Abstract
Objective:
Ischemia/reperfusion (I/R) injury plays a key role in the pathogenesis of hepatic failure in several clinical settings such as liver resection or transplantation. Lisinopril, a widely prescribed drug for various cardiovascular conditions, has been reported to have diverse pharmacological properties. The aim of this study, therefore, was to evaluate a potential protective effect of lisinopril on hepatic I/R injury in rats.
Materials and Methods:
In anesthetized rats, partial hepatic ischemia was applied for one hour followed by two hours reperfusion. Biochemical analysis of serum and hepatic tissue was done. Hepatic tissue was also subjected to electron microscopy.
Results:
Pre-ischemic treatment with lisinopril (10 mg/kg) exerted protection against I/R-induced hepatocellular injury as evident by significant decrease in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin levels, along with hepatic lipid peroxidation, expressed as malondialdehyde content, with a concurrent increase in hepatic nitric oxide content as compared to I/R group. The electron microscopic examination indicated that lisinopril can effectively protect against hepatic I/R injury.
Conclusion:
Lisinopril provides a protection against hepatic I/R injury in rats. The protective effect is associated with reduction of oxidative stress-induced lipid peroxidation level and enhancement of nitric oxide availability.
KEY WORDS: Ischemia/reperfusion, lisinopril, liver
Introduction
Hepatic I/R injury, a situation coupled with conditions such as liver transplantation and hepatic resection, is a serious clinical problem.[1] Mechanisms of hepatic I/R injury involve multiple and interdependent pathways, including oxidative stress,[2] and imbalance between endothelin and nitric oxide.[3] Lisinopril, an angiotensin converting enzyme inhibitor, has pleiotropic pharmacological effects that may be relevant in hepatoprotection against I/R injury. For example, in addition to inhibiting angiotensin converting enzyme, lisinopril also attenuates reactive oxygen species formation,[4] and increases nitric oxide bioavailability.[5] The present study, therefore, was undertaken to evaluate a possible hepato protective effect of lisinopril against hepatic I/R injury in rats.
Materials and Methods
Animals
Male Wistar rats, weighing 180-200 g, were obtained from National Research Center, Giza, Egypt. They were used after one week of acclimatization to the animal house conditions (12 h lighting cycle and 25±2 °C temperature) and had free access to standard rodent chow and water. All experimental procedures were conducted according to the ethical standards approved by the Institutional Animal Ethics Committee guidelines for animal care and use.
Chemicals
Lisinopril was purchased from Sigma Chemical Co., USA. All other chemicals were of analytical grade and were obtained from commercial sources.
Experimental Procedures
Animals were randomly divided into three groups of six animals each; sham group, I/R-untreated group, and I/R-lisinopril-treated group. Rats were deprived of food, but not water, for 18 h before experiment. They were anesthetized with urethane (1.6 g/kg, i.p.) and the body temperature was maintained at 37 °C using a heating pad. A midline incision was made to the abdomen, and the left branches of the portal vein and hepatic artery were clamped using a vascular clamp to induce ischemia of the median and left hepatic lobes. The right lobes remained perfused in order to prevent intestinal congestion and hemodynamic instability. After one hour of ischemia, the clamp was removed to initiate hepatic reperfusion. The sham-operated animals underwent the same protocol without vascular occlusion. Lisinopril (10 mg/kg, i.p.; the dose based on our preliminary experiments and as per Brower et al.,[6]) was administered 30 min prior to ischemic period. After two hours of reperfusion, blood and liver tissue samples were taken for biochemical analysis and hepatic ultra structural assessment. For preparing liver sample for biochemical analysis, it was homogenized in 20% w/v of ice-cold phosphate buffer (0.01 M, pH 7.4). The homogenate was centrifuged at 3000 rpm for 20 min and the supernatant was kept at -80°C till used.
Biochemical Analysis
Using commercially available colorimetric assay kits, serum ALT, AST (Randox Laboratories Ltd., Co. Antrim, UK) and total bilirubin (Spectrum Diagnostics, Egypt) levels were estimated. Hepatic lipid peroxidation was determined as thiobarbituric acid reacting substance and is expressed as equivalents of malondialdehyde, using 1,1,3,3-tetramethoxypropane as standard.[7] Hepatic nitric oxide level was measured as total nitrite/nitrate, the stable degradation products of nitric oxide, by reduction of nitrate into nitrite using copperized cadmium, followed by color development with Griess reagent in acidic medium.[8]
Electron Microscopic Examination
For transmission electron microscopy, liver tissue samples after reperfusion were prefixed in 2% glutaraldehyde in phosphate buffer (pH 7.2). The specimens were then post-fixed in 1% phosphate-buffered osmium tetroxide, dehydrated through ethanol and propylene oxide, and embedded in araldite. Semi-thin sections were stained with azure II and methylene blue. Ultra-thin sections were obtained from the selected blocks, stained with uranyl acetate and lead citrate, examined, and then photographed.
