore, conflict situations, in which a subject experiences two opposing impulses, are a common and clinically relevant feature of anxiety, and therefore employed in many models employed for the detection of anxiolytic agents in rodents. In conflict-based tests of anxiety in laboratory animals, subjects receive a punishment (mild electric shock) leading to suppression of a conditioned (learned) response for reinforcement (food or water) (Rodgers, 1997). Punishment-based conflict procedures have been employed for over 50 years in the identification and characterization of anxiolytic agents (Geller and Seifter, 1960). Studies demonstrated that when rats are trained to lever press for a food reward, during the ‘conflict’ component, responses are inhibited by concomitant, mild electric shocks. This paradigm is known as the Geller–Seifter test. Anxiolytic properties are deduced for drugs that selectively enhance punished responses in the presence of shock as compared to unpunished responses emitted in its absence. Benzodiazepines and barbiturates were initially demonstrated to exert specific anxiolytic properties active in the Geller–Seifter test, and, subsequently, many classes of potential anxiolytic agent have been characterized employing this procedure. However, major disadvantages remain: (i) the necessity for long-term (months) and daily training of subjects; and (ii) their repeated utilization. That is, exposure to drugs may modify the actions of those subsequently evaluated.
In an effort to overcome these problems, Vogel et al. (1971) developed a novel conflict procedure in which male rats were water deprived for 48 h and, during a test session of 3 min, drinking was punished by a mild, but aversive shock delivered via the spout of the bottle every 20 licks. Accordingly, a specific, drug-induced increase in the number of shocks taken was considered to reflect anxiolytic properties. Today, Vogel et al.'s (1971) test is one of the most widely used tests for assessing anxiolytic activity in rodents (Millan and Brocco, 2003).
A similar conflict procedure is the four-plate test, where the drive to explore a novel environment is conflicted with the drive to avoid floor-delivered foot shocks (Ripoll et al., 2006). Defensive marble and shock-probe burying tests, where animals bury novel or aversive items, differ from other tests of anxious behaviour in that an active behaviour (i.e. burying is used as an index of anxiety as opposed to other tests relying on passive avoidance behaviour). It should, however, be noted that controversy exists as to the precise nature of the behaviour elicited in the defensive marble burying assay in mice. It has been argued that this assay may be more ethologically relevant to obsessive–compulsive disorder than to the rest of the anxiety disorders (Witkin, 2008), or may represent a species-specific repetitive and perseverative behaviour with little correlation to anxiety levels of anxiety-like behaviour (Thomas et al., 2009). As such, they make valuable additions to anxiety test batteries (Broekkamp et al., 1986; Sluyter et al., 1996; 1999; Spooren et al., 2000; Jacobson et al., 2007).
Other anxiety tests in laboratory animals
Anxiolytic activity in many of the mentioned tests can be confounded by aspects of altered locomotor activity induced by genetic or pharmacological manipulations (Cryan and Holmes, 2005; Holmes and Cryan, 2006; Jacobson and Cryan, 2010). Thus, it is important to consider other tests in battery-style approaches that are less dependent on motor outputs. The following are some of the more widely used.
Hyponeophagia
Hyponeophagia, the suppression of eating due to anxiety-related states caused by novelty, can be assessed by measuring the latency to begin eating in a variety of potentially anxiogenic situations. Once again, it is a conflict-based model that has a long history in the assessment of emotionality and anxiety with Hall (1934) observing an inverse relationship between feeding and defecation in animals exposed to a novel environment. In hyponeophagia, tests the level of anxiety-related stimuli is manipulated by using novel food and by conducting the experiment in novel, potentially anxiogenic environments (Bannerman et al., 2002; 2003; Deacon and Rawlins, 2005; Dulawa and Hen, 2005; Finger et al., 2010). These paradigms are ethologically relevant and therefore do not require complex training procedures, are not confounded by painful stimuli, are simple to conduct and are relatively cost-effective. Hyponeophagia-based models are conducted either by presenting chow to food-deprived animals, or by presenting a highly palatable and familiar food to satiated animals, and measuring the latency to feed and/or the amount eaten in a novel environment. The same dependent measures should also be assessed in the home environment to control for effects of the independent variable on appetite. As in all anxiety assays, inbred mouse strains show baseline differences in levels of hyponeophagia (Trullas and Skolnick, 1993). A number of genetic manipulations resulting in anxious phenotypes including leptin-deficient mice (Finger et al., 2010), 5-HT1A receptor (Gross et al., 2000) and the NK1 receptor (Santarelli et al., 2002) have also increased hyponeophagia.
Several variations of hyponeophagia-based behavioural paradigms exist. These include the novelty-suppressed feeding paradigm where animals are presented with normal food in a novel anxiogenic environment, and the latency to begin eating is taken as an index of the anxiety state of the animal (Bodnoff et al., 1988; 1989; Gross et al., 2002). Repeated exposures to hyponeophagia paradigms in different environments and to different food stuffs can be used to modulate the levels of anxiety generated in these paradigms in order to optimize the sensitivity of the behavioural output in this test (Deacon and Rawlins, 2005; Finger et al., 2010). Another variant is the novelty-induced hypophagia model in which animals are trained to consume a highly palatable food, such as sweetened milk, and then later presented this food in a novel aversive environment (Soubríe et al., 1975; Gross et al., 2002; Santarelli et al., 2003).
Separation-induced ultrasonic vocalizations
Rodent pups produce vocalizations in the ultrasonic range when separated from their mother and littermates. This distress behaviour is intended to elicit maternal attention and retrieval, as well as to modulate maternal care behaviour by stimulating prolactin production (Noirot, 1972; Hashimoto et al., 2001; Farrell and Alberts, 2002). These distress behaviours can be recorded and analysed both quantitatively and qualitatively in order to measure levels of distress-like behaviour in both infant mice and rats (Groenink et al., 2008; Scattoni et al., 2009). Suppression of USV emission is an ethologically valid marker of anxiolytic drug efficacy (Groenink et al., 2008). Anxiolytic effects in this test can be elicited with administration of benzodiazepines, 5-HT1A receptor agonists and SSRIs. Agents acting on the noradrenergic system, such as tricyclic antidepressants, are, however, less consistent in their anxiolytic effects (Borsini et al., 2002). USV reduction is also seen with administration of a range of putative anxiolytic agents, including NK1 receptor antagonists (Groenink et al., 2008).
Measurement of USV production in response to painful or stressful stimuli has also been proposed as a potential measure of anxiety behaviour in adult rodents. 5-HT1A receptor antagonists and SSRI antidepressants are effective at suppressing USV emission in this model, although benzodiazepines have limited effects (Sánchez, 2003).
SIH
A key element of the adaptive anxiety response is activation of the autonomic nervous system and subsequent physiological responses including an increase in body temperature. This process is conserved across mammalian species, including rodents and humans. Measurement of the hypothermic response generated subsequent to stressful stimuli represents a translationally valid and useful approach to modelling anxiety disorders (Bouwknecht et al., 2007; Vinkers et al., 2008). The hypothermic response to stress can be attenuated using benzodiazepines, as well as buspirone and ethanol (Spooren et al., 2002), and chronic, but not acute, SSRI treatment (Conley and Hutson, 2007). The SIH paradigm is additionally sensitive to the effects of numerous putative anxiolytic agents (Spooren et al., 2002), as well as providing a useful technique for exploring the role of individual neurotransmitter systems in the anxiety response (Vinkers et al., 2010).
Fear conditioning-based models of anxiety
Alterations in conditioned fear learning and cognitive defects form an important facet of the clinical manifestation of anxiety disorders (American Psychiatric Association, 2000; Lang et al., 2000). These include inappropriate processing of potentially threatening stimuli in GAD, panic disorder and phobias, as well as the long-term salience of traumatic memories seen in PTSD. In order to model these aspects of anxiety disorders, several conditioned tests of anxiety, such as Pavlovian fear conditioning, have been developed and validated (Cryan and Holmes, 2005; Ledgerwood et al., 2005; Delgado et al., 2006; O'Connor et al., 2010). More recently, the discovery that insular cortex dysfunction may play a role in anxiety disorders (Paulus and Stein, 2006) has led to the insular cortex-dependent, conditioned taste aversion paradigm becoming more widely used (Bermúdez-Rattoni et al., 2004; Guitton and Dudai, 2004; Mickley et al., 2004; Yasoshima and Yamamoto, 2005; Jacobson et al., 2006; Hefner et al., 2008).
