Abstract
Background:
Topical therapy with comedolytics and antibiotics are often advocated for mild and moderate severity acne vulgaris. Nadifloxacin, a new fluoroquinolone with anti-Propionibacterium acnes activity and additional anti-inflammatory activity, is approved for use in acne. This randomized controlled assessor blind trial compared the clinical effectiveness and safety of eight weeks therapy of nadifloxacin 1% versus clindamycin 1% as add-on therapy to benzoyl peroxide (2.5%) in mild to moderate grade acne.
Materials and Methods:
The efficacy parameters were changes in the total, inflammatory and non-inflammatory lesion counts, Investigator Global Assessment (IGA), and Cardiff Acne Disability Index (CADI) scales from baseline to study end (eight weeks). All treatment emergent dermatological adverse events were evaluated for safety assessment.
Results:
Out of 84 randomized subjects (43-nadifloxacin arm) and (41-clindamycin) 42 in nadifloxacin group, 37 in clindamycin group completed the study. Reduction from baseline of total, inflammatory and non-inflammatory lesion counts were highly significant in both the groups (P<0.0001), but between group differences were not significant. Significant improvement in CADI and IGA scales were noted in both groups. Between-group comparison showed no significant differences. The safety and tolerability profile of both regimens were good and statistically comparable.
Conclusions:
Topical nadifloxacin, a new fluoroquinolone is effective, tolerable, and safe for mild o moderate facial acne. Its clinical effectiveness is comparable to clindamycin when used as add-on therapy to benzoyl peroxide.
KEY WORDS: Acne vulgaris, clindamycin, nadifloxacin, randomized controlled trial
Introduction
Acne vulgaris is a common dermatological disorder of the pilosebaceous unit presenting usually at puberty.[1] It is characterized by the formation of open and closed comedones (non-inflammatory lesions), papules, pustules, and nodulocystic lesions (inflammatory lesions) generally affecting the face, arms, and back. The pathogenesis is complex and multifactorial which includes abnormal sebum production, follicular hyperkeratinization, bacterial proliferation and inflammation.[2] The treatment goals are directed to reduce activity of the sebaceous glands, normalize follicular proliferation reduce bacterial colonization and control inflammation. Therefore, topical comedolytics, antibacterials and retinoids are mainly used for mild to moderate severe acne. Systemic therapy with antibacterials, retinoids and hormones are mainly indicated for severe cases. Topical therapy with antibiotics alone can induce or select resistance in coagulase negative staphylococcus and lead to widespread distribution of cross–resistant strains of Propionobacterium acnes.[2–5] Combination therapy with clindamycin and benzoyl peroxide is a well-accepted treatment regimen for mild to moderate acne as documented in clinical trials and meta-analysis reports.[6,7] The advantages of this combination therapy are - keratolytic action of benzoyl peroxide is possibly synergistic with the antibacterial activity of clindamycin. Secondly, benzoyl peroxide may reduce chances of antimicrobial resistance to topical antibacterials like erythromycin and clindamycin.[6,7]
Topical fluoroquinolone antibiotic, nadifloxacin, is a relatively new member of the family of anti-acne medications. It has been used for the therapy of mild to moderate acne in Japan and other European countries and has also been recently launched in India. This antimicrobial agent is effective against aerobic and anaerobic bacteria isolated from patients with infective skin disorders.[8] Several in-vitro studies have documented the antibacterial activity of nadifloxacin against Propionibacterium acnes, an important bacterial pathogen in acne.[9] Additionally, studies also suggest that the effectiveness of nadifloxacin in inflammatory acne lesions may be attributed to its inhibitory effect on pro-inflammatory cytokines like interleukin (IL)-1α, IL-6, and IL-8 which also play an important role in acne pathogenesis[10]. Since there are no published studies till date that evaluated the clinical effectiveness and safety of topical nadifloxacin and benzoyl peroxide compared to clindamycin and benzoyl peroxide (an accepted standard regimen) in mild to moderate severity facial acne, we undertook this randomized controlled trial.
Materials and Methods
This post-marketing, randomized, single (assessor) blind, controlled trial was undertaken at an outpatient setting of a dermatology clinic in a tertiary care hospital. Approval of the protocol and other study documents were taken from Institutional Ethics Committee before study commencement. After taking written informed consent, subjects with mild to moderate (grade I and II) acne vulgaris attending the dermatology out-patient clinic were screened for study selection criteria. The sample size was calculated considering the total lesion count as the primary efficacy parameter. Based on an effect size of four, standard deviation of five, significance level α of 0.05 and power of the study (1 - β) as 90%, the target number of “evaluable” subjects was 34 per group using Statistica version 6 software. Considering a 15% drop out rate, this translated to a target recruitment of 40 subjects in each group.