Statistical Analysis
The data are expressed as mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey-Kramar post analysis test for multiple comparisons with P < 0.05 being considered as statistically significant.
Results
Biochemical Findings
Lisinopril pretreatment significantly decreased the hepatic I/R-induced elevations in serum ALT, AST, and total bilirubin levels [Table 1].
Table 1.
Effect of lisinopril on serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin after two hours of reperfusion

The malondialdehyde content, as an index of lipid peroxidation, was significantly elevated after reperfusion in I/R group compared with sham group. Pretreatment with lisinopril significantly reduced malondialdehyde content compared with the I/R group [Figure 1a]. Correspondingly, I/R induction caused a significant decrease in hepatic nitric oxide, as assessed by reduced nitrite/nitrate content, as compared to the sham group. On the other hand, pretreatment with lisinopril increased I/R-induced decrease in hepatic nitric oxide content [Figure 1b].
Figure 1.
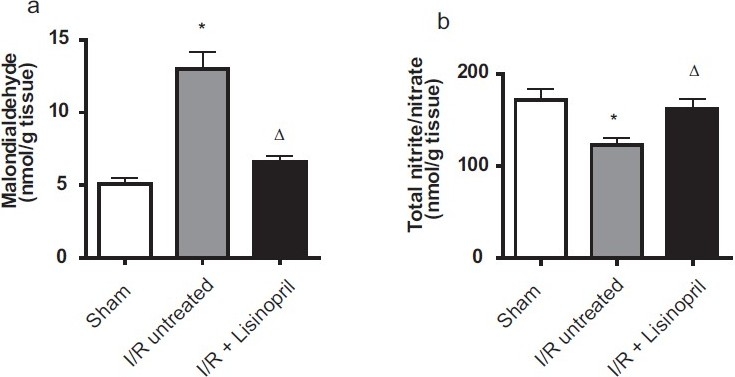
Effect of lisinopril on hepatic a) malondialdehyde and b) total nitrite/nitrate levels after two hours of reperfusion. Data shown are mean ± SEM of six rats. *,Δ Significantly different from sham and ischemia/reperfusion (I/R) untreated groups, respectively, at P < 0.05 (ANOVA)
Electron Microscopic Analysis
Sham hepatocytes had normal appearance of mitochondrion, rough endoplasmic reticulum, and nucleus structure [Figure 2a]. Hepatic I/R induced mitochondrial swelling (reflecting the generation of permeability transition) with indistinct cristae and vacuolization. The apoptotic nucleus appeared with irregular border and had increased amount of heterochromatin [Figures 2b and c]. In lisinopril-treated group, hepatocytes showed a marked improvement in architecture. The nucleus had a nearly smooth rounded contour with almost normally dispersed chromatin. The structure of the mitochondria was roughly normal. In addition, the cytoplasmic vacuoles were rarely seen; however, the typical structure of endoplasmic reticulum was still not clearly visible [Figure 2d].
Figure 2.
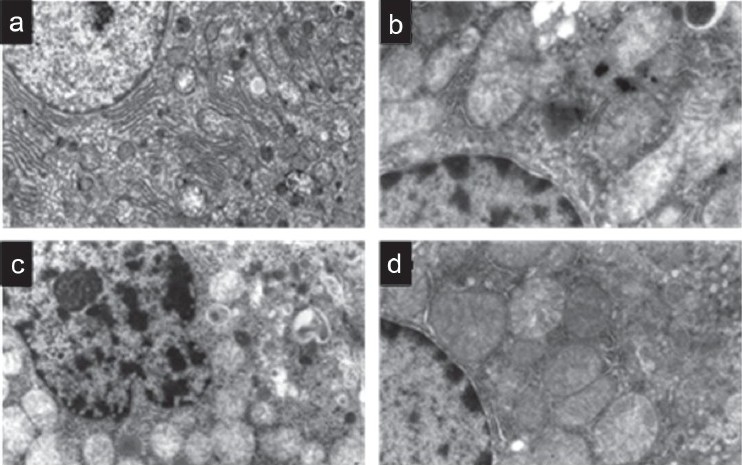
Electron micrographs depict the ultra structural changes in hepatocytes of a) sham, b,c) ischemia/reperfusion (I/R)-untreated and d) lisinopril-treated groups. Pretreatment of rats with lisinopril alleviated the deleterious effects of I/R
Discussion
Hepatic I/R via complex mechanisms lead to serious hepatocellular damage. In the present study, after two hours reperfusion, assessment of the classical markers of liver injury (ALT, AST, and total bilirubin), and hepatic malondialdehyde concentration as well as nitric oxide contents, along with the electron microscopic examination revealed that lisinopril is able to protect against hepatic I/R injury. El-Demerdash et al.,[9] reported that lisinopril reduced lipid peroxidation in carbon tetrachloride-induced hepatotoxicity in rats. Moreover, lisinopril has also been reported to decreased malondialdehyde levels on myocardial infarction in rats.[10] In addition, lisinopril significantly attenuated diabetes-induced increase in serum thiobarbituric acid reacting substance in rats.[11] Oxygen-derived free radicals generated during hepatic I/R have been reported to cause cell membrane damage by lipid peroxidation that eventually compromises cell viability, resulting in tissue injury.[12]