Conditioned fear paradigms revolve around the association of innocuous stimuli, such as a tone or palatable taste (conditioned stimulus), with a painful or stressful stimulus, such as a foot shock or chemically induced malaise (unconditioned stimulus). Levels of conditioned fear generated in these paradigms are indexed by a number of behavioural outputs, including conditioned freezing, fear-potentiated startle, active defensive behaviours, vocalizations, physiological responses, as well as alterations in sucrose preference in the conditioned taste aversion paradigm (Fendt and Fanselow, 1999; Cryan and Holmes, 2005). Sleep disturbances form part of the diagnostic criteria for several forms of anxiety disorder (American Psychiatric Association, 2000; Prut and Belzung, 2003), and also represent sensitive output in fear-conditioning paradigms (Sanford et al., 2003a,b).
Extinction of the fear response generated in fear conditioning and conditioned taste aversion paradigms is of particular use in modelling the persistence of traumatic memories associated with PTSD and panic disorder (Ressler et al., 2004; Barad, 2005; Cryan and Holmes, 2005; Ledgerwood et al., 2005; Delgado et al., 2006; Jacobson et al., 2006).The efficacy of the NMDA receptor antagonist d-cycloserine in facilitating fear extinction in both the rat fear potentiated startle paradigm and in human acrophobic patients indicate a predictive validity for this approach (Ressler et al., 2004; Ledgerwood et al., 2005; Davis et al., 2006). For further information, see review from Graham et al. (2010) in this issue.
Pharmacological validation
A major criticism of many of the behavioural tests discussed here is that while they display robust responsivity to the benzodiazepine anxiolytics and thus display predictive validity in this respect (Cryan and Holmes, 2005; Crawley, 2007), their predictive validity in respect of other classes of clinically used anxiolytic drugs, in particular SSRI antidepressants, is lacking (Borsini et al., 2002). This is based on the variable effects produced by SSRI across the spectrum of anxiety tests with the notable exceptions of the USV suppression, MDTB and defensive marble burying paradigms (Rodgers, 1997; Borsini et al., 2002; Blanchard, 2003; Markou et al., 2009). It should, however, be remembered that many of the currently used behavioural tests were developed based on prevailing clinical practice that clearly distinguished between the anxiety disorders and depression in terms of both symptom presentation and treatment, with the gold standard of predictive validity as benzodiazepine sensitivity (Treit et al., 2010). Nevertheless, a variety of putative anxiolytic compounds have shown efficacy in a variety of SSRI-sensitive behavioural tests. None, however, have made the transition to clinic as of yet (Table 2). Rodgers (2010) outlines several pitfalls in overemphasizing pharmacologically predictive validity as a marker of a valid test. Predictive validity is by its nature a retrospective assessment, a flaw in itself and that putting too much stead on it comes with the risk that active compounds will never be truly novel in their mode of action. An additional risk is that tests whose sole merit is their predictive validity for either benzodiazepine or chronic SSRI treatment may in reality be simple models of SERT or GABAA receptor pharmacology (Rodgers, 2010).
Pharmacological agents used to induce anxiety behaviour in animals include CCK (Rupprecht et al., 2009), sodium lactate (Shekhar et al., 1996), the serotonin antagonist mCPP (Gatch, 2003), as well as PTZ (Jung et al., 2002). CCK, mCPP and sodium lactate also provoke panic-like responses in humans (Liebowitz et al., 1984; Kahn et al., 1990; Koszycki et al., 1991), and thus are viewed as potential animal models of panic disorder. However, it should be noted that the lactate model needs the added manipulation in animals of surgically manipulating GABA in the dorsomedial hypothalamus to prime for the panic-inducing effects of lactate (Shekhar et al., 1996).
Caveats in preclinical models of anxiolytics action
Alterations across the entire behavioural repertoire following pharmacological or genetic intervention can often confound the analysis of anxiety behaviour (Bouwknecht and Paylor, 2008). It is critical that such confounding behaviours are taken into account in interpreting the effects of any agent in a test. Outputs, such as freezing or exploratory behaviour, and other locomotor-based behaviours are particularly vulnerable to obfuscation by alterations in locomotor function, making the assessment of the locomotor effects of any novel intervention paramount (Cryan and Holmes, 2005; Holmes and Cryan, 2006; Jacobson and Cryan, 2007). However, some tasks, such as the elevated plus maze, have in-built parameters that take into account any locomotor-altering effects of a drug (Hogg, 1996; Rodgers et al., 1997). Disruption of cognitive function by non-specific drug actions can disrupt learning-dependant and exploration-dependant behavioural outputs, confusing the analysis of data from assays of both innate and conditioned anxiety (Jacobson et al., 2007; Bouwknecht and Paylor, 2008). Alterations to the sensory system of the animal can have marked effects where nociception (foot shock-based paradigms) or olfactory (conditioned taste aversion) function is vital to proper responsiveness to the test. Alterations to basal temperature can confound results obtained from the SIH paradigm (Vinkers et al., 2008), whereas alterations to feeding behaviour and satiety function can influence behaviour in hyponeophagia paradigms (Dulawa and Hen, 2005).
Similar advice is needed for the assessment of genetically modified animals in tests of anxiety, and is vital in avoiding erroneous interpretations of behavioural data. A thorough determination of any confounding abnormalities present in genetically modified animals prior to behavioural testing is vital (Crawley, 2007). Other factors that can influence behaviour in anxiety tests are early life experience, previous test exposure and compensatory changes in the case of transgenic animals (Cryan and Holmes, 2005; Holmes and Cryan, 2006; Jacobson and Cryan, 2007). Use of a test battery encompassing multiple anxiety tests exploring different aspects of anxiety behaviour has thus been suggested as an approach to detect genuine behavioural effects (Cryan and Holmes, 2005; Arguello and Gogos, 2006; Bouwknecht and Paylor, 2008). It must be remembered, however, when designing such a battery, as prior test experiences can influence behaviour and markedly alter responsivity to pharmacological agents in a number of behavioural paradigms (Holmes and Rodgers, 1998; Holmes et al., 2001). Battery approaches where tests used progress from least stressful to most stressful, while considering which tests are more vulnerable to the effects of prior testing, and which allow for sufficient recovery time between tests, can overcome this potential caveat (Bouwknecht and Paylor, 2008).
Conclusions and future directions
Despite the present dizzying array of behavioural paradigms for use in anxiolytic drug discovery, recent advances in animal modelling are yet to translate into novel pharmacological therapies in the clinic. A multitude of reasons may underlie this apparent stagnation. These include concerns as to the ethological and predictive validity of the techniques at our disposal. The apparent insensitivity of many of the tests to commonly used clinical anxiolytics, such as the SSRIs, is a major concern (Borsini et al., 2002), and questions still linger as to the fact that the maintenance of anxiety disorders is ultimately based on cognitive processes only present in humans, as evidenced by the efficacy of cognitive treatments in humans (Steckler et al., 2008). In order to advance the efforts in drug discovery, we must both re-assess our concepts of validity (Treit et al., 2010). It is also important to remember that our animal models are only as valid as the knowledge of the neurobiology and pathophysiology of clinical anxiety disorders that underpin their design.
With this in mind, it is clear that animal models will play a role in the emergence of the next anxiolytic drug class, but only as a component of a broad multidisciplinary approach where advances in animal models are informed by insight into pathophysiological basis, as well as the neurobiological and behavioural correlates of the clinical disease. A recent example is that shown by Rupprecht et al. (2009), where in vitro electrophysiology, animal behavioural experiments and human studies are combined in the investigation of novel therapeutic avenue for panic disorder, translocator protein (18 kD) ligands.
Indeed, at a molecular and electrophysiological level, great inroads are being made into delineating the circuits underlying amygdala-dependent fear memory (Phelps and LeDoux, 2005; Sigurdsson et al., 2007; Herry et al., 2008; Ehrlich et al., 2009; Davis et al., 2010; Haubensak et al., 2010; Pape and Pare, 2010; Parsons and Davis, 2011; Sierra-Mercado et al., 2011), which are already being paralleled with imaging studies in humans (Phelps and LeDoux, 2005; Davis et al., 2010). It is only appropriate to mention here that despite the failings of animal research in generating a new anxiolytic drug class to date, it has proven a highly successful platform for advancing our knowledge of the neurobiology of anxiety and fear (Rodgers, 2010). The next step will be to advance such basic neuroscience approaches into clinical drug development. Interestingly, and for reasons not apparently clear, similar approaches in non-cognitive-based models have failed to advance in the same manner. If there is a continued reliance on behavioural outputs in such models for drug discovery efforts, it is crucial that knowledge is gleaned on how certain anxiolytic work (or not) in them. The combination of behaviour with imaging techniques, such as c-Fos immunohistochemistry, is becoming more sophisticated and will also play a role in the future delineation of anxiety circuits in the brain (Reijmers et al., 2007; Singewald, 2007).