After screening, 84 subjects fulfilled the subject selection criteria and were randomized (43 in nadifloxacin group and 41 in clindamycin group) to the two study groups using an unstratified computer generated randomization list. Allocation concealment was done by having sequentially numbered opaque sealed envelopes and medication dispensing was done accordingly.
The study inclusion criteria were: subjects of 12 to 40 years of age of either sex with ≥ 2 but ≤ 30 total lesions - inflammatory (papules and pustules) and/or non-inflammatory (open and closed comedones) lesions in the face which correspond to a baseline investigator global assessment (IGA) score of 2 or 3 (both inclusive). Exclusion criteria were: age out of range, total lesion count < 2 or >30, severe acne, subjects regularly using any anti-acne medications in the last 30 days before study entry or subjects with nodulo-cystic lesions, acne conglobata, acne fulminans, secondary acne (e.g. chloracne, drug-induced acne), or any acne requiring systemic treatment. Subjects were not allowed to use any other medication which might influence the study outcome and medicated cosmetics for the entire study duration.
Study was designed as a single-blind (assessor blind) study as the designated dermatologist who recruited and evaluated the subjects was unaware of the treatment allocation. The investigator who dispensed the study medications from the department of pharmacology was not involved in any subject related clinical assessment.
All enrolled subjects were instructed to apply a thin layer of the study medications over the lesions; benzoyl peroxide 2.5% gel once daily at bedtime and clindamycin 1% gel or nadifloxacin 1% gel twice daily. The patients were instructed to apply the study medications at least 10 minutes after the skin was gently washed, rinsed with water and patted dry. The patients were asked not to bathe, shower, wash or swim at least 4 hours after the application of the study medications.
The study medications were dispensed as 10 gm packs of benzoyl peroxide gel and as 5 gm packs of clindamycin or nadifloxacin gel, respectively. Accountability was assessed by evaluating the subject diary in which each subject was instructed to fill in the details of drug administration.
The primary efficacy parameter was change from baseline to study end of the total lesion count - both inflammatory and non-inflammatory lesions. Secondary efficacy parameters were the validated IGA,[11] on a six point scale: 0 - indicating clearance of inflammatory and non-inflammatory lesions, 1- almost clear, 2- mild severity, 3-moderate severity, 4-severe, and 5-very severe. Proportion of subjects in each group were considered as “improved” if there was at least two scale improvement in the IGA. Percentage of “improved” subjects in each group was compared for statistically significant difference if any. Changes in the Cardiff acne disability index (CADI)[12] was also evaluated for assessing the impact of disease on their quality of life. The CADI included assessment on a five question scale.
A modified intention to treat (MITT) analysis was done for the efficacy and safety data set where a subject was considered “evaluable” if the baseline and one post-baseline visit data was present. All treatment emergent dermatological adverse events were noted throughout the study period for safety analysis. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) for Windows (version 17, SPSS Inc., Chicago, IL, USA). The efficacy variables (total lesion, inflammatory, non-inflammatory lesion counts, and CADI scores) were tested for normality using the Kolmogorov-Smirnov Z test and were found to be normally distributed. Independent sample t-test was used to compare continuous parametric variables for between-group analysis while repeated measure ANOVA for within-group analysis with Bonferroni multiple comparison posthoc test. Categorical variables were analyzed using the χ2 test or Fisher's exact test, as appropriate. A P value of < 0.05 and <0.01 were considered as statistically significant and highly significant, respectively.
Results
Out of 84 randomized subjects (43-nadifloxacin arm) and (41-clindamycin) 42 in nadifloxacin (NADI) group, 37 in clindamycin (CLN) group completed the study. Four patients in the CLN group and one in the NADI group were lost to follow-up and did not attend any post baseline visit. Hence, there were 42 “evaluable subjects” in the NADI and 37 in CLN, respectively. Thus, the target number of evaluable subjects (34) in each group was achieved.
The mean age of subjects in the NADI group was 21.4 4.65 compared to 20.243.33 years in the CLN group, 42.85% were male in the NADI group while 54.05% in the CLN group. There were no significant differences in baseline demography and disease characteristics in the two treatment arms.
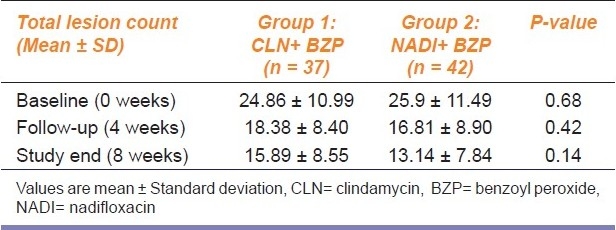
Table 1 clearly depicts that at baseline the two groups were comparable with respect to the total lesion count. At the end of 4 and 8 weeks, respectively, no statistically significant (P>0.05) difference of total lesion count was noted between two arms. Results show that 88.18% subjects in the NADI group while 62.16% in the CLN group had ≥ 50% reduction of baseline inflammatory lesion count at study end and this inter-group difference was statistically significant (P=0.009). However, similar comparison of the non-inflammatory counts showed statistically non-significant differences (P=0.636).