In the present study, hepatic I/R reduced the nitric oxide level. The decrease in nitric oxide production in the early phase of hepatic I/R is most probably due to decreased synthesis of nitric oxide caused by endothelial dysfunction, enhanced inactivation of nitric oxide by the overproduction of superoxide anion or both.[13] The results in the current study indicated that lisinopril increased hepatic nitric oxide content. Desideri et al.,[5] showed that lisinopril was able to increase nitric oxide bioavailability in human vascular endothelial cells. Moreover, lisinopril significantly attenuated diabetes-induced reduction in serum nitrite/nitrate concentration in rats.[11] Interventions that increase nitric oxide availability have been shown to be beneficial in hepatic I/R injury.[14,15] Several mechanisms have been proposed by which nitric oxide protects the liver against the injurious effects of I/R. For example, nitric oxide attenuates I/R injury by maintaining organ circulation as a vasoregulator, an inhibitor of platelet aggregation, and an attenuator of leukocyte adhesion.[16] The imbalance between nitric oxide and endothelin could cause microcirculatory disturbance in an organ thereby resulting in severe ischemia, which consequently damages its structure and function.[17] In addition, the toxic effects of nitric oxide are linked with the production of peroxynitrite, however, the beneficial effect of maintaining liver blood flow is more important than the potential for cell damage by peroxynitrite.[1]
Mitochondrial damage is a major participant in the development of I/R-induced hepatic injury.[18] In the present study, ultra structural examination revealed that I/R caused impairment of mitochondrial structure. On the other hand, lisinopril improved mitochondrial structural integrity and thereby it could probably protect against the I/R injury. Alternatively, nitric oxide which increased in the current study is reported to protect rat hepatocytes against reperfusion injury mediated by mitochondrial degeneration.[19]
Mechanism of hepatic I/R injury involves various pathways. Thus, it is difficult to attain effective protection by targeting individual mediators or mechanisms.[1] Lisinopril exerts multiple pharmacological actions that may be relevant in hepato protection against I/R injury. In addition to inhibiting angiotensin converting enzyme, lisinopril also attenuates reactive oxygen species formation,[4] increases prostacyclin synthesis,[20] blocks bradykinin degradation,[21] increases nitric oxide bioavailability,[5] and activates peroxisome proliferator-activated receptor gamma.[22] Therefore, many of these actions can be considered as potential protective targets against hepatic I/R injury.
Hypertension is common after liver transplantation.[23] Neal et al,[24] reported that lisinopril is an effective treatment for hypertensive patients after liver transplantation. In addition, lisinopril is the only angiotensin converting enzyme inhibitor that does not require hepatic metabolism.[25] In the present study, lisinopril showed a substantial hepato protection against the deleterious effects of hepatic I/R injury. Accordingly, lisinopril could be the treatment of choice for hypertensive patients before and after liver transplantation.
In conclusion, lisinopril can effectively attenuate I/R-induced liver injury possibly partly by, improving nitric oxide availability and reducing oxidative stress. Consequently, this may play a protective role in preserving the ultrastructural integrity of hepatocytes during I/R injury.