In conclusion, animal models have played a role in the development of some anxiolytic drugs, such as the benzodiazepines and buspirone; however, their relative contribution to future drug development will only be accentuated as part of a complete research program combining genetic signalling pathways, electrophysiology, brain neurochemistry, neuroimaging and behaviour. For this approach to bear fruit, there is also an onus on preclinical researchers to ensure that novel clinical insights into the aetiology of anxiety disorders appropriately inform both the design and use of animal models and tests (Rodgers, 2010). It is imperative to state that in addition to refining the predictive efficacy of the animal tests used in anxiety research, preclinical scientists are impressed on ethical grounds to actively innovate in replacement of current models and tests with lower species or non-animal techniques, refinement of procedures in order to minimize animal suffering and reducing the number of animals required to generate data – the 3Rs (Goldberg et al., 1996). Moreover, while we clearly point out some of the drawbacks of animal tests of anxiolytics action in this review, it would be remiss not to mention the impediment to preclinical research posed by the lack of conceptual clarity and stability at the clinical level. Reverse translation, from clinic to animal model in psychiatry, is not yet as fully developed as it should, and this will be crucially important in determining the true translational nature of animal models and aid in the development of novel treatment strategies that will counter the vast health problems in this age of anxiety.
Acknowledgments
The authors would like to thank Beate C. Finger, Richard M. O'Connor and Olivia F. O'Leary for careful reading of the manuscript and for valuable discussions. John F. Cryan and Fabian F. Sweeney are supported by the European Community's Seventh Framework Programme; grant number: FP7/2007–13, grant agreement 201714.
Glossary
Abbreviations
- 5-HT
5 hydroxytryptamine (serotonin)
- 8-OH-DPAT
8-hydroxy-N,N-dipropyl-2-aminotetralin
- CCK
cholecystokinin
- EPM
elevated plus maze
- GAD
generalized anxiety disorder
- GAT-1
GABA transporter 1
- LAB/HAB
low anxiety bred/high anxiety bred
- MCH1
melanin-concentrating hormone receptor 1
- mCPP
m-chlorophenylpiperazine
- MDTB
mouse defence test battery
- NK1
neurokinin 1
- NMDA
n-methyl-d-aspartate
- PTSD
post-traumatic stress disorder
- PTZ
pentylenetetrazol
- SSRI
selective serotonin re-uptake inhibitor
- TSST
trier social stress test
- USV
ultrasonic vocalization
Conflict of interest
The authors declare no conflict of interest.
References
- Acevedo SF, Pfankuch T, Ohtsu H, Raber J. Anxiety and cognition in female histidine decarboxylase knockout (Hdc–/–) mice. Behav Brain Res. 2006;168:92–99. doi: 10.1016/j.bbr.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Adler A. Understanding Human Nature: The Psychology of Personality. New York: Fawcett Books; 1954. [Google Scholar]
- Alonso J, Lépine J. Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD) J Clin Psychiatry. 2007;68:s3–s9. [PubMed] [Google Scholar]
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;109:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Roesler R. Targeting the NMDA receptor for fear-related disorders. Recent Pat CNS Drug Discov. 2008;3:166–178. doi: 10.2174/157488908786242470. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition 4th Ed. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- de Angelis L. Experimental anxiety and antidepressant drugs: the effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:379–383. doi: 10.1007/BF00171072. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Aprison MH, Ferster CB. Neurochemical correlates of behavior II. Correlation of brain monoamine oxidase activity with behavioural changes after iproniazid and 5-hydroxytryptophan administration. J Neurochem. 1961;6:350–357. doi: 10.1111/j.1471-4159.1961.tb13486.x. [DOI] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Aron C, Simon P, Larousse C, Boissier JR. Evaluation of a rapid technique for detecting minor tranquilizers. Neuropharmacology. 1971;10:459–469. doi: 10.1016/0028-3908(71)90074-8. [DOI] [PubMed] [Google Scholar]
- Atack J, Wafford K, Street L, Dawson G, Tye S, Van Laere K, et al. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J Psychopharmacol. 2011;25:314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- Auden WH. The Age of Anxiety: A Baroque Eclogue. New York: Random House; 1947. [Google Scholar]
- Azevedo TM, Volchan E, Imbiriba LA, Rodrigues EC, Oliveira JM, Oliveira LF, et al. A freezing-like posture to pictures of mutilation. Psychophysiology. 2005;42:255–260. doi: 10.1111/j.1469-8986.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Ajel KI, Garner M. Pharmacological treatment of generalized anxiety disorder. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Heidelberg, Germany: Springer-Verlag; 2010. pp. 453–467. Current Topics in Behavioural Neurosciences. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Woolley ML, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology (Berl) 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- Balon R, Pohl R, Yeragani VK, Rainey JM, Weinberg P. Lactate- and isoproterenol-induced panic attacks in panic disorder patients and controls. Psychiatry Res. 1988;23:153–160. doi: 10.1016/0165-1781(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RMJ, Offen S, Friswell J, Grubb M, Rawlins JNP. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Barros M, Maior RS, Huston JP, Tomaz C. Predatory stress as an experimental strategy to measure fear and anxiety-related behaviors in non-human primates. Rev Neurosci. 2008;19:157–169. doi: 10.1515/revneuro.2008.19.2-3.157. [DOI] [PubMed] [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: general emotional or disorder specificity? J Anxiety Disord. 2001;15:147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Benvenga MJ, Overshiner CD, Monn JA, Leander JD. Disinhibitory effects of LY354740, a new mGluR2 agonist, on behaviors suppressed by electric shock in rats and pigeons. Drug Dev Res. 1999;47:37–44. [Google Scholar]
- Bermúdez-Rattoni F, Ramírez-Lugo L, Gutiérrez R, Miranda MI. Molecular signals into the insular cortex and amygdala during aversive gustatory memory formation. Cell Mol Neurobiol. 2004;24:25–36. doi: 10.1023/B:CEMN.0000012722.45805.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Adams N. The Maudsley reactive and nonreactive strains: a new perspective. Behav Genet. 2002;32:277–299. doi: 10.1023/a:1020206120248. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- van Bogaert MJV, Groenink L, Oosting RS, Westphal KGC, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Boren JJ, Sidman M, Herrnstein RJ. Avoidance, escape, and extinction as functions of shock intensity. J Comp Physiol Psychol. 1959;52:420–426. doi: 10.1037/h0042727. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Bourin M, Redrobe JP, Hascoet M, Baker GB, Colombel MC. A schematic representation of the psychopharmacological profile of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1389–1402. doi: 10.1016/s0278-5846(96)00134-0. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav Pharmacol. 2008;19:385–402. doi: 10.1097/FBP.0b013e32830c3658. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38(Pt 3):267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D. Cholecystokinin and panic disorder: past and future clinical research strategies. Scand J Clin Lab Invest Suppl. 2001;234:19–27. [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Mannoury la Cour C, Touzard M, Girardon S, Veiga S, et al. Cellular and behavioural profile of the novel, selective neurokinin1 receptor antagonist, vestipitant: a comparison to other agents. Eur Neuropsychopharmacol. 2008;18:729–750. doi: 10.1016/j.euroneuro.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Camp M, Norcross M, Whittle N, Feyder M, D'Hanis W, Yilmazer-Hanke D, et al. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes Brain Behav. 2009;8:744–752. doi: 10.1111/j.1601-183X.2009.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, et al. Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto E. Use of the elevated T-maze to study anxiety in mice. Behav Brain Res. 2004;148:119–132. doi: 10.1016/s0166-4328(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Breier A. Noradrenergic function in panic anxiety. Effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch Gen Psychiatry. 1984;41:751–763. doi: 10.1001/archpsyc.1984.01790190025003. [DOI] [PubMed] [Google Scholar]
- Chiu C, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka-Wójcik E, Kłodzinska A, Pilc A. Glutamate receptor ligands as anxiolytics. Curr Opin Investig Drugs. 2001;2:1112–1119. [PubMed] [Google Scholar]
- Commissaris RL, McCloskey TC, Harrington GM, Altman HJ. MR/Har and MNRA/Har Maudsley rat strains: differential response to chlordiazepoxide in a conflict task. Pharmacol Biochem Behav. 1989;32:801–805. doi: 10.1016/0091-3057(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Commissaris RL, Franklin L, Verbanac JS, Altman HJ. Maudsley reactive (MR/Har) and nonreactive (MNRA/Har) rats: performance in an operant conflict paradigm. Physiol Behav. 1992;52:873–878. doi: 10.1016/0031-9384(92)90364-8. [DOI] [PubMed] [Google Scholar]
