Abstract
Objective:
The aim of the present study was to evaluate the sedative and antiepileptic activities of ethanolic extract of Anthocephalus cadamba (ACE) bark in various experimental animal models.
Materials and Methods:
ACE was tested at three doses viz. 100, 200 and 400 mg/kg p.o. We used ketamine-induced sleeping time model to test the sedative property of the extract where, onset and duration of sleep were observed. A paradigm of anticonvulsant models (pentylenetetrazole, isoniazid and maximal electroshock-induced seizures) were used to evaluate its protective effect against absence and generalized types of seizures. Onset of clonic convulsions, tonic extension and time of death were observed in PTZ and INH-induced seizure models. In MES model, duration of tonic hind leg extension and onset of stupor were observed.
Results:
ACE showed significant increase in ketamine induced sleeping time. It also exhibited significant increase (P<0.05, 0.01 and 0.001) in latency to clonic convulsion, tonic extension and time of death in PTZ and INH models at all tested doses, whereas in the MES model, the lower dose was found to be effective when compared with the higher doses (200 and 400 mg/kg, p.o.).
Conclusion:
The results of the present investigation demonstrated that ACE possesses sedative and antiepileptic activities.
KEY WORDS: Anthocephalus cadamba, isoniazid, maximal electro shock, pentylenetetrazole, sedation, seizures
Introduction
Epilepsy is a serious neurological disorder, which does not have any boundaries such as age, race, social class or nationality. The incidence of the disease in developing countries is higher than that in developed countries and is reported to be 190 per one lakh people. The currently available anticonvulsant drugs suffer from drawbacks like teratogenic and other dose-related side effects. In spite of daily treatment, nearly 30% of patients continue to have convulsions.[1] The ancient systems of medicines provide a wide range of options for these problems with vast source of medicinal plants, which are devoid of undesirable effects and are gaining popularity in most of the developing countries.[2]
Anthocephalus cadamba Roxb. (Syn: Neolamarckia cadamba, Anthocephalus indicus; Family: Rubiaceae), commonly known as Kadamba, is a large tree widely distributed throughout Indian subcontinent. The ancient Indian systems of medicine have used various parts of this tree as a remedy for pain, fever, uterine complaints, hematological and dermatological problems and for gastrointestinal disorders.[3] Indole alkaloids cadambine, 3α-dihydrocadambine, cadamine, isocadamine, isodihydrocadambine, and triterpenes, tripernoid glycosides and saponins are reported to be present in bark.[4] The earlier studies have demonstrated antimicrobial, wound healing, antioxidant, antimalarial, hepatoprotective, analgesic, anti-inflammatory, antipyretic, anthelmintic, diuretic, laxative and antidiabetic activities of the plant.[4–6] Recently, Mondal et al. has reported that the bark extract produced sedation during the acute toxicity testing.[5] Hence, based on this observation, the present investigation was undertaken to evaluate the sedative and antiepileptic effects of the Anthocephalus cadamba ethanolic extract (ACE) using different animal models.
Material and Methods
Animals
Male Swiss albino mice (28 ± 6 g) and male Wistar rats (210 ± 30 g) bred in Animal House of Sree Siddaganga College of Pharmacy, Tumkur, Karnataka, India were used. The animals were fed standard laboratory diet and tap water ad libitum and were maintained at 12 h/12 h, light/dark cycle at an ambient temperature of 25 ± 2°C. The experiments were carried out after the approval by the Institutional Animal Ethics Committee (No: SSCPT/IAEC-86/2010-11) constituted as per the guidelines of CPCSEA, India.
Drugs and Chemicals
Ketamine hydrochloride (Neon laboratories Ltd, Mumbai, India) diazepam (Ranbaxy, New Delhi, India), phenytoin (Zydus Neuroscience, Ahmedabad, India), isoniazid (Sd-fine-chem, Mumbai, India) and pentylenetetrazole (Sigma Aldrich, St. Louis, USA) were used in the present study. Pentylenetetrazole (PTZ) and isoniazid (ISO) were dissolved in distilled water. ACE was suspended in 1% (v/v) Tween 80 and used.