Table 1.
Between group comparison of total lesion count
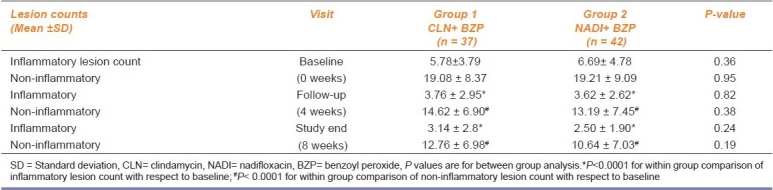
A within group analysis of the total lesion count at different time points (baseline, 4 and 8 weeks) showed a highly significant difference in both the groups. For both treatment groups, a progressive decline in the number of inflammatory and non-inflammatory lesion counts was observed. A between-group analysis of lesion counts at the first follow up (P=0.82 for inflammatory; P=0.38 for non-inflammatory) and at study end (P=0.24 for inflammatory; P=0.19 for non-inflammatory) did not show any statistically significant difference.
A within group comparison of inflammatory lesion and non-inflammatory lesion count from baseline to different time points (4 and 8 weeks) showed a highly significant reduction of scores (P<0.0001) [Table 2].
Table 2.
Between group comparison of inflammatory and non-inflammatory lesion count
The percentage of subjects at study end who demonstrated at least two scale improvements in the IGA were 54.05% (20 out of 37) in the CLN group versus 73.8% (31 out of 42) in the NADI group at the study end visit. Though the proportion of subjects in the NADI group showed better improvement, the difference did not reach statistically significant (P=0.067) values. No subjects in either group demonstrated worsening of the scores.
Between groups comparison of CADI is shown in Table 3. The treatment groups were comparable at baseline and the first follow up scores also showed no significant differences (P=0.43), but at the study end visit a statistically significant difference (P=0.04) was observed in favor of the NADI group.
Table 3.
Between group comparison of Cardiff Acne Disability Index
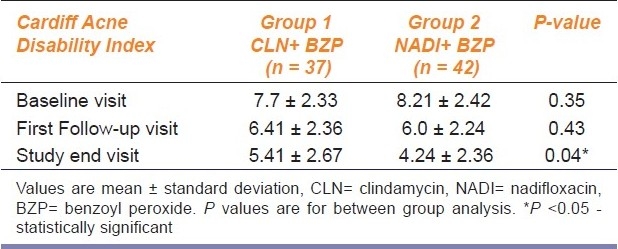
Within group comparison of CADI at different time points (baseline, 1st follow-up and study end) showed a highly significant reduction (P<0.001) of scores for both the groups.
In the safety and tolerability assessment, both treatments were well tolerated with only minor differences 24.3% (9 out of 37) patients in CLN group and 14.3% (6 out of 42) in the NADI group experienced at least one treatment emergent adverse event (AE). There was no statistically significant difference in the incidence of AEs in the two treatment arms (P=0.39). The adverse events were post-inflammatory hyperpigmentation, dryness, pruritus, burning sensation, and erythema. None of the patients needed treatment modification for the AEs and they resolved spontaneously. Causality assessment as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) adverse drug reaction (ADR) causality scale showed that most of the events were in “probable or possible” category. Treatment compliance was comparable in both the treatment arms.
Discussion
The results from this study demonstrate a reduction in both the inflammatory and non-inflammatory lesions of acne over an eight-week treatment period with two topical therapies (clindamycin with benzoyl peroxide and nadifloxacin with benzoyl peroxide). No differences between two therapies were observed in the total, inflammatory or non-inflammatory lesion counts.
This study provides us with scientific evidence that a combination topical therapy of nadifloxacin and benzoyl peroxide is effective, tolerable and safe in cases of mild to moderate severity facial acne vulgaris and comparable to that of existing clindamycin-benzoyl peroxide regimen. Our findings were similar to previous published literature in this domain. Nadifloxacin inhibits activation of T cells and keratinocytes which could partly be responsible for its beneficial effects in inflammatory acne.[13] A phase III, regulatory trial (non-inferiority study design), published from Japan with clindamycin (test drug) versus nadifloxacin (control) reported non-inferiority of clindamycin to nadifloxacin in terms of its efficacy in reducing inflammatory lesion count and improving global assessment scores.[14] Our study has shown similar effectiveness and safety of nadifloxacin with that of clindamycin.
A study by Veronnica et al., from Germany[10] was conducted to evaluate susceptibility of clinical isolates of P. acnes and the results have shown that nadifloxacin was superior to erythromycin and clindamycin as the MIC (minimal inhibitory concentration) values of nadilfloxacin against P. acnes were the least compared to the others.