- Commissaris RL, Verbanac JS, Markovska VL, Altman HJ, Hill TJ. Anxiety-like and depression-like behavior in Maudsley reactive (MR) and non-reactive (NMRA) rats. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:491–501. doi: 10.1016/0278-5846(96)00012-7. [DOI] [PubMed] [Google Scholar]
- Commissaris RL, Ardayfio PA, McQueen DA, Gilchrist GA, Overstreet DH. Conflict behavior and the effects of 8-OHDPAT treatment in rats selectively bred for differential 5-HT(1A)-induced hypothermia. Pharmacol Biochem Behav. 2000;67:199–205. doi: 10.1016/s0091-3057(00)00314-2. [DOI] [PubMed] [Google Scholar]
- Conley RK, Hutson PH. Effects of acute and chronic treatment with fluoxetine on stress-induced hyperthermia in telemetered rats and mice. Eur J Pharmacol. 2007;564:138–145. doi: 10.1016/j.ejphar.2007.02.063. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Roth BL. Opportunities and challenges of psychiatric drug discovery: roles for scientists in academic, industry, and government settings. Neuropsychopharmacology. 2008;33:2048–2060. doi: 10.1038/sj.npp.1301638. [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Craft RM, Howard JL, Pollard GT. Conditioned defensive burying as a model for identifying anxiolytics. Pharmacol Biochem Behav. 1988;30:775–780. doi: 10.1016/0091-3057(88)90098-6. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley-Liss; 2007. [Google Scholar]
- Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull. 1982;8:609–612. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don't worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Masur J. Evaluation of psychotropic drugs with a modified open field test. Pharmacology. 1978;16:259–267. doi: 10.1159/000136777. [DOI] [PubMed] [Google Scholar]
- Czech DA, Quock RM. Nitrous oxide induces an anxiolytic-like effect in the conditioned defensive burying paradigm, which can be reversed with a benzodiazepine receptor blocker. Psychopharmacology (Berl) 1993;113:211–216. doi: 10.1007/BF02245699. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Descent of Man. London: Penguin Classics; 1871. [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals. New York: General Books LLC; 1872. [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, Heinrichs M. The trier social stress test for groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2010;36:514–522. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156:241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Furlan C. The anxiolytic-like properties of two selective MAOIs, moclobemide and selegiline, in a standard and an enhanced light/dark aversion test. Pharmacol Biochem Behav. 2000;65:649–653. doi: 10.1016/s0091-3057(99)00237-3. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Brocco M, Adhumeau A, Gobert A, Millan MJ. The selective serotonin (5-HT)1A receptor ligand, S15535, displays anxiolytic-like effects in the social interaction and Vogel models and suppresses dialysate levels of 5-HT in the dorsal hippocampus of freely-moving rats. A comparison with other anxiolytic agents. Psychopharmacology (Berl) 2000;152:55–66. doi: 10.1007/s002130000449. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Mannoury la Cour C, Gobert A, Brocco M, Lejeune F, Serres F, et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology (Berl) 2008;199:549–568. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Depino AM, Tsetsenis T, Gross C. GABA homeostasis contributes to the developmental programming of anxiety-related behavior. Brain Res. 2008;1210:189–199. doi: 10.1016/j.brainres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, et al. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. J Pharmacol Exp Ther. 1986;237:649–658. [PubMed] [Google Scholar]
- Dierssen M, Gratacòs M, Sahún I, Martín M, Gallego X, Amador-Arjona A, et al. Transgenic mice overexpressing the full-length neurotrophin receptor TrkC exhibit increased catecholaminergic neuron density in specific brain areas and increased anxiety-like behavior and panic reaction. Neurobiol Dis. 2006;24:403–418. doi: 10.1016/j.nbd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Does the melanin-concentrating hormone antagonist SNAP-7941 deserve 3As? Expert Opin Investig Drugs. 2003;12:1035–1038. doi: 10.1517/13543784.12.6.1035. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33:1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Pfankuch T, Wilson JM, Grabell J, Chhajlani V, Brown DG, et al. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Behav Brain Res. 2010;212:168–173. doi: 10.1016/j.bbr.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr Pharm Des. 2009;15:1647–1674. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Increased levels of anxiety-related behaviors in a tsc2 dominant negative transgenic mouse model of tuberous sclerosis. Behav Genet. 2010 doi: 10.1007/s10519-010-9398-1. [Epub ahead of print] doi: 10.1007/s10519-010-9398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Behavioral and genetic investigations of low exploratory behavior in Il18r1(–/–) mice: we can't always blame it on the targeted gene. Brain Behav Immun. 2010;24:1116–1125. doi: 10.1016/j.bbi.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin GN, Cooper BR. Use of conditioned taste aversion as a conflict model: effects of anxiolytic drugs. J Pharmacol Exp Ther. 1988;245:137–146. [PubMed] [Google Scholar]
- Erzegovesi S, Martucci L, Henin M, Bellodi L. Low versus standard dose mCPP challenge in obsessive–compulsive patients. Neuropsychopharmacology. 2001;24:31–36. doi: 10.1016/S0893-133X(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Eser D, Schüle C, Baghai T, Floesser A, Krebs-Brown A, Enunwa M, et al. Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study. Psychopharmacology (Berl) 2007;192:479–487. doi: 10.1007/s00213-007-0738-7. [DOI] [PubMed] [Google Scholar]
- España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, et al. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Farrell WJ, Alberts JR. Stimulus control of maternal responsiveness to norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol. 2002;116:297–307. doi: 10.1037/0735-7036.116.3.297. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, et al. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Filip M, Baran L, Siwanowicz J, Chojnacka-Wójcik E, Przegaliński E. The anxiolytic-like effects of 5-hydroxytryptamine3 (5-HT3) receptor antagonists. Pol J Pharmacol Pharm. 1992;44:261–269. [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010;210:559–568. doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- Finn DA, Rutledge-Gorman MT, Crabbe JC. Genetic animal models of anxiety. Neurogenetics. 2003;4:109–135. doi: 10.1007/s10048-003-0143-2. [DOI] [PubMed] [Google Scholar]
- Frantz S. Therapeutic area influences drug development costs. Nat Rev Drug Discov. 2004;3:466–467. doi: 10.1038/nrd1436. [DOI] [PubMed] [Google Scholar]
- Freud S. Introductory Lectures on Psychoanalysis Standard Edition. New York: Liveright; 1966. [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB. Discriminative stimulus effects of m-chlorophenylpiperazine as a model of the role of serotonin receptors in anxiety. Life Sci. 2003;73:1347–1367. doi: 10.1016/s0024-3205(03)00422-3. [DOI] [PubMed] [Google Scholar]
- Geller I, Seifter S. The effects of meprobamate, barbiturate, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacologia. 1960;1:482–492. [Google Scholar]
- Geller I, Seifter J. The effects of mono-urethans, di-urethans and barbiturates on a punishment discrimination. J Pharmacol Exp Ther. 1962;136:284–288. [PubMed] [Google Scholar]
- Geller EB, Kulak JT, Seifter J. The effects of chlordiazepoxide and chlorpromazine on a punishment discrimination. Psychopharmacologia. 1962;3:374–385. doi: 10.1007/BF00408322. [DOI] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychophamacology: The Fourth Generation of Progress. New York: Raven; 2000. pp. 787–798. [Google Scholar]
- Geyer MA, Markou A. The role of preclinical models in the development of psychotropic drugs. In: Davis KL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. New York: Lippincott Williams & Wilkins; 2002. p. 445. [Google Scholar]
- Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, Hermans D, et al. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol Learn Mem. 2008;90:103–111. doi: 10.1016/j.nlm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Manian AA, Efron DH. A comparative study of certain pharmacologic responses following acute and chronic administrations of chlordiazepoxide. Life Sci. 1967;6:481–491. doi: 10.1016/0024-3205(67)90051-3. [DOI] [PubMed] [Google Scholar]