Plant Material
The barks of A. cadamba Roxb. were collected locally in the month of June 2010. The plant was authenticated by Prof. K. Siddappa, Department of Botany, Sree Siddaganga College of Arts, Science and Commerce for Boys, Tumkur, Karnataka, India and a voucher specimen (#SSCP/Pcol-Pcog/10/02) of the bark was deposited in the college herbarium.
Preparation of Extract
The coarsely powdered material (100 g) of the shade-dried barks was extracted with 95% ethanol in a Soxhlet apparatus for 10 h. Then the extractive solution was concentrated to a small volume and evaporated to get a brown colored sticky mass (yield: 3.7g). The extract was stored at 4° C until use.
Evaluation of Sedative Activity
Ketamine-induced sleeping time
The method as described by Rabbani et al. was employed to measure the effect of ACE on ketamine induced sleeping time.[7] Thirty mice were divided into control (Group I) and treatment groups (Group II, III, IV and V) containing six animals each. Group I received saline and Groups III, IV and V recieved ACE 100, 200 and 400 mg/kg, respectively, 1 h prior to the intraperitoneal administration of ketamine (100 mg/kg). In case of Group II, mice were treated with diazepam (1.5 mg/kg, i.p.) 30 min prior to ketamine injection. The time between loss and gain of righting reflex, (duration of sleeping time) was recorded.
Evaluation of Anticonvulsant Activity
PTZ-induced seizures
Rats were divided into five groups consisting of six animals in each. Group I received saline, Group II received diazepam (4 mg/kg, i.p) and Groups III, IV and V received ACE 100, 200 and 400 mg/kg respectively. All the animals received PTZ (85 mg/kg, i.p.) 1 h after their respective treatment, except Group II that received PTZ after 30 min. The animals were observed for the following 30 min, in a separate cage for latency of clonic convulsion, tonic extension and time of death.[8]
Isonaiazid (INH)-induced seizures
ACE was administered orally to groups of mice at doses of 100, 200 and 400 mg/kg 1 h before i.p. administration of INH (300 mg/kg, i.p.) whereas, in Group II INH was injected after 30 min of diazepam (4 mg/kg, i.p) treatment. The animals were observed for the following 1 h for the onset of clonic convulsion, tonic extension and death latency.[9]
Maximal electroshock (MES)-induced seizures
Rats were divided into five groups of six animals in each. Groups I, III, IV and V were treated with saline, ACE at doses of 100, 200 and 400 mg/kg, respectively, and electroshock was applied after 1 h of the respective treatments, whereas Group II animals received phenytoin (25 mg/kg, i.p.) 30 min prior to the electroshock. Seizure was induced by current stimulus of 150 mA for 0.2 s duration intraaurically through electroconvulsiometer (INCO, Ambala Pvt. Ltd., India). Duration of tonic hind leg extension and onset of stupor were recorded.[8,10]
Statistical Analysis
All values are expressed as Mean±SEM. Statistical comparisons were performed by using One-way ANOVA followed by Tukey's Post-test using Graph Pad Prism version 5.0, USA. P-value less than 0.05 was considered statistically significant.
Results
Ketamine-Induced Sleeping Time
Treatment with ACE at the dose of 100 mg/kg exhibited significant reduction in sleep latency time (88.8 ± 8.188 s) when compared with the saline treated group (152.0 ± 9.048 s), whereas at higher doses, the extract failed to do so [Figure 1A]. However, ACE at all tested doses potentiated the sleeping time induced by ketamine and it was dose dependent. ACE showed prolongation of sleeping time at 200 and 400 mg/kg significantly (P<0.05; 0.01), which is comparable with diazepam as shown in Figure 1B.
Figure 1.