A recently published randomized, vehicle controlled trial from Korea[15] has demonstrated that nadifloxacin 1% cream brought about a significant reduction of the inflammatory, non-inflammatory lesion counts in facial acne along-with a decrease in inflammation severities and IL-8 staining intensities in immuno-histochemistry studies. In contrast, no significant differences were observed for TGF-β intensity patterns.
The limitations of our study could be overcome by a double blind study of longer duration with simultaneous microbial susceptibility testing. This could further reinforce the scientific evidence.
The results of this study might be helpful for manufacturers to consider undertaking pharmacokinetic feasibility studies for preparing combination formulation of nadifloxacin and benzoyl peroxide for treatment of acne.
Acknowledgments
The authors are thankful to the Medical officers of the Department of Dermatology, IPGMER, and SSKM Hospital. Complimentary samples of nadifloxacin 1% gel were provided by Wockhardt Limited, Mumbai
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of Acne, A report from a global alliance to improve outcomes of acne. J Am Acad Dermatol. 2003;49(suppl):S1–37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 2.Zaenglein AL, Graber EM, Thiboutot DM, Strauss JS. In: Acne vulgaris and acneiform eruption in Fitzpatrick's Dermatology in general medicine. 7th ed. Wolff K, et al., editors. New York: McGraw Hill; 2008. pp. 687–703. [Google Scholar]
- 3.Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: A report from a global alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49:S1–37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 4.Ross JI, Snelling AM, Caenegie E, Coates P, Cunliffe WJ, Bettoli V, et al. Antibiotic resistant acne: lessons from Europe. Br J Dermatol. 2003;148:467–78. doi: 10.1046/j.1365-2133.2003.05067.x. [DOI] [PubMed] [Google Scholar]
- 5.Weiss J. Current options of topical treatment of acne vulgaris. Pediatr Dermatol. 1997;14:480–8. doi: 10.1111/j.1525-1470.1997.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 6.Degitz K, Ochsendorf F. Pharmacotherapy of acne. Pharmacother. 2008;9:955–71. doi: 10.1517/14656566.9.6.955. [DOI] [PubMed] [Google Scholar]
- 7.Kubba A, Bajaj AK, Thappa DM, Sharma R, Vedamurthy M, Dhar S. Acne in India: Guidelines for management - IAA consensus document: Topical antibacterials. Indian J Dermatol Venereol Leprol. 2009;75(Suppl 1):1–62. [PubMed] [Google Scholar]
- 8.Schfer H, Gollner A, Kusche W, Schwantes U. Effectiveness and tolerance of topical nadifloxacin in the therapy of acne vulgaris (grade I-II): Results of non-interventional trial in 555 patients. J Appl Res. 2009;9:44–51. [Google Scholar]
- 9.Nenoff P, Haustein UF, Hittel N. Activity of nadifloxacin (OPC-7251) and seven other antimicrobial agents against aerobic and anaerobic gram positive bacteria isolated from bacterial skin infections. Chemotherapy. 2004;50:196–201. doi: 10.1159/000081032. [DOI] [PubMed] [Google Scholar]
- 10.Alba V, Urban E, Angeles Dominguez M, Nagy E, Nord CE, Palacin C. In vitro activity of nadifloxacin against several gram positive bacteria and analysis of possible evolution of resistance after two years of use in Germany. Int J Antimicrob Agents. 2009;33:272–5. doi: 10.1016/j.ijantimicag.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi N, Akamatsu H, Kawashima M. Establishment of grading criteria for acne severity. J Dermatol. 2008;35:255–60. doi: 10.1111/j.1346-8138.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M, Johnson AM, Gupta AK. The development of an acne quality of life scale: Reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78:451–6. doi: 10.1080/000155598442773. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara K, Kitazawa T, Kitagaki H, Tsukamoto T, Kikuchi M. Nadifloxacin, an antiacne quinolone antimicrobial, inhibits the production of proinflammatory cytokines by human peripheral blood mononuclear cells and normal human keratinocytes. J Dermatol Sci. 2005;38:47–55. doi: 10.1016/j.jdermsci.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Cldmtkenkyukai Phase III Clinical Study of Clindamycin Phosphate Topical Gel (CLDM-T) in the treatment of acne vulgaris-randomized comparative study with nadifloxacin cream as a control drug. J Clin Therap Med. 1999;15:603–28. [Google Scholar]
- 15.Jung YJ, Kwon HH, Yeom KB, Yoon MY, Suh DH. Clinical and histological evaluation of 1% nadifloxacin cream in the treatment of acne vulgaris in Korean patients. Int J Dermatol. 2011;50:350–7. doi: 10.1111/j.1365-4632.2010.04701.x. [DOI] [PubMed] [Google Scholar]