- Goldberg AM, Zurlo J, Rudacille D. The three Rs and biomedical research. Science. 1996;272:1403. doi: 10.1126/science.272.5267.1403. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Marrer E, Kehren J, Kinnunen A, Imbert G, Hillebrand R, et al. Central nervous system drug development: an integrative biomarker approach toward individualized medicine. NeuroRx. 2005;2:683–695. doi: 10.1602/neurorx.2.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Netto CF, Zangrossi H. The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Parente A, Del-Ben CM, Guimarães FS. Pharmacology of human experimental anxiety. Braz J Med Biol Res. 2003;36:421–432. doi: 10.1590/s0100-879x2003000400003. [DOI] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: preclinical models. Br J Pharmacol. 2010;164:1230–1247. doi: 10.1111/j.1476-5381.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Agnes RS, Blanchard RJ. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology (Berl) 1995a;120:57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard D, Jung A, Blanchard R. A model of ‘antipredator’ defense in Swiss-Webster mice: effects of benzodiazepine receptor ligands with different intrinsic activities. Behav Pharmacol. 1995b;6:732–745. [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Behavioural profiles in the mouse defence test battery suggest anxiolytic potential of 5-HT(1A) receptor antagonists. Psychopharmacology (Berl) 1999;144:121–130. doi: 10.1007/s002130050984. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Verdouw PM, van Oorschot R, Olivier B. Models of anxiety: ultrasonic vocalizations of isolated rat pups. In: Enna S, Williams M, Barret JF, Ferkany JW, Kenakin T, Porsolt RD, editors. Current Protocols in Pharmacology. Hoboken, NJ: John Wiley & Sons; 2008. DOI: 10.1002/0471141755.ph0518s43. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Dudai Y. Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry. 2004;56:901–904. doi: 10.1016/j.biopsych.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Hall C. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Hanson D. Librium. Chem Eng News. 2005;83:80. [Google Scholar]
- Härter MC, Conway KP, Merikangas KR. Associations between anxiety disorders and physical illness. Eur Arch Psychiatry Clin Neurosci. 2003;253:313–320. doi: 10.1007/s00406-003-0449-y. [DOI] [PubMed] [Google Scholar]
- Hascoët M, Bourin M, Nic Dhonnchadha BA. The influence of buspirone, and its metabolite 1-PP, on the activity of paroxetine in the mouse light/dark paradigm and four plates test. Pharmacol Biochem Behav. 2000a;67:45–53. doi: 10.1016/s0091-3057(00)00293-8. [DOI] [PubMed] [Google Scholar]
- Hascoët M, Bourin M, Colombel MC, Fiocco AJ, Baker GB. Anxiolytic-like effects of antidepressants after acute administration in a four-plate test in mice. Pharmacol Biochem Behav. 2000b;65:339–344. doi: 10.1016/s0091-3057(99)00191-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Furuichi T, Yoshida T, Endoh K, Kato K, Sado M, et al. Transgenic up-regulation of alpha-CaMKII in forebrain leads to increased anxiety-like behaviors and aggression. Mol Brain. 2009;2:6. doi: 10.1186/1756-6606-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Saito TR, Furudate S, Takahashi KW. Prolactin levels and maternal behavior induced by ultrasonic vocalizations of the rat pup. Exp Anim. 2001;50:307–312. doi: 10.1538/expanim.50.307. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S, Qian X, Velleca M. Genetically engineered mouse models for drug discovery: new chemical genetic approaches. Curr Drug Discov Technol. 2004;1:13–26. doi: 10.2174/1570163043484806. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Min H, Tamraz S, Carmouché M, Boehme SA, Vale WW. Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology. 1997;22:215–224. doi: 10.1016/s0306-4530(97)00030-9. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Davis M, Ratti E, Corsi M, Trist D, Ressler KJ. Anxiolytic-like effects of the neurokinin 1 receptor antagonist GR-205171 in the elevated plus maze and contextual fear-potentiated startle model of anxiety in gerbils. Behav Pharmacol. 2009;20:584–595. doi: 10.1097/FBP.0b013e32832ec594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hoffman EJ, Mathew SJ. Anxiety disorders: a comprehensive review of pharmacotherapies. Mt Sinai J Med. 2008;75:248–262. doi: 10.1002/msj.20041. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Holden C. Psychiatry. Behavioral addictions debut in proposed DSM-V. Science. 2010;327:935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- Holmes A. Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev. 2001;25:261–273. doi: 10.1016/s0149-7634(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Cryan JF. Measuring anxiety- and depression-related behaviors in the mouse. In: Fisch GS, Flint J, editors. Transgenic and Knockout Models of Neuropsychiatric Disorders. New York, NY: Human Press; 2006. pp. 237–264. [Google Scholar]
- Holmes A, Rodgers RJ. Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav. 1998;60:473–488. doi: 10.1016/s0091-3057(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ, Rodgers RJ. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res. 2001;122:159–167. doi: 10.1016/s0166-4328(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, Krasowski MD, Rick CE, de Blas AL, et al. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the gamma2 subunit of the gamma-aminobutyrate type A receptor. Neuropharmacology. 1999;38:253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Chlordiazepoxide modified exploration in rats. Psychopharmacologia. 1972;24:462–469. doi: 10.1007/BF00423436. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Genetic approaches to modeling anxiety in animals. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Current Topics in Behavioural Neurosciences. Heidelberg, Germany: Springer-Verlag; 2010. pp. 161–201. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26:8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Bettler B, Kaupmann K, Cryan JF. Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology (Berl) 2007;190:541–553. doi: 10.1007/s00213-006-0631-9. [DOI] [PubMed] [Google Scholar]
- Joel D. Current animal models of obsessive compulsive disorder: a critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:374–388. doi: 10.1016/j.pnpbp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, et al. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl) 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Costall B, Domeney AM, Kelly ME, Naylor RJ, Oakley NR, et al. The potential anxiolytic activity of GR38032F, a 5-HT3-receptor antagonist. Br J Pharmacol. 1988;93:985–993. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Sedivy JM. Gene Targeting: A Practical Approach. 2nd edn. Oxford: Oxford University Press; 2000. [Google Scholar]
- Jung ME, Lal H, Gatch MB. The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments. Neurosci Biobehav Rev. 2002;26:429–439. doi: 10.1016/s0149-7634(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Wetzler S, Asnis GM, Kling MA, Suckow RF, Praag HM. Effects of m-chlorophenylpiperazine in normal subjects: a dose–response study. Psychopharmacology. 1990;100:339–344. doi: 10.1007/BF02244603. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. The Suok (‘ropewalking’) murine test of anxiety. Brain Res Brain Res Protoc. 2005;14:87–99. doi: 10.1016/j.brainresprot.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Keisala T, Minasyan A, Tuohimaa P. Pharmacological modulation of anxiety-related behaviors in the murine Suok test. Brain Res Bull. 2007;74:45–50. doi: 10.1016/j.brainresbull.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Keisala T, Minasyan A, Kumar SR, LaPorte JL, Murphy DL, et al. The regular and light–dark Suok tests of anxiety and sensorimotor integration: utility for behavioral characterization in laboratory rodents. Nat Protoc. 2008;3:129–136. doi: 10.1038/nprot.2007.516. [DOI] [PubMed] [Google Scholar]
- Kantor S, Anheuer ZE, Bagdy G. High social anxiety and low aggression in Fawn-Hooded rats. Physiol Behav. 2000;71:551–557. doi: 10.1016/s0031-9384(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, Seong E, et al. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci U S A. 1999;96:11595–11600. doi: 10.1073/pnas.96.20.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65 kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne J, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Targets CNS Neurol Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- Kellner M, Muhtz C, Stark K, Yassouridis A, Arlt J, Wiedemann K. Effects of a metabotropic glutamate(2/3) receptor agonist (LY544344/LY354740) on panic anxiety induced by cholecystokinin tetrapeptide in healthy humans: preliminary results. Psychopharmacology (Berl) 2005;179:310–315. doi: 10.1007/s00213-004-2025-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. J Clin Psychiatry. 2007;68(Suppl 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR. The national comorbidity survey replication (NCS-R): background and aims. Int J Methods Psychiatr Res. 2004;13:60–68. doi: 10.1002/mpr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Messenger T, Ferris CF. Seed finding in golden hamsters: a potential animal model for screening anxiolytic drugs. Neuropsychobiology. 2002;45:150–155. doi: 10.1159/000054956. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘trier social stress test’– a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Kłodzińska A, Chojnacka-Wójcik E, Pałucha A, Brański P, Popik P, Pilc A. Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models. Neuropharmacology. 1999;38:1831–1839. doi: 10.1016/s0028-3908(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ohta M, Miyasaka K, Funakoshi A. Decrease in exploratory behavior in naturally occurring cholecystokinin (CCK)-A receptor gene knockout rats. Neurosci Lett. 1996;214:61–64. doi: 10.1016/0304-3940(96)12881-0. [DOI] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, et al. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszycki D, Bradwejn J, Bourin M. Comparison of the effects of cholecystokinin-tetrapeptide and carbon dioxide in health volunteers. Eur Neuropsychopharmacol. 1991;1:137–141. doi: 10.1016/0924-977x(91)90715-7. [DOI] [PubMed] [Google Scholar]