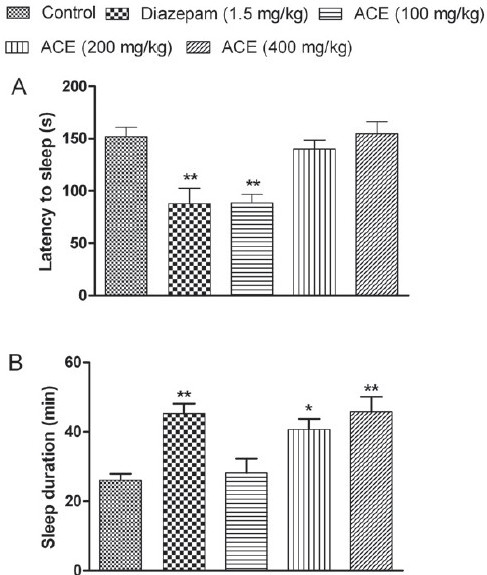
Effect of Anthocephalus cadmaba ethanolic extract (ACE) on (A) latency and on (B) duration of sleep in ketamine-induced sleeping time. Each bar represent Mean ± SEM (n=6). *P<0.05; **P<0.01 compared to control group
PTZ-Induced Seizures
Intraperitoneal injection of PTZ elicited various phases of convulsions such as myoclonic jerking, repeated forelimb clonus and forelimb/hindlimb tonic extension and finally death. A significant increase (P<0.05) in the latency to clonic convulsions was observed with the ACE-treated groups compared with the control [Figure 2A]. Treatment with ACE (100 and 200 mg/kg) showed a significant increase (P<0.001) in latency to tonic phase, whereas ACE (400 mg/kg) exhibited higher latency period, but statistically insignificant. A significant increase in latency to death was observed with all tested doses of ACE; however, the effect was not dose-dependent [Figure 2B]. In the diazepam-treated group none of the animals showed any phases of convulsions or death.
Figure 2.
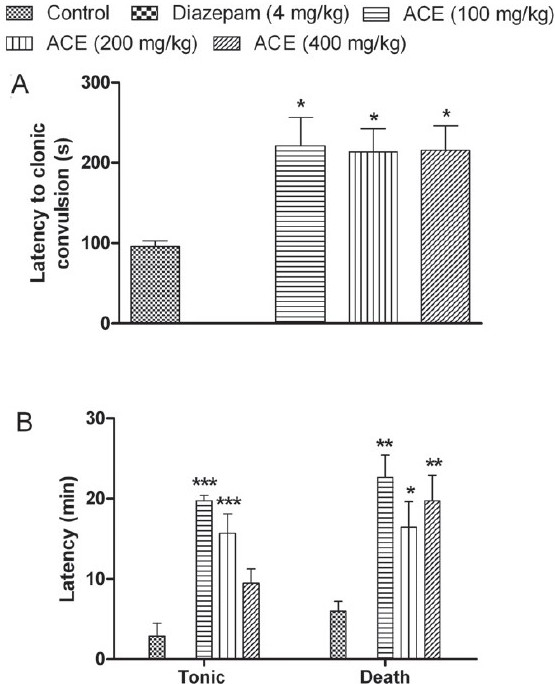
Effect of Anthocephalus cadmaba ethanolic extract (ACE) on (A) latency to clonic convulsions and on (B) tonic extension and death in Pentylenetetrazole-induced seizures. Each bar represent Mean ± SEM (n=6). *P<0.05; **P<0.01; ***P<0.001 compared to control group
INH-Induced Seizures
Isoniazid produced clonic followed by tonic seizures in all the animals used. ACE showed a dose dependent delay in the onset of clonic convulsions when compared with the control group. Treatment with higher doses of ACE (200 and 400 mg/kg) showed significant increase (P<0.001) in latency of clonic convulsions whereas lower dose failed to do so. Latency to tonic extensor phase was significantly increased at all doses. At 400 mg/kg, ACE showed protection against the tonic extensor phase, but statistically insignificant. At all tested doses, the extract was able to show significant increase (P<0.05) in latency to death. These results were comparable with the diazepam treatment as shown in Figure 3.
Figure 3.