Acknowledgments
Thanks to Dr. Wafaa M. Zahran, professor of zoology, faculty of science, El-Minia University, for her assistance in the electron microscopic analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 2.González-Flecha B, Cutrin JC, Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993;91:456–64. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlmann D, Uhlmann S, Spiegel HU. Endothelin/nitric oxide balance influences hepatic ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2000;36(5 Suppl 1):S212–4. doi: 10.1097/00005344-200036051-00064. [DOI] [PubMed] [Google Scholar]
- 4.Fiordaliso F, Cuccovillo I, Bianchi R, Bai A, Doni M, Salio M, et al. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79:121–9. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Desideri G, Grassi D, Croce G, Bocale R, Tiberti S, Evangelista S, et al. Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: Evidence of an oxidant-sensitive pathway. Mediat Inflamm. 2008;2008:305087. doi: 10.1155/2008/305087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower GL, Levick SP, Janicki JS. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H3057–64. doi: 10.1152/ajpheart.00447.2006. [DOI] [PubMed] [Google Scholar]
- 7.Buege JA, Aust SD. Microsomal lipid peroxidation. Met Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 8.Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 9.el-Demerdash E, Salam OM, el-Batran SA, Abdallah HM, Shaffie NM. Inhibition of the renin-angiotensin system attenuates the development of liver fibrosis and oxidative stress in rats. Clin Exp Pharmacol Physiol. 2008;35:159–67. doi: 10.1111/j.1440-1681.2007.04797.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang B, Wu W, Li M, Xu L, Sun K, Yang M, et al. Cardioprotection and matrix metalloproteinase-9 regulation of salvianolic acids on myocardial infarction in rats. Planta Med. 2009;75:1286–92. doi: 10.1055/s-0029-1185669. [DOI] [PubMed] [Google Scholar]
- 11.Balakumar P, Chakkarwar VA, Singh M. Ameliorative effect of combination of benfotiamine and fenofibrate in diabetes-induced vascular endothelial dysfunction and nephropathy in the rat. Mol Cell Biochem. 2009;320:149–62. doi: 10.1007/s11010-008-9917-z. [DOI] [PubMed] [Google Scholar]
- 12.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, Hines I, Zibari G, Grisham MB. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch Biochem Biophys. 2009;484:232–7. doi: 10.1016/j.abb.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricciardi R, Foley DP, Quarfordt SH, Saavedra JE, Keefer LK, Wheeler SM, et al. V-PYRRO/NO: A hepato-selective nitric oxide donor improves porcine liver hemodynamics and function after ischemia reperfusion. Transplantation. 2001;71:193–8. doi: 10.1097/00007890-200101270-00004. [DOI] [PubMed] [Google Scholar]
- 15.Katsumi H, Nishikawa M, Yamashita F, Hashida M. Prevention of hepatic ischemia/reperfusion injury by prolonged delivery of nitric oxide to the circulating blood in mice. Transplantation. 2008;85:264–9. doi: 10.1097/TP.0b013e31815e902b. [DOI] [PubMed] [Google Scholar]
- 16.Aiba M, Takeyoshi I, Ohwada S, Kawashima Y, Iwanami K, Sunose Y, et al. Novel nitric oxide donor (FK409) ameliorates liver damage during extended liver resection with warm ischemia in dogs. J Am Coll Surg. 2001;193:264–71. doi: 10.1016/s1072-7515(01)01002-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WH, Li JY, Zhou Y. Melatonin abates liver ischemia/reperfusion injury by improving the balance between nitric oxide and endothelin. Hepatobil Pancreat Dis Int. 2006;5:574–9. [PubMed] [Google Scholar]
- 18.Elimadi A, Sapena R, Settaf A, Le Louet H, Tillement J, Morin D. Attenuation of liver normothermic ischemia-reperfusion injury by preservation of mitochondrial functions with S-15176, a potent trimetazidine derivative. Biochem Pharmacol. 2001;62:509–16. doi: 10.1016/s0006-2952(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004;39:1533–43. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann G, Düsing R. ACE-inhibition, kinins, and vascular PGI2 synthesis. Eicosanoids. 1992;5(Suppl):S60–2. [PubMed] [Google Scholar]
- 21.Gräfe M, Bossaller C, Graf K, Auch-Schwelk W, Baumgarten CR, Hildebrandt A, et al. Effect of angiotensin-converting-enzyme inhibition on bradykinin metabolism by vascular endothelial cells. Am J Physiol. 1993;264:H1493–7. doi: 10.1152/ajpheart.1993.264.5.H1493. [DOI] [PubMed] [Google Scholar]
- 22.Storka A, Vojtassakova E, Mueller M, Kapiotis S, Haider DG, Jungbauer A, et al. Angiotensin inhibition stimulates PPAR gamma and the release of visfatin. Eur J Clin Invest. 2008;38:820–6. doi: 10.1111/j.1365-2362.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 23.Guckelberger O, Bechstein WO, Neuhaus R, Luesebrink R, Lemmens HP, Kratschmer B, et al. Cardiovascular risk factors in long-term follow-up after orthotopic liver transplantation. Clin Transplant. 1997;11:60–5. [PubMed] [Google Scholar]
- 24.Neal DA, Brown MJ, Wilkinson IB, Byrne CD, Alexander GJ. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation. 2004;77:748–50. doi: 10.1097/01.tp.0000116418.78963.dc. [DOI] [PubMed] [Google Scholar]
- 25.Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57(Suppl 1):S3–7. doi: 10.1093/ajhp/57.suppl_1.S3. [DOI] [PubMed] [Google Scholar]