- Kromer SA, Kessler MS, Milfay D, Birg IN, Bunck M, Czibere L, et al. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;25:4375–4384. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Berger P, Tauber RF, Bandelow B, Henkel V, Heuser I. Randomized, double-blind study of SR142801 (osanetant). A novel neurokinin-3 (NK3) receptor antagonist in panic disorder with pre- and posttreatment cholecystokinin tetrapeptide (CCK-4) challenges. Pharmacopsychiatry. 2005;38:24–29. doi: 10.1055/s-2005-837768. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Pagano A, Stoehr N, Vranesic I, Flor PJ, Lingenhöhl K, et al. In vitro and in vivo characterization of MPEP, an allosteric modulator of the metabotropic glutamate receptor subtype 5: review article. Amino Acids. 2002;23:207–211. doi: 10.1007/s00726-001-0130-6. [DOI] [PubMed] [Google Scholar]
- Lähdesmäki J, Sallinen J, MacDonald E, Kobilka BK, Fagerholm V, Scheinin M. Behavioral and neurochemical characterization of alpha(2A)-adrenergic receptor knockout mice. Neuroscience. 2002;113:289–299. doi: 10.1016/s0306-4522(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Lakoff A. The mousetrap: managing the placebo effect in antidepressant trials. Mol Interv. 2002;2:72–76. doi: 10.1124/mi.2.2.72. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Keßler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. Alzheimers Dis. 2010;20(Suppl 1):S239–S248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lee C, Rodgers RJ. Antinociceptive effects of elevated plus-maze exposure: influence of opiate receptor manipulations. Psychopharmacology. 1990;102:507–513. doi: 10.1007/BF02247133. [DOI] [PubMed] [Google Scholar]
- Lee C, Parks GS, Civelli O. Anxiolytic effects of the MCH1R antagonist TPI 1361-17. J Mol Neurosci. 2010;43:132–137. doi: 10.1007/s12031-010-9425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Leite JR, Seabra MDL, Sartori VA, Andreatini R. The video-recorded Stroop color-word test as a new model of experimentally-induced anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:809–822. doi: 10.1016/s0278-5846(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Appleby IL, Levy G, et al. Lactate provocation of panic attacks. I. Clinical and behavioral findings. Arch Gen Psychiatry. 1984;41:764–770. doi: 10.1001/archpsyc.1984.01790190038004. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer AJ, Levitt M, Dillon D, Levy G, et al. Lactate provocation of panic attacks. II. Biochemical and physiological findings. Arch Gen Psychiatry. 1985;42:709–719. doi: 10.1001/archpsyc.1985.01790300077010. [DOI] [PubMed] [Google Scholar]
- Line SJ, Barkus C, Coyle C, Jennings KA, Deacon RM, Lesch KP, et al. Opposing alterations in anxiety and species-typical behaviours in serotonin transporter overexpressor and knockout mice. Eur Neuropsychopharmacol. 2011;21:108–116. doi: 10.1016/j.euroneuro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu G, Cai G, Cai Y, Sheng Z, Jiang J, Mei Z, et al. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32:1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- Lopes FL, Azevedo TM, Imbiriba LA, Freire RC, Valença AM, Caldirola D, et al. Freezing reaction in panic disorder patients associated with anticipatory anxiety. Depress Anxiety. 2009;26:917–921. doi: 10.1002/da.20593. [DOI] [PubMed] [Google Scholar]
- López-Aumatell R, Blázquez G, Gil L, Aguilar R, Cañete T, Giménez-Llort L, et al. The Roman high- and low-avoidance rat strains differ in fear-potentiated startle and classical aversive conditioning. Psicothema. 2009;21:27–32. [PubMed] [Google Scholar]
- López-Muñoz F, Ucha-Udabe R, Alamo C. The history of barbiturates a century after their clinical introduction. Neuropsychiatr Dis Treat. 2005;1:329–343. [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoromethylphenyl)piperazine on locomotor activity. J Pharmacol Exp Ther. 1989;249:155–164. [PubMed] [Google Scholar]
- McAuley JD, Stewart AL, Webber ES, Cromwell HC, Servatius RJ, Pang KCH. Wistar-Kyoto rats as an animal model of anxiety vulnerability: support for a hypervigilance hypothesis. Behav Brain Res. 2009;204:162–168. doi: 10.1016/j.bbr.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WT, Bunney WE. Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Tõru I, Mäemets K, Sepp S, Vasar V, Shlik J, et al. CCK-4-induced anxiety but not panic is associated with serum brain-derived neurotrophic factor in healthy subjects. J Psychopharmacol (Oxford) 2009;23:460–464. doi: 10.1177/0269881108089600. [DOI] [PubMed] [Google Scholar]
- Maron E, Kallassalu K, Tammiste A, Kolde R, Vilo J, Tõru I, et al. Peripheral gene expression profiling of CCK-4-induced panic in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:269–274. doi: 10.1002/ajmg.b.30898. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Ayuse T, Oi K, Kataoka Y. Propofol produces anticonflict action by inhibiting 5-HT release in rat dorsal hippocampus. Neuroreport. 1997;8:3087–3090. doi: 10.1097/00001756-199709290-00016. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. Implications of genomics for public health: the role of genetic epidemiology. Cold Spring Harb Symp Quant Biol. 2003;68:359–364. doi: 10.1101/sqb.2003.68.359. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, McMullen CA, Yocom AM, Valentine EL, Dengler-Crish CM, et al. Dynamic processing of taste aversion extinction in the brain. Brain Res. 2004;1016:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M. The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- Miller G, Holden C. Psychiatry. Proposed revisions to psychiatry's canon unveiled. Science. 2010;327:770–771. doi: 10.1126/science.327.5967.770-a. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Molinengo L, Ricci-Gamalero S. The ‘staircase maze’ and the ‘simple staircase’ in the analysis of the psychopharmacological action of CNS depressants. Pharmacology. 1970;4:169–178. doi: 10.1159/000136134. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16:307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens DeltaFosB accumulation. J Pharmacol Exp Ther. 2007;321:172–177. doi: 10.1124/jpet.106.116228. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Marsden CA. Effect of central and peripheral administrations of cholecystokinin-tetrapeptide on panic-like reactions induced by stimulation of the dorsal periaqueductal grey area in the rat. Biol Psychiatry. 1997;42:335–344. doi: 10.1016/S0006-3223(96)00407-6. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Moser PC, Tricklebank MD, Middlemiss DN, Mir AK, Hibert MF, Fozard JR. Characterization of MDL 73005EF as a 5-HT1A selective ligand and its effects in animal models of anxiety: comparison with buspirone, 8-OH-DPAT and diazepam. Br J Pharmacol. 1990;99:343–349. doi: 10.1111/j.1476-5381.1990.tb14706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE. Using functional neuroimaging to investigate the mechanisms of action of selective serotonin reuptake inhibitors (SSRIs) Curr Pharm Des. 2010;16:1990–1997. doi: 10.2174/138161210791293051. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Downham C, Cowen PJ, Harmer CJ. Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology (Berl) 2008;199:503–513. doi: 10.1007/s00213-008-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Valença AM, Mezzasalma MA, Lopes FL, Nascimento I, Veras AB, et al. 35% Carbon dioxide and breath-holding challenge tests in panic disorder: a comparison with spontaneous panic attacks. Depress Anxiety. 2006;23:236–244. doi: 10.1002/da.20165. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Valença AM, Lopes FL, de-Melo-Neto VL, Freire RC, Veras AB, et al. Caffeine and 35% carbon dioxide challenge tests in panic disorder. Hum Psychopharmacol. 2007;22:231–240. doi: 10.1002/hup.840. [DOI] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain PF, Haug M. The effects of 5-HT receptor ligands on ultrasonic calling in mouse pups. Neurosci Biobehav Rev. 1991a;15:483–487. doi: 10.1016/s0149-7634(05)80136-8. [DOI] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain P. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol. 1991b;2:121–128. [PubMed] [Google Scholar]
- Netto CF, Guimarães FS. Anxiogenic effect of cholecystokinin in the dorsal periaqueductal gray. Neuropsychopharmacology. 2004;29:101–107. doi: 10.1038/sj.npp.1300334. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Kessler RC, Alonso J, Benbow A, Lecrubier Y, Lépine J, et al. Consensus statement on the benefit to the community of ESEMeD (European Study of the Epidemiology of Mental Disorders) survey data on depression and anxiety. J Clin Psychiatry. 2007;68(Suppl 2):42–48. [PubMed] [Google Scholar]
- O'Connor RM, Finger BC, Flor PJ, Cryan JF. Metabotropic glutamate receptor 7: at the interface of cognition and emotion. Eur J Pharmacol. 2010;639:123–131. doi: 10.1016/j.ejphar.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Ohl F, Holsboer F, Landgraf R. The modified hole board as a differential screen for behavior in rodents. Behav Res Methods Instrum Comput. 2001a;33:392–397. doi: 10.3758/bf03195393. [DOI] [PubMed] [Google Scholar]