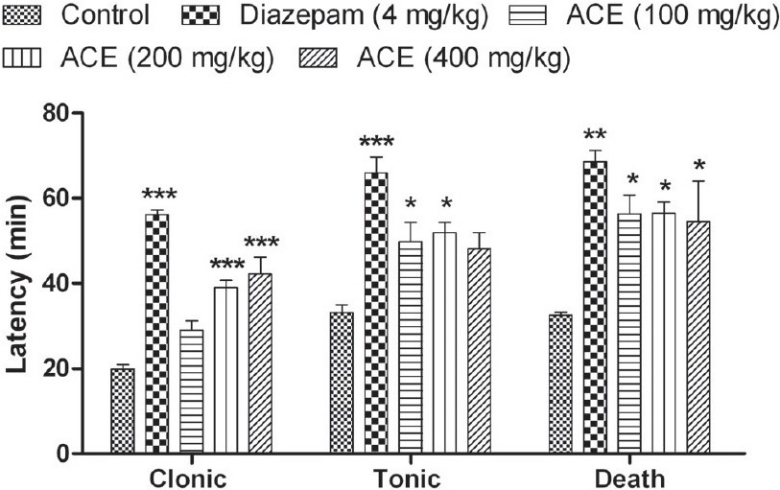
Effect of Anthocephalus cadmaba ethanolic extract (ACE) on latency to clonic convulsions, tonic extension and death in Isoniazidinduced seizures. Each bar represent Mean ± SEM (n=6). *P<0.05; **P<0.01; ***P<0.001 compared to control group
MES-Induced Seizures
Following electrical stimulus, an immediate severe tonic seizure with maximal extension of the anterior and posterior limbs was observed. Treatment with ACE (100 and 200 mg/kg) significantly decreased (P<0.05) the duration of tonic hind limb extensor phase, whereas higher dose (400 mg/kg) did not produce any reduction in the tonic extension period [Figure 4A]. Onset of stupor was decreased significantly (P<0.001) at all tested doses and the results were comparable with standard phenytoin-treated group [Figure 4B].
Figure 4.
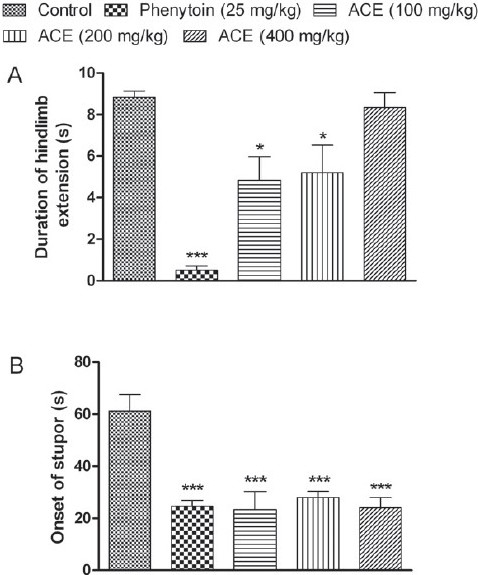
Effect of Anthocephalus cadmaba ethanolic extract (ACE) on (A) duration of tonic extensor phase and on (B) onset of stupor in Maximal electro shock-induced seizures. Each bar represent Mean ± SEM (n=6). *P<0.05; ***P<0.001 compared to control group
Discussion
The present study was designed to evaluate the sedative and antiepileptic activities of A. cadamba ethanolic extract in ketamine-induced sleeping time and MES, PTZ and INH-induced seizure models. Ketamine-induced sedation is an extensively used model to screen sedatives and anesthetics.[11] Ketamine is an antagonist for N-Methyl-D-Aspartate (NMDA) type of excitatory receptor system, which have very significant role in seizures. It has been reported to cause sedation with additional γ-Amino Butyric Acid-A (GABA-A) receptor potentiation.[12] In our study, ACE potentiated ketamine-induced sedation, since a significant increase in sleeping time was observed. Hence, the potentiation of ketamine-induced sleeping time by ACE may be due to modulation of GABA or NMDA system, proving the sedative potential. Moreover, the extract also showed protection against PTZ-induced convulsions, thus supporting the sedative mode of action, since PTZ is believed to be an antagonist at GABA-A receptor complex and produces seizures by inhibiting the GABA pathway in the CNS resulting in an imbalance between the ionic concentrations of the membrane. In addition, protection of ACE against PTZ-induced seizures implies efficacy against absence seizures.[13]
Treatment with ACE, at all the doses deyaled the latency for clonic convulsions, tonic extensor and death in INH model. INH is an antitubercular drug, which induces seizures at higher doses by interfering with the synthesis of GABA, through the inhibition of pyridoxal–5–phosphate, a cofactor for the Glutamic Acid Decarboxylase (GAD), an enzyme that catalyzes the synthesis of GABA from glutamic acid.[14,15] The compounds that can facilitate GABAergic transmission may show protective action in this model. Sedatives act through modulation of GABA receptors, which is also the mode of action for benzodiazepine and barbiturate class of anticonvulsants.[15] Similar mechanism can be drawn for ACE, since it showed protection against INH-induced convulsions and also potentiated ketamine-induced sedation.
