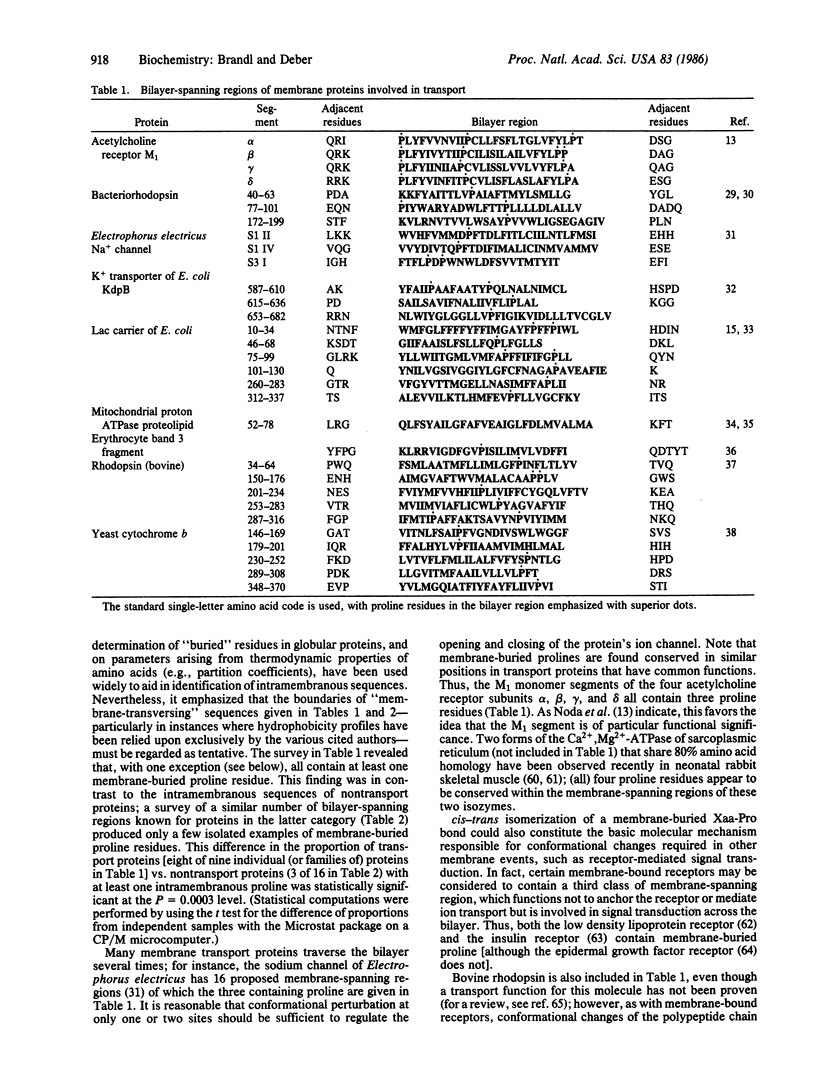
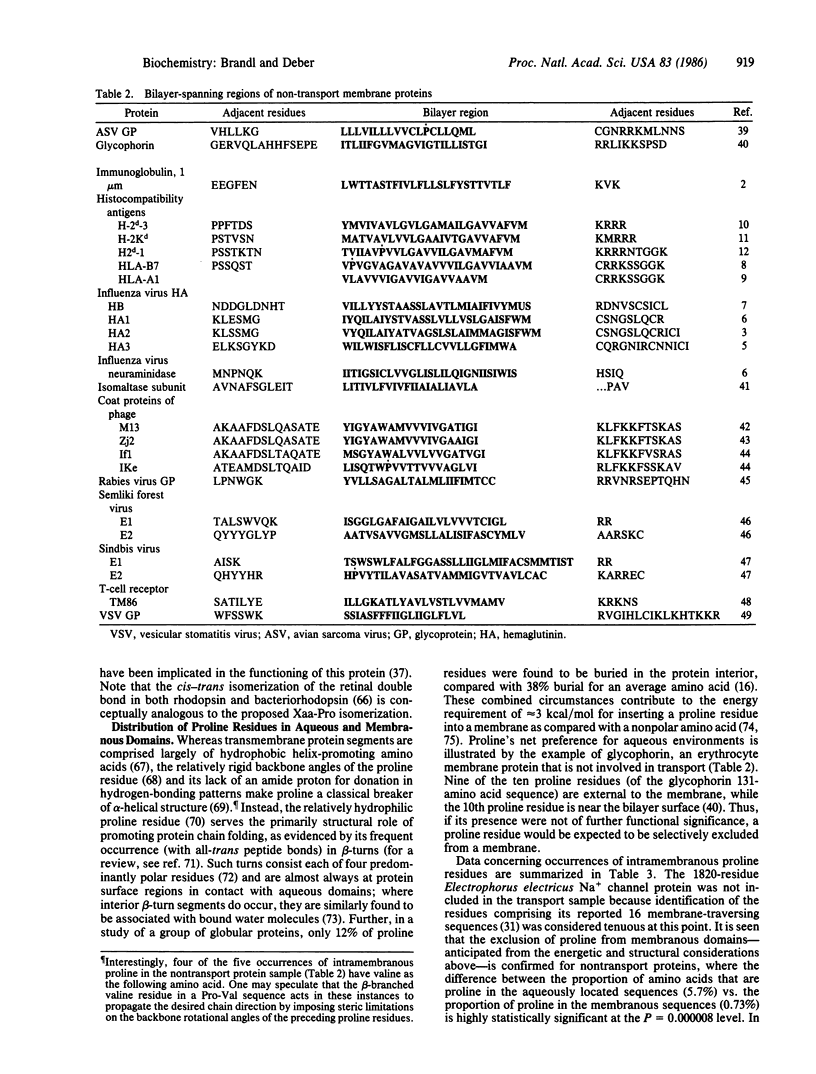
Abstract
In a survey of the bilayer-spanning regions of integral membrane proteins, membrane-buried proline residues were found in nearly all transport proteins examined, whereas membrane-buried regions of nontransport proteins were largely devoid of intramembranous proline residues. When amino acids from the complete sequences of representative sets of transport and nontransport membrane proteins were analyzed for the distribution of proline residues between aqueous vs. membranous domains, proline was shown to be selectively excluded from membranous domains of the nontransport proteins, in accord with expectation from energetic and structural considerations. In contrast, proline residues in transport proteins were evenly distributed between aqueous and membranous domains, consistent with the notion that functional membrane-buried proline residues are selectively included in transport proteins. As cis peptide bonds involving proline arise in proteins and have been implicated in protein dynamic processes, the cis-trans isomerization of an Xaa-Pro peptide bond (Xaa = unspecified amino acid) buried within the membrane--and the resulting redirection of the protein chain--is proposed to provide the reversible conformational change requisite for the regulation (opening/closing) of a transport channel. Parallel to this function, the relatively negative character of the carbonyl groups of Xaa-Pro peptide bonds may promote their participation as intramembranous liganding sites for positive species in proton/cation transport processes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Argos P., Rao J. K., Hargrave P. A. Structural prediction of membrane-bound proteins. Eur J Biochem. 1982 Nov 15;128(2-3):565–575. doi: 10.1111/j.1432-1033.1982.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Argos P., Rao J. K., Hargrave P. A. Structural prediction of membrane-bound proteins. Eur J Biochem. 1982 Nov 15;128(2-3):565–575. doi: 10.1111/j.1432-1033.1982.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J., Kempf C. The human erythrocyte anion-transport protein. Partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J. 1983 Sep 1;213(3):577–586. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brégégère F., Abastado J. P., Kvist S., Rask L., Lalanne J. L., Garoff H., Cami B., Wiman K., Larhammar D., Peterson P. A. Structure of C-terminal half of two H-2 antigens from cloned mRNA. Nature. 1981 Jul 2;292(5818):78–81. doi: 10.1038/292078a0. [DOI] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clément J. M. On the insertion of proteins into membranes. Biochimie. 1983 Jun;65(6):325–338. doi: 10.1016/s0300-9084(83)80154-0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Min Jou W., Huylebroeck D., Devos R., Fiers W. Complete structure of A/duck/Ukraine/63 influenza hemagglutinin gene: animal virus as progenitor of human H3 Hong Kong 1968 influenza hemagglutinin. Cell. 1981 Aug;25(2):315–323. doi: 10.1016/0092-8674(81)90049-0. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Alger J. R., Liakopoulou-Kyriakides M. s-Cis...and s-trans isomerism in acylproline analogs. Models for conformationally locked proline peptides. Int J Pept Protein Res. 1982 Feb;19(2):123–132. doi: 10.1111/j.1399-3011.1982.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry M. A., Gutowsky H. S., Oldfield E. Surface dynamics of the integral membrane protein bacteriorhodopsin. 1984 Jan 26-Feb 1Nature. 307(5949):383–386. doi: 10.1038/307383a0. [DOI] [PubMed] [Google Scholar]