- Ohl F, Sillaber I, Binder E, Keck ME, Holsboer F. Differential analysis of behavior and diazepam-induced alterations in C57BL/6N and BALB/c mice using the modified hole board test. J Psychiatr Res. 2001b;35:147–154. doi: 10.1016/s0022-3956(01)00017-6. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2010;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Martin BR. Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:963–976. doi: 10.1016/0278-5846(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Pape H, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMζ inhibition in the amygdala. Nat Neurosci. 2011;14:295–296. doi: 10.1038/nn.2745. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Hanania T. The modified Geller–Seifter test in rats was insensitive to GABAB receptor positive modulation or blockade, or 5-HT1A receptor activation. Behav Brain Res. 2010;208:258–264. doi: 10.1016/j.bbr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Siegel SJ, Shields AE, Patterson F, Gould TJ, Strasser AA, et al. Translating basic science to improve pharmacotherapy for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S583–S598. doi: 10.1080/14622200701691755. [DOI] [PubMed] [Google Scholar]
- Picazo O, Fernández-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680:135–141. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pobbe RLH, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2010;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl R, Ettedgui E, Bridges M, Lycaki H, Jimerson D, Kopin I, et al. Plasma MHPG levels in lactate and isoproterenol anxiety states. Biol Psychiatry. 1987;22:1127–1136. doi: 10.1016/0006-3223(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein e in anxiety. Neural Plast. 2007 doi: 10.1155/2007/91236. 2007: 91236. doi: 10.1155/2007/91236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LO. Pharmacology of methaminodiazepoxide. Dis Nerv Syst. 1960;21:7–10. [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Voits M, Fink H. Pharmacological evaluation of a modified open-field test sensitive to anxiolytic drugs. Pharmacol Biochem Behav. 1998;59:677–683. doi: 10.1016/s0091-3057(97)00461-9. [DOI] [PubMed] [Google Scholar]
- Rickels K, Csanalosi I, Chung HR, Case WG, Pereira-Ogan JA, Downing RW. Amitriptyline in anxious-depressed outpatients: a controlled study. Am J Psychiatry. 1974;131:25–30. doi: 10.1176/ajp.131.1.25. [DOI] [PubMed] [Google Scholar]
- Rickels K, Downing R, Schweizer E, Hassman H. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50:884–895. doi: 10.1001/archpsyc.1993.01820230054005. [DOI] [PubMed] [Google Scholar]
- Ripoll N, Hascoët M, Bourin M. The four-plates test: anxiolytic or analgesic paradigm? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:873–880. doi: 10.1016/j.pnpbp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Robichaud RC, Sledge KL. The effects of p-chlorophenylalanine on experimentally induced conflict in the rat. Life Sci. 1969;8:965–969. doi: 10.1016/0024-3205(69)90427-5. [DOI] [PubMed] [Google Scholar]
- Rodgers R. Animal tests for anxiety. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Oxford: Academic Press; 2010. pp. 90–100. [Google Scholar]
- Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. discussion 497–504. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJG, Goodwin RD, Kubzansky L, et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30:208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Salomé N, Viltart O, Darnaudéry M, Salchner P, Singewald N, Landgraf R, et al. Reliability of high and low anxiety-related behaviour: influence of laboratory environment and multifactorial analysis. Behav Brain Res. 2002;136:227–237. doi: 10.1016/s0166-4328(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Salomons AR, van Luijk JAK, Reinders NR, Kirchhoff S, Arndt SS, Ohl F. Identifying emotional adaptation: behavioural habituation to novelty and immediate early gene expression in two inbred mouse strains. Genes Brain Behav. 2010a;9:1–10. doi: 10.1111/j.1601-183X.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- Salomons AR, Kortleve T, Reinders NR, Kirchhoff S, Arndt SS, Ohl F. Susceptibility of a potential animal model for pathological anxiety to chronic mild stress. Behav Brain Res. 2010b;209:241–248. doi: 10.1016/j.bbr.2010.01.050. [DOI] [PubMed] [Google Scholar]
- Sánchez C. Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur J Pharmacol. 2003;463:133–143. doi: 10.1016/s0014-2999(03)01277-9. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl) 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003a;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003b;26:527–540. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Blier P, Hen R. Behavioral and physiologic effects of genetic or pharmacologic inactivation of the substance P receptor (NK1) J Clin Psychiatry. 2002;63(Suppl 11):11–17. [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sass SM, Heller W, Stewart JL, Silton RL, Edgar JC, Fisher JE, et al. Time course of attentional bias in anxiety: emotion and gender specificity. Psychophysiology. 2010;47:247–259. doi: 10.1111/j.1469-8986.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. The internal conflict model: a theoretical framework for integration. Humanist Psychol. 2005;33:33–44. [Google Scholar]
- Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: effects of genetic background and stress duration. Front Behav Neurosci. 2011;5 doi: 10.3389/fnbeh.2011.00013. DOI: 10.3389/fnbeh.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci (Regul Ed) 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils M, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Hiemke C. Combination of open field and elevated plus-maze: a suitable test battery to assess strain as well as treatment differences in rat behavior. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1197–1215. doi: 10.1016/s0278-5846(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiyama K, Hashimoto O, Ushiro Y, Adachi C, Kikusui T, Tanemura K, et al. Abnormalities in aggression and anxiety in transgenic mice overexpressing activin E. Biochem Biophys Res Commun. 2009;385:319–323. doi: 10.1016/j.bbrc.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Fitz SD, Nakazato A, Chaki S, Steckler T, et al. A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int J Neuropsychopharmacol. 2010;14:355–365. doi: 10.1017/S1461145710001355. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sherif F, Oreland L. Effect of the GABA-transaminase inhibitor vigabatrin on exploratory behaviour in socially isolated rats. Behav Brain Res. 1995;72:135–140. doi: 10.1016/0166-4328(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Shibata S, Yamashita K, Yamamoto E, Ozaki T, Ueki S. Effects of benzodiazepine and GABA antagonists on anticonflict effects of antianxiety drugs injected into the rat amygdala in a water-lick suppression test. Psychopharmacology (Berl) 1989;98:38–44. doi: 10.1007/BF00442003. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Yoshimizu T, Chaki S. Melanin-concentrating hormone MCH1 receptor antagonists: a potential new approach to the treatment of depression and anxiety disorders. CNS Drugs. 2006;20:801–811. doi: 10.2165/00023210-200620100-00002. [DOI] [PubMed] [Google Scholar]
- Shorter E, Tyrer P. Separation of anxiety and depressive disorders: blind alley in psychopharmacology and classification of disease. BMJ. 2003;327:158–160. doi: 10.1136/bmj.327.7407.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Avoidance conditioning with brief shock and no exteroceptive warning signal. Science. 1953;118:157–158. doi: 10.1126/science.118.3058.157. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Panhuysen M, Henniger MSH, Ohl F, Kühne C, Pütz B, et al. Profiling of behavioral changes and hippocampal gene expression in mice chronically treated with the SSRI paroxetine. Psychopharmacology (Berl) 2008;200:557–572. doi: 10.1007/s00213-008-1232-6. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane PE, Morre M. The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology (Berl) 1984;84:48–53. doi: 10.1007/BF00432023. [DOI] [PubMed] [Google Scholar]
- Singewald N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci Biobehav Rev. 2007;31:18–40. doi: 10.1016/j.neubiorev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Van Baal GC, De Ruiter AJ, Van Oortmerssen GA. Y chromosomal and sex effects on the behavioral stress response in the defensive burying test in wild house mice. Physiol Behav. 1999;67:579–585. doi: 10.1016/s0031-9384(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Soubríe P, Kulkarni S, Simon P, Boissier JR. [Effects of antianxiety drugs on the food intake in trained and untrained rats and mice (author's transl)] Psychopharmacologia. 1975;45:203–210. doi: 10.1007/BF00429062. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spooren W, Gasparini F. mGlu5 receptor antagonists: a novel class of anxiolytics? Drug News Perspect. 2004;17:251–257. doi: 10.1358/dnp.2004.17.4.829052. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, et al. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Spooren WPJ, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608) Eur J Pharmacol. 2002;435:161–170. doi: 10.1016/s0014-2999(01)01562-x. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Zarotsky V, Cutler NR. Generalised anxiety disorder: treatment options. Drugs. 2002;62:1635–1648. doi: 10.2165/00003495-200262110-00005. [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Brañski P, Kłak K, van der Putten H, Cryan JF, Flor PJ, et al. Selective activation of metabotropic G-protein-coupled glutamate 7 receptor elicits anxiolytic-like effects in mice by modulating GABAergic neurotransmission. Behav Pharmacol. 2008;19:597–603. doi: 10.1097/FBP.0b013e32830cd839. [DOI] [PubMed] [Google Scholar]