Further to know the profile of protection of ACE, we carried out MES-induced convulsion model, which is a widely used tool to screen drugs for generalized tonic-clonic seizures. MES causes several changes at the cellular level, which can disrupt the signal transduction in the neurons. One of the most important mechanisms by which it causes cellular damage is facilitation of Ca2+ entry into the cells in large amounts and thus, prolonging the duration of convulsions.[16] Apart from Ca2+ ions, MES may also facilitate the entry of other positive ions like Na+, blockade of which, can prevent the MES-induced tonic extension.[2,17] Currently available anticonvulsant drugs like valproate and phenytoin act by modulation of these ion channels.[15] On the other hand, potentiation of opioid and GABA receptors are also reported to protect against MES-induced seizures.[9,18] ACE exhibited protection against tonic extensor phase at lower tested doses (100 and 200 mg/kg), whereas at higher dose (400 mg/kg) failed to do so. Moreover, the protection offered by 100 and 200 mg/kg ACE treatments was similar and were not statistically different. This observed effect suggests that the protection of ACE is maximum at 100 mg/kg. However, ACE was successful in decreasing the time taken for stupor at all tested doses, which may be because of modulation of sedative mechanisms.
It has been reported that A. cadamba bark contains alkaloids like cadambine and its derivatives, saponins, glycosides, triterpinoids, cadambagic acid, quinovic acid and β-sitosterol.[3,4] Several reports suggest that alkaloids, triterpenic steroids and flavonoids have potent antiepileptic effect in various seizure models.[2] In addition to this, saponins have also been able to modulate the neurotransmitter levels in the brain and to possess potent anti-convulsant activity.[19] Therefore, the presence of such compounds in the extract may be responsible for the sedative and anticonvulsant activities.
Conclusions
The present investigation demonstrated that A. cadamba ethanolic extract has sedative and anticonvulsant activities in different tested animal models. Further, these observations suggest that ACE may be potentiating GABAergic transmission or modulation of ion channels in CNS. However, further research is required to elucidate its specific mechanism of action and responsible active principles.
Acknowledgments
We are gratefully acknowledging the financial support by the Vision Group on Science and Technology, Government of Karnataka under the programme for Establishment of Centre of Excellence in Herbal Drug Development sanctioned to us (VGST/PRMG/CESEM-1/2009-10/199). SBD and NP thank to AICTE for the award of GATE fellowship and Dr. VPV for CAYT fellowship of AICTE.
Footnotes
Source of Support: Vision Group on Science and Technology, Government of Karnataka.
Conflict of Interest: None declared.