- Kennedy S. J. Structures of membrane proteins. J Membr Biol. 1978 Sep 19;42(3):265–279. doi: 10.1007/BF01870362. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981 Apr 9;290(5806):449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Bregegere F., Rask L., Cami B., Garoff H., Daniel F., Wiman K., Larhammar D., Abastado J. P., Gachelin G. cDNA clone coding for part of a mouse H-2d major histocompatibility antigen. Proc Natl Acad Sci U S A. 1981 May;78(5):2772–2776. doi: 10.1073/pnas.78.5.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature. 1981 Jul 16;292(5820):201–204. doi: 10.1038/292201a0. [DOI] [PubMed] [Google Scholar]
- Lewin R. Unexpected Progress in Photoreception: A recent gathering of photobiologists has replaced their major guiding hypothesis of the past 15 years by a new one. Science. 1985 Feb 1;227(4686):500–503. doi: 10.1126/science.227.4686.500. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Determination of cis-trans proline isomerization by trypsin proteolysis. Application to a model pentapeptide and to oxidized ribonuclease A. Biochemistry. 1983 Feb 1;22(3):553–559. doi: 10.1021/bi00272a005. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Isomerization of proline-93 during the unfolding and refolding of ribonuclease A. Biochemistry. 1983 Feb 1;22(3):559–563. doi: 10.1021/bi00272a006. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Mechanism for the unfolding and refolding of ribonuclease A. Kinetic studies utilizing spectroscopic methods. Biochemistry. 1983 Feb 1;22(3):564–573. doi: 10.1021/bi00272a007. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Mao D., Wachter E., Wallace B. A. Folding of the mitochondrial proton adenosinetriphosphatase proteolipid channel in phospholipid vesicles. Biochemistry. 1982 Sep 28;21(20):4960–4968. doi: 10.1021/bi00263a020. [DOI] [PubMed] [Google Scholar]
- Meek J. L. Prediction of peptide retention times in high-pressure liquid chromatography on the basis of amino acid composition. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1632–1636. doi: 10.1073/pnas.77.3.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Frangione B., Wiseman R. L., Konigsberg W. H. Primary structure of the major coat protein of the filamentous bacterial viruses, If1 and Ike. J Biol Chem. 1981 Jun 10;256(11):5792–5797. [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Lopez de Castro J. A., Parham P., Ploegh H. L., Strominger J. L. Comparison of amino acid sequences of two human histocompatibility antigens, HLA-A2 and HLA-B7: location of putative alloantigenic sites. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4395–4399. doi: 10.1073/pnas.76.9.