- Steckler T, Stein MB, Holmes A. Developing novel anxiolytics: improving preclinical detection and clinical assesment. In: McArthur RA, Borsini F, editors. Animals and Translational Models for CNS Drug Discovery. San Diego, CA: Academic Press; 2008. pp. 117–132. [Google Scholar]
- Stefański R, Pałejko W, Kostowski W, Płaznik A. The comparison of benzodiazepine derivatives and serotonergic agonists and antagonists in two animal models of anxiety. Neuropharmacology. 1992;31:1251–1258. doi: 10.1016/0028-3908(92)90053-r. [DOI] [PubMed] [Google Scholar]
- Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100. doi: 10.1080/1025389031000111320. [DOI] [PubMed] [Google Scholar]
- Steimer T, la Fleur S, Schulz PE. Neuroendocrine correlates of emotional reactivity and coping in male rats from the Roman high (RHA/Verh)- and low (RLA/Verh)-avoidance lines. Behav Genet. 1997;27:503–512. doi: 10.1023/a:1021448713665. [DOI] [PubMed] [Google Scholar]
- Sternbach LH. The benzodiazepine story. J Med Chem. 1979;22:1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- Stork O, Yamanaka H, Stork S, Kume N, Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2003;2:65–70. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Bucan M. Dissection of behavior and psychiatric disorders using the mouse as a model. Hum Mol Genet. 2000;9:953–965. doi: 10.1093/hmg/9.6.953. [DOI] [PubMed] [Google Scholar]
- Taylor DP, Moon SL. Buspirone and related compounds as alternative anxiolytics. Neuropeptides. 1991;19(Suppl):15–19. doi: 10.1016/0143-4179(91)90078-w. [DOI] [PubMed] [Google Scholar]
- Tecott LH. The genes and brains of mice and men. Am J Psychiatry. 2003;160:646–656. doi: 10.1176/appi.ajp.160.4.646. [DOI] [PubMed] [Google Scholar]
- Thoeringer CK, Erhardt A, Sillaber I, Mueller MB, Ohl F, Holsboer F, et al. Long-term anxiolytic and antidepressant-like behavioural effects of tiagabine, a selective GABA transporter-1 (GAT-1) inhibitor, coincide with a decrease in HPA system activity in C57BL/6 mice. J Psychopharmacol (Oxford) 2010;24:733–743. doi: 10.1177/0269881109103091. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tõru I, Aluoja A, Võhma U, Raag M, Vasar V, Maron E, et al. Associations between personality traits and CCK-4-induced panic attacks in healthy volunteers. Psychiatry Res. 2010;178:342–347. doi: 10.1016/j.psychres.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Treit D, Engin E, McEown K. Animal models of anxiety and anxiolytic drug action. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Waltham MA: Academic Press; 2010. pp. 121–160. Current Topics in Behavioral Neurosciences. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Tulen JH, Moleman P, van Steenis HG, Boomsma F. Characterization of stress reactions to the Stroop color word test. Pharmacol Biochem Behav. 1989;32:9–15. doi: 10.1016/0091-3057(89)90204-9. [DOI] [PubMed] [Google Scholar]
- Umehara F, Mishima K, Egashira N, Ogata A, Iwasaki K, Fujiwara M. Elevated anxiety-like and depressive behavior in desert hedgehog knockout male mice. Behav Brain Res. 2006;174:167–173. doi: 10.1016/j.bbr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Van Bogaert M, Oosting R, Toth M, Groenink L, van Oorschot R, Olivier B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: modulation by serotonergic and GABAA-ergic drugs. Eur J Pharmacol. 2006;550:84–90. doi: 10.1016/j.ejphar.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Varty GB, Cohen-Williams ME, Morgan CA, Pylak U, Duffy RA, Lachowicz JE, et al. The gerbil elevated plus-maze II: anxiolytic-like effects of selective neurokinin NK1 receptor antagonists. Neuropsychopharmacology. 2002;27:371–379. doi: 10.1016/S0893-133X(02)00313-5. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology. 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Takahashi RN, Brüske GR, Ramos A. Evaluation of the anxiolytic-like effect of NKP608, a NK1-receptor antagonist, in two rat strains that differ in anxiety-related behaviors. Psychopharmacology (Berl) 2003;170:287–293. doi: 10.1007/s00213-003-1545-4. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like. Behavior Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, van Bogaert MJV, Klanker M, Korte SM, Oosting R, Hanania T, et al. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur J Pharmacol. 2008;585:407–425. doi: 10.1016/j.ejphar.2008.02.097. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Cryan JF, Olivier B, Groenink L. Elucidating GABAA and GABAB receptor functions in anxiety using the stress-induced hyperthermia paradigm: a review. Open Pharmacol J. 2010;4:1–14. [Google Scholar]
- Vogel JR, Beer B, Clody DE. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- Voigt J, Hörtnagl H, Rex A, van Hove L, Bader M, Fink H. Brain angiotensin and anxiety-related behavior: the transgenic rat TGR(ASrAOGEN)680. Brain Res. 2005;1046:145–156. doi: 10.1016/j.brainres.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Võikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wacker DA, Miller KJ. Agonists of the serotonin 5-HT2C receptor: preclinical and clinical progression in multiple diseases. Curr Opin Drug Discov Devel. 2008;11:438–445. [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Acher F, Brański P, Pilc A. Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology. 2010;59:627–634. doi: 10.1016/j.neuropharm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Porter JH. Effects of four antipsychotics on punished responding in rats. Pharmacol Biochem Behav. 1993;45:263–267. doi: 10.1016/0091-3057(93)90237-n. [DOI] [PubMed] [Google Scholar]
- Wilks LJ, File SE. Evidence for simultaneous anxiolytic and aversive effects several hours after administration of sodium phenobarbitone to the rat. Neuropsychobiology. 1988;19:86–89. doi: 10.1159/000118440. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Witkin JM. Animal models of obsessive–compulsive disorder. 2008. Curr Protoc Neurosci, Chapter 9:Unit 9.30. [DOI] [PubMed]
- Wong M, Licinio J. From monoamines to genomic targets: a paradigm shift for drug discovery in depression. Nat Rev Drug Discov. 2004;3:136–151. doi: 10.1038/nrd1303. [DOI] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- Wu L, Ko SW, Toyoda H, Zhao M, Xu H, Vadakkan KI, et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS ONE. 2007;2:e167. doi: 10.1371/journal.pone.0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Akiyoshi J, Kiyota A, Katsuragi S, Tsutsumi T, Isogawa K, et al. Increased anxiety behavior in OLETF rats without cholecystokinin-A receptor. Brain Res Bull. 2000;53:789–792. doi: 10.1016/s0361-9230(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Effects of midazolam on the expression of conditioned taste aversion in rats. Brain Res. 2005;1043:115–123. doi: 10.1016/j.brainres.2005.02.070. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Rainey JM, Berchou R, Ortiz A. Sodium lactate infusions after treatment with tricyclic antidepressants: behavioral and physiological findings. Biol Psychiatry. 1988;24:767–774. doi: 10.1016/0006-3223(88)90252-1. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Bär K, Chokka P, Tancer M. Exaggerated beat-to-beat R amplitude variability in patients with panic disorder after intravenous isoproterenol. Neuropsychobiology. 2007;55:213–218. doi: 10.1159/000108380. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Faber-Zuschratter H, Linke R, Schwegler H. Contribution of amygdala neurons containing peptides and calcium-binding proteins to fear-potentiated startle and exploration-related anxiety in inbred Roman high- and low-avoidance rats. Eur J Neurosci. 2002;15:1206–1218. doi: 10.1046/j.1460-9568.2002.01945.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Liberzon I. Stress and anxiety disorders. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 443–465. [Google Scholar]
- Zanoveli JM, Netto CF, Guimarães FS, Zangrossi H. Systemic and intra-dorsal periaqueductal gray injections of cholecystokinin sulfated octapeptide (CCK-8s) induce a panic-like response in rats submitted to the elevated T-maze. Peptides. 2004;25:1935–1941. doi: 10.1016/j.peptides.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, et al. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22:1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanzger P, Jarry H, Eser D, Padberg F, Baghai T, Schule C, et al. Plasma gamma-amino-butyric acid (GABA) levels in cholecystokinine-tetrapeptide (CCK-4) induced anxiety. J Neural Transm. 2003;110:313–316. doi: 10.1007/s00702-002-0774-8. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Eser D, Romeo E, di Michele F, Baghai TC, Pasini A, et al. Changes in CCK-4 induced panic after treatment with the GABA-reuptake inhibitor tiagabine are associated with an increase in 3alpha,5alpha-tetrahydrodeoxycorticosterone concentrations. Psychoneuroendocrinology. 2009;34:1586–1589. doi: 10.1016/j.psyneuen.2009.04.017. [DOI] [PubMed] [Google Scholar]