References
- 1.Neurological disorders: Public health challenges. Switzerland: World Health Organization; 2006. Anonymous. [Google Scholar]
- 2.Hegde K, Thakker SP, Joshi AB, Shastry CS, Chandrashekhar KS. Anticonvulsant activity of Carissa carandas Linn. root extract in experimental mice. Trop J Pharm Res. 2009;8:117–25. [Google Scholar]
- 3.Kumar V, Khan MM, Khanna AK, Singh R, Singh S, Chander R, et al. Lipid lowering activity of Anthocephalus indicus root in hyperlipidemic rats. Evid Based Complement Alternat Med. 2010;7:317–22. doi: 10.1093/ecam/nen001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umachigi SP, Kumar GS, Jayaveera K, Kishore KD, Ashok KC, Dhanapal R. Antimicrobial, wound healing and antioxidant activities of Anthocephalus cadamba. Afr J Tradit Complement Altern Med. 2007;4:481–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Mondal S, Dash GK, Acharyya A. Analgesic, anti-inflammatory and antipyretic studies of Neolamarckia cadamba barks. J Pharm Res. 2009;2:1133–6. [Google Scholar]
- 6.Acharyya S, Dash GK, Mondal S, Dash SK. Studies on glucose lowering efficacy of the Anthocephalus cadamba (roxb.) Miq. roots. Int J Pharm Bio Sci. 2010;1:1–9. [Google Scholar]
- 7.Rabbani M, Sajjadi SE, Mohammadi A. Evaluation of the anxiolytic effect of Nepeta persica Boiss.in mice. Evid Based Complement Alternat Med. 2008;5:181–6. doi: 10.1093/ecam/nem017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahendran S, Thippeswamy BS, Veerapur VP, Badami S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine. 2011;18:186–8. doi: 10.1016/j.phymed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Sandrini M, Marrama D, Vergoni AV, Bertolini A. Repeated administration of triiodothyronine enhances the susceptibility of rats to isoniazid- and picrotoxin-induced seizures. Life Sci. 1992;51:765–70. doi: 10.1016/0024-3205(92)90486-9. [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, Nagori BP, Sasmal D. Sedative and anticonvulsant effects of an alcoholic extract of Capparis deciduas. J Nat Med. 2009;63:375–9. doi: 10.1007/s11418-009-0339-3. [DOI] [PubMed] [Google Scholar]
- 11.Tose R, Kushikata T, Yoshida H, Kudo M, Furukawa K, Ueno S, et al. Orexin A decreases ketamine-induced anesthesia time in the rat: The relevance to brain noradrenergic neuronal activity. Anesth Analg. 2009;108:491–5. doi: 10.1213/ane.0b013e31819000c8. [DOI] [PubMed] [Google Scholar]
- 12.Henschel O, Gipson KE, Bordey A. GABAA receptors, anesthetics and anticonvulsants in brain development. CNS Neurol Disord Drug Targets. 2008;7:211–24. doi: 10.2174/187152708784083812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozbakis-Dengiz G, Bakirci A. Anticonvulsant and hypnotic effects of amiodarone. J Zhejiang Univ Sci B. 2009;10:317–22. doi: 10.1631/jzus.B0820316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajender V, Saluja J. INH induced status epilepticus: Response to pyridoxine. Indian J Chest Dis Allied Sci. 2006;48:205–6. [PubMed] [Google Scholar]
- 15.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Philadelphia: Churchil Livingstone, Elsevier science Ltd; 2003. [Google Scholar]
- 16.Inan SY, Buyukafsar K. Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br J Pharmacol. 2008;155:44–51. doi: 10.1038/bjp.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bum EN, Nkantchoua GN, Njikam N, Taiwe GS, Ngoupaye GT, Pelanken MM, et al. Anticonvulsant and sedative activity of leaves Senna spectabilis in mice. Int J Pharmacol. 2010;6:123–8. [Google Scholar]
- 18.Manocha A, Sharma KK, Mediratta PK. The anticonvulsant effect of pentazocine against maximal electroshock seizure in mice. Indian J Pharmacol. 1997;29:194–7. [Google Scholar]
- 19.Pal D, Sannigrahi S, Mazumder UK. Analgesic and anticonvulsant effects of saponins isolated from the leaves of Clerodendrum infortunatum Linn in mice. Indian J Exp Biol. 2007;47:743–7. [PubMed] [Google Scholar]