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Roy S. Hydrophobic basis of packing in globular proteins. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4643–4647. doi: 10.1073/pnas.77.8.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. D., Young W. B., Gierasch L. M. Interior turns in globular proteins. Nature. 1983 Aug 18;304(5927):654–657. doi: 10.1038/304654a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Welch W. J., Sefton B. M., Esch F. S., Ling N. C. Vesicular stomatitis virus glycoprotein is anchored in the viral membrane by a hydrophobic domain near the COOH terminus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3884–3888. doi: 10.1073/pnas.77.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Radhakrishnan R., Robson R. J., Khorana H. G. The transmembrane domain of glycophorin A as studied by cross-linking using photoactivatable phospholipids. J Biol Chem. 1982 Apr 25;257(8):4152–4161. [PubMed] [Google Scholar]
- Sarkar S. K., Young P. E., Sullivan C. E., Torchia D. A. Detection of cis and trans X-Pro peptide bonds in proteins by 13C NMR: application to collagen. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4800–4803. doi: 10.1073/pnas.81.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. Secondary and tertiary structure of membrane proteins involved in proton translocation. Biochim Biophys Acta. 1983 Jul 15;726(2):81–95. doi: 10.1016/0304-4173(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Thermodynamics, the structure of integral membrane proteins, and transport. J Supramol Struct. 1977;6(3):313–323. doi: 10.1002/jss.400060304. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Pease L. G. Reverse turns in peptides and proteins. CRC Crit Rev Biochem. 1980;8(4):315–399. doi: 10.3109/10409238009105470. [DOI] [PubMed] [Google Scholar]
- Snell D. T., Offord R. E. The amino acid sequence of the B-protein of bacteriophage ZJ-2. Biochem J. 1972 Mar;127(1):167–178. doi: 10.1042/bj1270167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz T. A., Goldman A., Engelman D. M. Quantitative application of the helical hairpin hypothesis to membrane proteins. Biophys J. 1982 Jan;37(1):124–125. doi: 10.1016/S0006-3495(82)84633-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T. C., Weissman L., Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities. Biophys J. 1982 Jan;37(1):353–361. doi: 10.1016/S0006-3495(82)84683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., Di Corato A., Wong S. C., Deber C. M., Blout E. R. Cyclic peptides. II. Solution conformations of cyclo(ProlyLserylglycylprolylserylglycyl) from nuclear magnetic resonance. J Am Chem Soc. 1972 Jan 26;94(2):609–615. doi: 10.1021/ja00757a048. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Veis A., Nawrot C. F. Basicity differences among peptide bonds. J Am Chem Soc. 1970 Jul 1;92(13):3910–3914. doi: 10.1021/ja00716a013. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. S., Scheraga H. A. Stability of cis, trans, and nonplanar peptide groups. Macromolecules. 1976 May-Jun;9(3):408–416. doi: 10.1021/ma60051a005. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]



